Abstract
Background
Ambient particulate matter (PM) derived from coal-fired power plants may have important cardiovascular effects, but existing toxicological studies are inadequate for understanding these effects. The Toxicological Evaluation of Realistic Emissions of Source Aerosols (TERESA) study aims to evaluate the toxicity of primary and secondary PM derived from coal-fired power plants. As part of this effort, we evaluated in susceptible animals the effect of stack emissions on cardiac electrophysiology and respiratory function under exposure conditions intended to simulate an aged plume with unneutralized acidity and secondary organic aerosols (POS exposure scenario).
Methods
Rats with acute myocardial infarction were exposed to either stack emissions (n=15) or filtered air (n=14) for 5 hours at a single power plant. Respiration and electrocardiograms were continuously monitored via telemetry and heart rate, heart rate variability (HRV), premature ventricular beat (PVB) frequency, electrocardiographic intervals, and respiratory intervals and volumes were evaluated. Similar experiments at another power plant were attempted but were unsuccessful.
Results
POS exposure (fine particle mass = 219.1 μg/m3; total sulfate = 172.5 μg/m3; acidic sulfate = 132.5 μg/m3; organic carbon = 50.9 μg/m3) was associated with increased PVB frequency and decreased respiratory expiratory time and end-inspiratory pause, but not with changes in heart rate, HRV, or electrocardiographic intervals. Results from a second power plant were uninterpretable.
Conclusions
Short-term exposure to primary and unneutralized secondary particulate matter formed from aged emissions from a coal-fired power plant, as simulated by the POS scenario, may be associated with increased risk of ventricular arrhythmias in susceptible animals.
Keywords: coal-combustion, particulate matter, cardiac, myocardial infarction, arrhythmia, heart rate variability, respiratory
Introduction
Ambient air pollution is a recognized risk factor for cardiovascular morbidity and mortality (Brook et al., 2004). Short-term elevations in ambient particulate matter (PM) have been specifically implicated in the triggering of acute cardiovascular events including myocardial infarction (MI) (Peters et al., 2001; Zanobetti and Schwartz, 2005), ventricular arrhythmias (Peters et al., 2000; Rich et al., 2005), heart failure exacerbations (Schwartz and Morris, 1995; Dominici et al., 2006; Wellenius et al., 2006), and ischemic stroke (Tsai et al., 2003; Wellenius et al., 2005).
Because the toxicity of PM likely varies by source, quantifying the effects of PM from sources such as biomass burning, power plants, and gasoline and diesel emissions is of substantial public health interest. In the US, coal-fired power plants are used to generate the majority of electricity consumed (US Energy Information Administration, 2009). Ambient PM consist of primary particles emitted directly from sources and secondary particles formed by chemical reaction of particles and gases in the atmosphere. Source apportionment studies in a number of US cities have found that coal-fired power plants contribute significantly to ambient PM levels, primarily in the form of secondary sulfates formed from oxidation of SO2 and nitrogen oxides (NOx) from stack emissions (Laden et al., 2000; Maykut et al., 2003; Brown et al., 2007; Duvall et al., 2008; Sarnat et al., 2008). For example, Duvall et al. (2008) estimated that secondary sulfate from coal combustion contributed 24-48% of fine PM mass at 6 sites across the US.
There are limited published results from animal experiments examining the cardiovascular effects of primary particles emitted from coal-fired power plants. Studies using inhaled or instilled coal fly ash in laboratory animals suggest that this material can elicit a pulmonary and systemic inflammatory response (Gilmour et al., 2004; Smith et al., 2006; Finnerty et al., 2007). However, the current relevance of these studies to human health is unclear since emissions of primary particles from coal-fired power plants are regulated and ambient concentrations of these primary particles are quite low.
The toxicological effects of secondary PM, in addition to residual primary PM, have not been examined in detail. Epidemiologic studies provide some evidence that emissions from coal-fired power plants may have important cardiovascular health effects. First, some (Ito et al., 2006; Mar et al., 2006), but not all (Sarnat et al., 2008), source apportionment studies have found an association between ambient PM from coal-fired power plants and cardiovascular morbidity and mortality. Second, short-term changes in ambient levels of sulfates have been associated with cardiovascular hospitalizations (Burnett et al., 1995), adverse changes in predictors of cardiovascular events including markers of inflammation, coagulation, autonomic function, and vascular reactivity (O'Neill et al., 2005; Luttmann-Gibson et al., 2006; Chuang et al., 2007), and increased rate of supraventricular cardiac arrhythmias (Sarnat et al., 2006). Additionally, a source apportionment study in animals exposed to concentrated ambient air particles (CAPs) identified associations between secondary sulfates and changes in heart rate and heart rate variability (Lippmann et al., 2005). On the other hand, a number of time-series and source apportionment studies have not detected statistically significant relationships between health endpoints and sulfate or other markers of coal combustion (Reviewed by: Reiss et al., 2007).
Laboratory-generated sulfates on their own do not appear to have significant health effects in normal humans (Schlesinger and Cassee, 2003). This finding suggests that the potential health effects of secondary sulfate created by the conversion of SO2 and NOx from stack emissions in the presence of residual primary particles and biogenic organic material warrants further investigation. The Toxicological Evaluation of Realistic Emissions of Source Aerosols (TERESA) study is a comprehensive effort to evaluate the toxicity of both primary and secondary PM derived from coal-fired power plants. Importantly, this study attempted to simulate the complex atmospheric chemical reactions that stack emissions undergo during transport from the source to distant sites. As part of this effort, we evaluated the effect of stack emissions from coal-fired power plants on electrocardiographic changes in a rat model of acute myocardial infarction.
Methods
Overview
In the TERESA study, emissions were drawn from the stack at three separate power plants into a mobile laboratory where oxidants were added to oxidize SO2 to sulfate. Other atmospheric constituents (e.g., NH3, secondary organic aerosol) were added in pre-specified combinations in order to study the effects of different atmospheric conditions. Laboratory rats were then exposed to the resulting atmospheric mixtures, with PM present at environmentally relevant concentrations and hematologic, respiratory, and cardiovascular effects were evaluated. This report focuses on the cardiovascular effects of primary particles emitted directly from coal-fired power plants in conjunction with unneutralized secondary particles formed from emitted SO2 (POS scenario), as assessed by measuring changes in electrocardiographic parameters in a rat model of acute MI. The overall TERESA study design is described in detail elsewhere (Godleski et al., 2010b; Kang et al., 2010). Results for other biologic outcomes are published separately (Diaz et al., 2010; Godleski et al., 2010a; Lemos et al., 2010).
In the TERESA study, most biologic outcomes were assessed under a variety of experimental scenarios and at 3 different power plants (Power Plants 1, 2, and 3). Specifically, in order to simulate atmospheric transformations that coal power plant emissions undergo in a plume the following scenarios were chosen: (1) primary emissions only (“P”); (2) the oxidation of SO2 to form H2SO4 aerosol, along with primary particles (“PO”); (3) the oxidation of SO2 plus the reaction of α-pinene with ozone to form secondary organic aerosols, along with primary particles (“POS”); and (4) the neutralization of H2SO4 aerosol by NH3, along with primary particles and secondary organic aerosols (“PONS”).
Due to the complexity of the acute MI animal model, we chose a priori to conduct these experiments only under the exposure scenario producing the greatest effects in normal rats. Logistical considerations necessitated that the decision of which exposure to use be made shortly after the findings of the initial exposures of normal animals to each scenario were completed. Preliminary analyses of heart chemiluminescence data from Power Plant 1 suggested no health effects under any scenario, so experiments using the acute MI animal model were not carried out at Power Plant 1. Preliminary analyses of data from Power Plant 2 suggested similar increases in heart chemiluminescence with the POS and PONS exposure scenarios, and we chose to use the POS exposure scenario for experiments using the acute MI animal model. The same scenario was chosen for experiments at Power Plant 3 to be consistent. Thus, results from the POS scenario at Power Plants 2 and 3 are reported here.
Animals
Adult, male Sprague-Dawley rats weighing ~250g (Charles River Laboratories, Inc., Wilmington, MA) were maintained and studied in accordance with the National Institutes of Health guidelines for the care and use of animals in research. Animals were housed (12-h light/dark cycle) in plastic cages with pine chip bedding (Northeastern Products Corp., Warrensburg, NY) and received food (LabDiet, PMI Nutrition International, Inc., Brentwood, MO) and water ad libitum. All protocols were approved by the Harvard Medical Area Standing Committee on Animals.
Surgical Protocol
Rats were implanted with a radio telemetry transmitter (DSI PhysioTel® Transmitter ETA-F20, Data Sciences International, Inc., Saint Paul, MN) for measurement of the ECG. Electrodes were implanted subcutaneously in a Lead II configuration, as previously described (Wellenius et al., 2004). At least 2 weeks post-implantation, left-ventricular myocardial infarction was induced by thermocoagulation as previously described (Wellenius et al., 2002). Briefly, under inhalation anesthesia, a left thoracotomy was performed via the third or fourth intercostal space to gain access to the left ventricular wall of the heart. Myocardial infarction was induced by briefly and repeatedly applying the tip (0.5″ fine electrode) of a portable thermocautery unit (2200°C, Aaron Medical Industries, Inc., St. Petersburg, FL) to one or more visible branches of the left coronary artery. Visible discoloration of the affected region indicated that blood flow had been successfully interrupted. Each animal was allowed to recover for at least 12h, and then exposure was carried out so that evaluation on cardiac changes would be within the 24 hour post-MI window of greatest vulnerability.
Exposure Technology and Characterization
Details of the exposure set up and exposure assessment are provided elsewhere (Ruiz et al., 2007a; Ruiz et al., 2007b; Godleski et al., 2010b; Kang et al., 2010). Briefly, stack emissions composed primarily of SO2, NO and primary PM were diluted by addition of filtered (dry) air as needed and delivered to photochemical chambers. In a first chamber, OH radicals were generated by the photolysis of ozone, and SO2 was oxidized to form sulfuric acid. Subsequently, in a second chamber, the oxidized plume was mixed with secondary organic aerosols by addition of α-pinene and ozone (POS scenario). The mixture was then passed through a counter-current parallel plate membrane denuder (Ruiz et al., 2006) to remove gaseous pollutants and the resulting aerosols were delivered to the animal exposure chambers. Control animals with acute MI were exposed to room air filtered through a HEPA filter (using Millipore Opticap filters at a flow of 1.5 liters per min) and are designated as Sham animals in this paper.
Electrocardiographic (ECG) Data Acquisition and Analysis
Real-time ECG waveforms were continuously displayed and recorded using a PC-based system (Dataquest ART, Data Sciences International, Inc.). Offline, ECG signals were reviewed and analyzed using customized software scripts in Matlab (Mathworks, Inc., Natick, MA). Premature ventricular beats (PVBs) were identified and annotated by an investigator blinded to the exposure status of each animal and the number of PVBs for each exposure hour recorded. If there were >50 PVBs in a given hour, the total number for that hour was estimated based on review of 3 1-min windows positioned 5, 30, and 55-min into that hour.
To assess heart rate and heart rate variability, normal sinus beats were automatically labeled and subsequently verified by an investigator. We calculated heart rate as the reciprocal of the 3-min mean normal beat-to-beat interval, SDNN, a measure of total heart rate variability, as the standard deviation of all normal beat-to-beat intervals within a 3-min interval, and RMSSD, a measure of heart rate variability that reflects parasympathetic nervous system activity, as the root-mean-square of successive differences among all normal beat-to-beat intervals within a 3-min interval. Heart rate and heart rate variability were assessed at 0, 60, 120, 180, and 240 min after the start of the exposure. If the ECG within 10 min of each time point could not be automatically labeled by the software or was otherwise of insufficient quality, no value was reported for that time point for that rat.
ECG intervals were estimated every 10 sec using Ponemah Physiology Platform version 4.8 (DSI Ponemah, Valley View, OH) Because T-wave morphology was distorted by the acute myocardial infarct in these animals, we restricted analysis of ECG intervals to the PR interval (defined as the time in ms from the beginning of the P wave to the start of the Q wave) and P-wave duration (defined as the time in ms from the beginning to the end of the P wave). Statistical analyses were based on the mean PR interval and P wave duration for each rat for each hour.
Respiratory Function
During exposures, continuous respiratory data were collected using a whole body plethysmography system (Buxco Electronics Inc., Wilmington, NC), as described in detail elsewhere (Diaz et al., 2010). Briefly, flow through each chamber was maintained at 1.5LPM and Buxco air flow transducers (TRD5700) were connected to the chambers and to a reference chamber to compensate for changes in pressure in the system. Each chamber was calibrated to its respective transducer using a 1.5 LPM flow at the beginning of each scenario (week), a daily check of the accuracy of these calibrations were performed before each exposure. A sampling protocol was designed to collect continuous data and 10 minute averages of the following parameters: frequency, tidal volume, inspiratory time, expiratory time, enhanced pause (Penh), minute volume, peak inspiratory flow, peak expiratory flow, end inspiratory pause, end expiratory pause, expiratory flow at 50%, and pause. A rejection algorithm was automatically included in the breath by breath analysis.
Histopathology
Histopathologic confirmation of the MI was carried out in all animals within 1 week after infarction. Animals were euthanized using an overdose of sodium pentobarbital. The heart was removed and placed in 10% buffered formalin (Fisher Scientific, Pittsburgh, PA). After at least several days of fixation, the heart was dissected into serial 2–3 mm cross sections from the apex to base. These cross sections of the ventricles were processed routinely for paraffin histology, sectioned at 4 mm thickness, stained with hematoxylin and eosin, and examined by light microscopy to assess the presence and extent of infarction. This histopathological assessment was done by an investigator blinded with regard to exposure group and outcomes.
Statistical analysis
The statistical analysis of arrhythmia count data can be methodologically challenging when there are more subjects with no arrhythmias than would be expected under a Poisson model (Lambert, 1992). First, we tested the hypothesis that exposure to POS increases PVB frequency as compared to control animals exposed to filtered air. We used repeated-measures Poisson regression (Diggle et al., 2002) fit using generalized estimating equations (GEE) to model PVB frequency during each exposure hour. This model included indicator variables for time (5 indicator variables) and group (exposed versus filtered air). As in previous analyses (Wellenius et al., 2004) we initially excluded animals with >50 PVBs in a single hour. We allowed for Poisson overdispersion in the data, assumed a first-order autoregressive covariance structure, and based inferences on empirical (robust) standard errors. As a sensitivity analysis we also considered a more flexible model with group-by-time interactions. Second, we tested the related hypothesis that exposure to POS increases the risk of observing one or more PVB as compared to control animals exposed to filtered air using repeated-measures logistic regression. As above, this model was fit using GEE and included indicator variables for time (5 indicator variables) and exposure group. An exchangeable covariance structure was assumed and inferences were based on empirical (robust) standard errors.
We modeled ECG intervals, heart rate and heart rate variability parameters using linear mixed models with indicator variables for time (5 indicator variables) and exposure group as fixed effects and random subject-specific intercepts. As a sensitivity analysis we also considered a more flexible model with group-by-time interactions.
To evaluate the effects of exposure on respiratory outcomes, we applied the ANOVA analysis described in detail elsewhere (Coull et al., 2010; Diaz et al., 2010). Briefly, for 10-minute averaged BUXCO data, additive mixed models were applied to estimate the effects of exposure while accounting for the correlation among repeated measurements taken on the same animal during the exposure period. This approach is analogous to that used in the analysis of electrocardiographic endpoints described above, but allows for more flexible control of the time trends in the outcomes.
Because animals were exposed in groups on a small number of days, there was insufficient variability in mass concentration or the concentration of specific constituents to explore dose-response relationships with any of the outcomes. All statistical analyses were performed in SAS version 9.1 (SAS Institute, Cary, NC). Statistical significance for all models was based on a two-sided α = 0.05.
Results
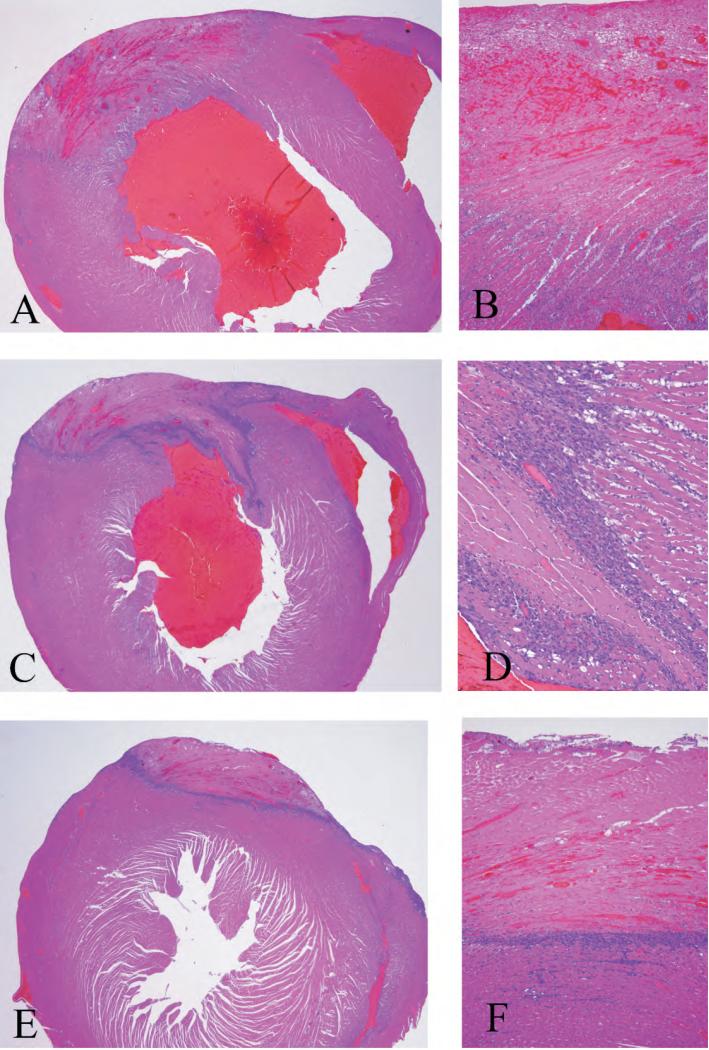
At each of Power Plants 2 and 3 we attempted to expose over 4 experimental days a total of 32 rats with acute myocardial infarction to either stack emissions under the POS scenario or filtered air. At Power Plant 2, 30 rats survived the MI surgery and were randomized to either filtered air or exposure under the POS scenario (Table 1). High quality ECG data were available from 29 (97%) of animals. As expected based on our past experience with this animal model, most rats at Power Plant 2 had transmural left ventricular myocardial infarcts as assessed by histopathology (Fig. 1).
Table 1.
Summary of animal characteristics and sample size.
| Power Plant 2 |
Power Plant 3 |
|||
|---|---|---|---|---|
| Filtered Air | POS | Filtered Air | POS | |
| Randomized | 15 | 15 | 7 | 8 |
| Died during exposure | 0 | 0 | 0 | 2† |
| Histopathology | ||||
| Transmural left ventricular infarct | 9 | 13 | 1 | 1 |
| Transmural right ventricular infarct | 2 | 1 | 3 | 5 |
| Subepicardial left ventricular infarct | 4 | 1 | 3 | 0 |
| Electrocardiographic outcomes | 14‡ | 15 | 2‡ | 6 |
| Respiratory outcomes | 15 | 15 | 5 | 6 |
1 animal died for technical problems unrelated to the exposure.
1 animal from Power Plant 2 and 5 animals from Power Plant 3 were excluded from analysis due to poor signal quality.
Figure 1.
H&E-stained heart sections established the presence of MI. These show the infarctions to be in the distribution of the anterior descending coronary, and all have histological characteristics indicating ages of at least 3 days old, but less than one week. (A) Low magnification (20x) picture of a large transmural infarction with extensive cardiac myocyte necrosis surrounded by an influx of inflammatory cells. (B) Same infarction shown at higher magnification (100x). A large area of necrotic myocytes with hemorrhage into the necrotic area and inflammation extending from the epicardium to the subendocardial myocardium is shown. (C) Low magnification (20x) picture of another transmural infarction. The area of this infarction is slightly smaller than that illustrated in A, but has a more dense inflammatory infiltrate surrounding the area of infarction. (D) Same infarction shown at higher magnification (200x). Necrotic myocytes without nuclei and the inflammatory infiltrate of macrophages and neutrophils surrounding the necrotic cells can be seen. Normal myocytes with typical cardiac myocyte nuclei are on the right side of the inflammatory cells. (E) Low magnification (20x) picture of an infarct that is not transmural. This infarction has the similar histological characteristics as the others illustrated except that it extends from the epicardium to the mid point of the myocardium. The distribution of these subepicardial infactions are the same as those reported previously in the form of two week old infarctions (Wellenius et al 2002). (F) Same infarction at higher magnification (100x) showing the necrosis and inflammatory infiltrate in this non-transmural infaction
At Power Plant 3 we experienced a number of unexpected complications. First, due to an unplanned power plant shutdown on 2 of the 4 experimental days, we were only able to expose a total of 15 animals over 2 days. Second, contrary to our past experience with this animal model, many rats used at this power plant had transmural right ventricular myocardial infarcts which could not be explained by apparent differences in surgical technique, surgeon, animal size, or other controllable factors. Third, due to poor signal quality from the telemetry system, high quality ECG data were available from only 8 of the 15 (53%) experimental animals. This high rate of technical complications could also not be explained by identifiable differences in equipment, surgical technique, implantation surgeon, animal size, or other controllable factors. These complications resulted in a small, highly unbalanced dataset obtained from an animal model that differed materially from the intended model and from the animal model used at Power Plant 2. For this reason, we only report the results from Power Plant 2.
Power Plant 2
The POS scenario is intended to simulate an aged plume with unneutralized acidity and secondary organic aerosol derived from biogenic emissions. Exposure data for the experiments at Power Plant 2 are summarized in Table 2. Exposure characteristics were comparable to those observed for other POS scenario studies at the same plant (Kang et al., 2010), with one notable exception. On 3 of the 4 experimental days levels of iron, chromium and nickel were unexpectedly high.
Table 2.
Daily mean exposure characteristics under the POS scenario at Power Plant 2.†
| Power Plant 2 |
|||||
|---|---|---|---|---|---|
| Exposure Metric | Units | 7/8/2005 | 7/13/2005 | 9/7/2005 | 9/8/2005 |
| O3 | ppb | 4.7 | 6.9 | 3.4 | 4.1 |
| NO | ppb | 3.6 | 4.3 | 4.2 | 3.2 |
| NO2 | ppb | 0.0 | 3.0 | 0.1 | 0.1 |
| SO2 | ppb | 24.3 | 32.5 | 21.2 | 26.9 |
| Formaldehyde | μg/m3 | 19.6 | 21.5 | 14.0 | 11.5 |
| Acetaldehyde | μg/m3 | 3.6 | 5.9 | 2.9 | 3.2 |
| Acetone | μg/m3 | 1.9 | 14.9 | 2.6 | 13.4 |
| Total Aldehydes | μg/m3 | 25.1 | 42.3 | 19.4 | 28.1 |
| Pinene | μg/m3 | 15.2 | 2.3 | 8.9 | 6.0 |
| Particle Mass | μg/m3 | na | 252.7 | 187.4 | 217.2 |
| Particle Number | 1000/cm3 | 16.1 | 13.8 | 3.5 | 13.3 |
| SO42- | μg/m3 | na | 170.5 | 156.9 | 190.2 |
| NO3- | μg/m3 | na | 0.5 | 0.0 | 0.0 |
| NH4+ | μg/m3 | na | 9.4 | 9.1 | 10.0 |
| Acid SO42- | μg/m3 | na | 127.8 | 125.3 | 144.4 |
| Neutralized SO42- | μg/m3 | na | 42.7 | 31.6 | 45.7 |
| Organic Carbon | μg/m3 | 50.9 | 50.8 | na | na |
| Elemental Carbon | μg/m3 | 23.6 | 18.3 | 18.0 | 13.7 |
| Total Carbon | μg/m3 | 74.5 | 69.1 | 18.0 | 13.7 |
| Na | ng/m3 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mg | ng/m3 | 0.0 | 0.0 | 0.0 | 0.1 |
| Al | ng/m3 | 0.0 | 0.7 | 2.4 | 5.7 |
| Si | ng/m3 | 0.0 | 3.8 | 0.0 | 1.2 |
| P | ng/m3 | 0.0 | 4.6 | 0.0 | 0.0 |
| S | μg/m3 | na | 56.8 | 52.3 | 63.4 |
| K | ng/m3 | 0.0 | 0.0 | 0.0 | 0.1 |
| Ca | ng/m3 | 0.0 | 0.0 | 3.3 | 1.2 |
| Ti | ng/m3 | 0.3 | 0.0 | 0.1 | 0.2 |
| Cr | ng/m3 | 0.0 | 45.5 | 92.1 | 31.3 |
| Fe | ng/m3 | 0.0 | 446.2 | 707.0 | 241.6 |
| Ni | ng/m3 | 0.0 | 67.2 | 56.5 | 18.9 |
| Zn | ng/m3 | 0.0 | 0.6 | 0.0 | 0.0 |
| Se | ng/m3 | 0.0 | 0.5 | 0.0 | 0.0 |
| Pb | ng/m3 | 0.0 | 0.0 | 0.0 | 0.0 |
| Temperature | °C | 22.7 | 24.0 | 22.4 | 22.4 |
| Relative Humidity | % | 52.9 | 51.8 | 51.4 | 48.2 |
See Kang et al. (2010) for details of exposure characterization. na: not available.
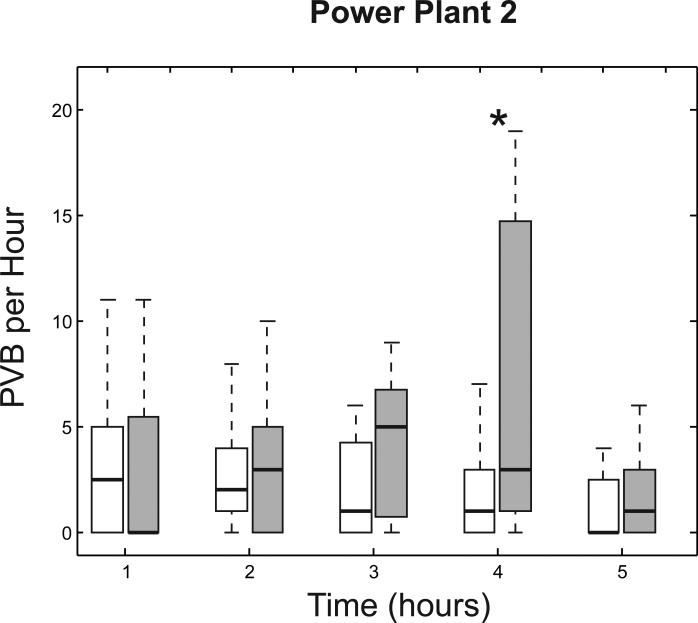
PVB frequency was greater among POS-exposed animals versus filtered air controls (Fig. 2). We used repeated-measures Poisson regression to model the number of PVBs observed in each hour. When averaged across the entire exposure period PVB frequency was 68.9% (p=0.11) higher in POS-exposed animals versus filtered air controls. At each time point, POS exposure was associated with increased PVB frequency, but this difference was statistically significant only during the 4th exposure hour where PVB frequency was 3.9 times greater among POS-exposed animals versus filtered air controls (p=0.045). During the 3rd exposure hour, PVB frequency was 2.6 times greater among the POS-exposed animals, but this difference did not reach statistical significance (p=0.067).
Figure 2.
Box plots summarizing the number of premature ventricular beats per hour (PVB/h) observed in animals exposed to filtered air (white boxes) or stack emissions under the POS scenario (gray boxes) at Power Plant 2. Each box has lines at the lower quartile, median, and upper quartile values. The whiskers are lines extending from each end of the box to show the extent of the rest of the data (up to 1.5 times the interquartile range). *: p<0.05 comparing the response under the POS scenario versus filtered air controls at the same time point.
As a sensitivity analysis we excluded from analysis rats exposed on either of the 2 days with the highest levels of chromium, iron, and nickel (2nd and 3rd days of exposure) and found that the effect estimates were of similar magnitude to those in the main analysis, but the standard errors were larger. As an additional sensitivity analysis, we used repeated-measures logistic regression to model the odds of observing ≥1 PVB in each hour. During the fourth and fifth exposure hours, POS-exposed animals were significantly more likely to have ≥1 PVB versus animals exposed to filtered air (p=0.031 and 0.029 for the fourth and fifth hours, respectively).
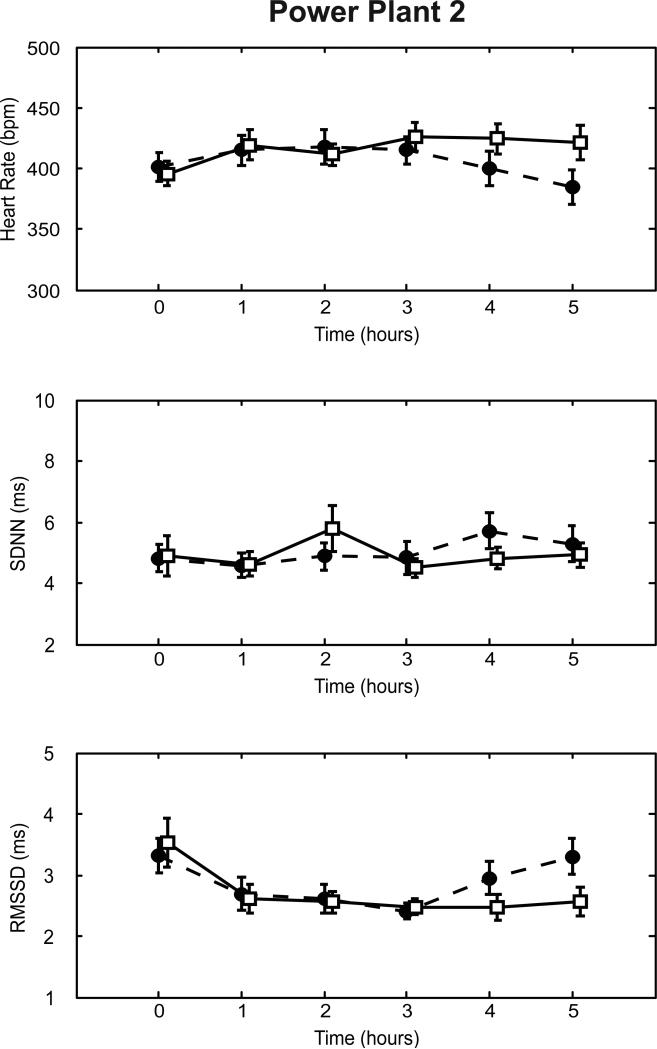
We assessed heart rate and time-domain measures of heart rate variability at 0, 60, 120, 180, 240, and 300 min after the start of each exposure (Fig. 3). Heart rate, SDNN, and RMSSD at the start of exposure were similar in the two exposure groups. POS exposure was not associated with statistically significant changes in heart rate, SDNN or RMSSD, either overall (Table 2) or at any given hour (Fig. 3). Similarly, PR interval and P-wave duration did not differ significantly between the two exposure groups.
Figure 3.
Effect of POS exposure on heart rate and measures of heart rate variability at Power Plant 2. The mean heart rate (top panels), SDNN (middle panels), and RMSSD (bottom panels) at different time points after the start of exposure is shown for animals exposed to either filtered air (solid lines) or stack emissions under the POS scenario (dashed lines). Error bars indicate standard errors. *: p<0.05 comparing the response under the POS scenario versus filtered air controls at the same time point.
We assessed respiratory outcomes in each rat throughout the exposure. POS exposure was associated with a statistically significant decrease in expiratory time and end-inspiratory pause (Table 3).
Table 3.
Estimated average effect of exposure to POS scenario on electrocardiographic outcomes in rats with acute myocardial infarction.
| Power Plant 2 (N=29) |
||||
|---|---|---|---|---|
| Outcome | Units | Estimate | SE | P-Value† |
| Heart Rate Variability | ||||
| Heart Rate | bpm | -10.92 | 12.27 | 0.38 |
| SDNN | msec | 0.09 | 0.46 | 0.85 |
| RMSSD | msec | 0.17 | 0.24 | 0.47 |
| ECG Intervals | ||||
| PR Interval | msec | -1.26 | 1.52 | 0.41 |
| P-Wave Duration | msec | 0.33 | 0.78 | 0.67 |
Values in bold are statistically significant at the a=0.05 level. bpm: beats per minute.
Discussion
The TERESA project represents a novel approach for investigating the toxicity of PM derived from coal combustion. Past toxicological studies of coal combustion emissions have evaluated the health effects of primary emissions such as coal fly ash. Given that coal-fired power plants in the US have adopted controls to reduce the emission of primary particles, the relevance of toxicological studies using primary emission PM is unclear. However, even with such controls, stack emissions of SO2 and NOx still contribute substantially to ambient PM in the form of secondary particles. The health effects of these secondary particles, in combination with any residual primary PM, are largely unknown. The goal of the current study was to evaluate the effects of aged stack emissions from coal-fired power plants on electrocardiographic changes in a rat model of acute myocardial infarction.
We carried out these experiments at two power plants (Power Plants 2 and 3). At Power Plant 2, we found that exposure to a scenario reflecting an aged plume with unneutralized acidity and secondary organic aerosol was associated with increased frequency of ventricular arrhythmias, decreased respiratory expiratory time and decreased end-inspiratory pause. We did not observe statistically significant changes in heart rate, heart rate variability, or ECG intervals. As discussed below, the experiments from Power Plant 3 were uninterpretable.
We had chosen a priori to carry out these experiments using the exposure scenario that demonstrated the most substantial change in cardiac in vivo chemiluminescence in normal animals. This decision was based on studies showing important changes in this measure following exposure to concentrated ambient particles (Gurgueira et al., 2002) that may be abrogated with the anti-oxidant N-acetyl cysteine (Rhoden et al., 2004). Preliminary analyses of data from Power Plant 2 suggested similar increases in cardiac chemiluminescence in normal animals exposed under the POS and PONS scenarios and we chose to use the POS scenario for the MI experiments. In the final analysis, the change in heart chemiluminescence at Power Plant 2 was more pronounced for the PONS scenario than for the POS scenario. On the other hand, across all 3 power plants, exposure to the POS scenario increased heart chemiluminescence more than did exposure to the PONS scenario. Therefore, in retrospect, either the POS or PONS exposure scenarios would have been an acceptable choice for the MI studies.
Our choice to use a complex exposure scenario rather than a scenario involving only primary particles was based on the fact that emissions from coal-fired power plants interact with natural and anthropogenic organics in the atmosphere. Although the final products in the atmosphere are not strictly from the power plant, it can be argued that if power plant emissions were not present in that form, different reactions might take place in the environment. Because the reactions that we studied do in fact take place, we believe their use in a study of health effects is a strength of this project.
The experiment with MI animals at Power Plant 3 failed due to unplanned power plant shutdowns, unexpected variations in infarct location in our animal model, and technical difficulties obtaining ECG signals of sufficient quality. Plant shutdowns were uncommon in this series, and usually had minor impacts in experiments. The shutdowns at Power Plant 3 had greater impact because they occurred after the surgical preparation of the MI animals but prior to their exposure. Because exposure needed to occur within 24 hrs of the surgery, the period of greatest myocardial ectopic vulnerability (Wellenius et al., 2002; Wellenius et al., 2004), delaying exposures was not feasible. Unfortunately, extending our planned stay at Power Plant 3 or returning to the power plant at a later date to carry out additional experiments was not possible.
The predominant site of myocardial infarction (left ventricle at Power Plant 2, right ventricle in Power Plant 3) is important in this animal model, as evidenced by the much higher rate of ventricular ectopy among the sham-exposed animals at Power Plant 2 versus Power Plant 3. The difference in infarct distribution is not explained by the technical factors mentioned above. One possible explanation is that Sprague-Dawley is an outbred strain of rats, and variations in coronary artery distribution can occur. That it occurred in a series of animals all received at the same time may suggest that this variation could well have been a feature of this cohort of animals, many of which could have been siblings. Coupled with an unexpectedly high rate of technical failure in ECG recording equipment, these problems resulted in the data set from Power Plant 3 being small and highly unbalanced. This not only limited the analyses that we could perform on these data, but also casts serious doubts on the assumptions underlying the statistical models applied. Specifically, it is unlikely that data were missing at random or that the two exposure groups were successfully randomized. For these reasons, we do not believe that valid conclusions can be drawn from the data from Power Plant 3..
Of note, this rat model of acute vulnerability to arrhythmia after MI requires survival thoracic surgery on an animal that has developed a large, transmural myocardial infarction during the surgery. Infarcts of the size typically observed in this model are associated with a high peri-operative mortality rate. The challenges of successfully producing and using this model in a mobile laboratory in a field setting are substantial. Given these challenges, it is disappointing, but not entirely unexpected, that we would experience more complications at some sites than others.
The results from Power Plant 2 may suggest that in this susceptible animal model, emissions from coal-fired power plants that are photochemically aged in the presence of pinene, a naturally occurring pollutant, can increase ventricular ectopy. If causal, this effect may be mediated by changes in autonomic function, as suggested by a number of toxicologic and epidemiologic studies of ambient particles (Godleski et al., 2000; Gold et al., 2000; Devlin et al., 2003). However, in this study we did not observe any substantial or statistically significant changes in heart rate or heart rate variability. This could be either because the POS scenario had no effect on autonomic nervous system function or because such changes were difficult to observe in this animal model which already has reduced heart rate variability. Alternatively, the increased arrhythmia frequency could reflect increased oxidative stress. In normal animals, the POS exposure at Power Plant 2 led to increased cardiac oxidative stress as measured by in vivo cardiac chemiluminescence, although this difference did not reach statistical significance (Lemos et al., 2010).
We have previously used this animal model to evaluate the effects of concentrated ambient particles (CAPs) and found that CAPs exposure was associated with a non-significant 64.2%, (95% CI: –17.7, 227.6%; p = 0.16) increase in arrhythmia frequency during a 1 hr exposure (Wellenius et al., 2004). We have also shown that a 1 hr inhalation exposure to residual oil fly ash significantly increased arrhythmia frequency among animals with preexisting arrhythmias (Wellenius et al., 2002). In the current study, at Power Plant 2 the frequency of ventricular premature beats peaked during the 4th exposure hour where it was almost three fold higher among animals exposed to POS scenario as compared to filtered air controls. Because of differences in the duration of exposure and experimental design, direct comparison of these results to those of previous studies is not possible.
At Power Plant 2, the respiratory effects of POS exposure were quite different in normal animals as compared to animals with MI. Specifically, in normal animals POS exposure led to statistically significant decreases in tidal volume, pause, and Penh (Diaz et al., 2010). In contrast, in the current study in animals with MI, POS exposure was associated with decreased expiratory time and end-inspiratory pause. Whether these divergent results are due to differences in the animal models or observed differences in the POS exposure atmosphere is not estimable from the existing data. Alternatively, given the small magnitude of the effects and the large number of respiratory outcomes examined, the changes observed in respiratory parameters in the MI animals may reflect chance findings.
This study has several potential limitations that warrant discussion. First, for unknown reasons we observed unexpectedly high levels of iron, chromium, and nickel at Power Plant 2 on three of the four exposure days. The source of these trace elements is unclear, but assessment of these particles by single particle analyses using scanning electron microscopy and energy dispersive X-ray analyses suggested they were derived from the emissions of the plant rather than contamination by corrosion of the sampling line from stack (Kang et al., 2010). How (or if) the elevated concentrations may have affected the measured outcomes is unknown, although there is recent evidence to suggest that some of these elements may play a role in cardiovascular effects (Chen and Lippmann, 2009). However, the results were not materially different when we excluded from analyses the 2 days with the highest levels of these metals. A second limitation is that the duration of exposure was limited to 5 h. Thus, it is not known whether a longer exposure would produce similar results. Third, to reduce biologic variability, only mature, male, Sprague-Dawley rats were studied. Thus, it is unknown how the effect of exposure to a POS scenario might vary by gender or age. Fourth, even at Power Plant 2 there were some differences in infarct size and location across the exposure groups. It is unclear how these differences might have affected the results. Fifth, there are important differences between the rat and human heart, including differences in the degree of collateral blood flow, ventricular mass, and electrical properties (Janse et al., 1998) which make extrapolation of these findings to human populations difficult.. Finally, our conclusions are based on results from 4 exposure days at a single power plant. Thus, we were unable to evaluate how variations in power plant and coal characteristics may affect these results.
The overall goal of the TERESA project is to investigate the adverse health effects of specific emission sources and components by examining the relative toxicity of coal combustion and mobile sources (gasoline and/or diesel engine) emissions and their oxidative products. The first phase of the project was to evaluate the health effects of emissions from coal combustion. The second phase of the project evaluating the health effects of emissions from mobile sources is currently in progress. It will be important to compare the relative toxicity of these two important sources of ambient fine PM.
Conclusions
The goal of the this study was to evaluate the effects of aged stack emissions from coal-fired power plants on electrocardiographic changes in a rat model of acute myocardial infarction. Results from a single power plant suggest that exposure to an aged plume with unneutralized acidity and secondary organic aerosols was associated with increased frequency of ventricular arrhythmias and decreased respiratory expiratory time and end-inspiratory pause, but not with changes in heart rate, heart rate variability, or electrocardiographic intervals.
Stack emissions from coal-fired power plants contribute substantially to ambient PM through the formation of secondary particles. However, direct evaluation of the health effects of secondary particles from coal-fired power plants has not been previously possible. The TERESA project represents a novel approach for investigating the health effects of primary and secondary particles derived from coal combustion. The results of this study provide important insights into the cardiovascular and respiratory toxicity of these emissions in normal and susceptible animals. More broadly, applying the approach illustrated here to the study of the health effects of primary and secondary particles from a number of other potentially important sources has the potential to advance our understanding of the health effects of ambient particulate matter and inform future policy decisions.
Table 4.
Estimated average effect of exposure to POS scenario on respiratory outcomes in rats with acute myocardial infarction.
| Power Plant 2 (N=30) |
|||||
|---|---|---|---|---|---|
| Outcome | Units | Mean Value Among Sham Animals | Estimate | SE | P-Value† |
| Frequency | Breaths/min | 176.4 | 17.8 | 11.7 | 0.13 |
| Tidal Volume | ml | 2.08 | -0.10 | 0.11 | 0.37 |
| Inspiratory Time | sec | 0.179 | -0.021 | 0.012 | 0.085 |
| Expiratory Time | sec | 0.242 | -0.025 | 0.013 | 0.049 |
| Enhanced Pause | dimensionless | 1.34 | 0.01 | 0.15 | 0.97 |
| End Expiratory Pause | msec | 51.5 | -7.5 | 6.3 | 0.23 |
| End Inspiratory Pause | msec | 10.2 | -1.0 | 0.5 | 0.037 |
| Minute Volume | ml | 333.5 | 27.8 | 23.3 | 0.23 |
| Expiratory Flow at 50% | ml/min | 1.28 | 0.10 | 0.12 | 0.40 |
| Pause | dimensionless | 1.15 | 0.08 | 0.08 | 0.31 |
| Peak Expiratory Flow | ml/min | 20.3 | 0.5 | 1.3 | 0.71 |
| Peak Inspiratory Flow | ml/min | 21.6 | 1.3 | 1.5 | 0.38 |
Values in bold are statistically significant at the α=0.05 level.
Acknowledgements
The authors thank the power plant personnel, local universities, veterinary clinics, and suppliers who made an extraordinary effort to make a logistically very complex project possible.
Declaration of Interest: This project was supported by the Electric Power Research Institute (Contract EP-P10983/C5530/56546), the U.S. Environmental Protection Agency Center for Particle Health Effects at the Harvard School of Public Health (grants R827353 and 832416), and the Harvard NIEHS Center for Environmental Health (grant ES00002). This work was also prepared with the support from grant number ES015774 from the NIEHS, NIH, award DE-FC26-03NT41902 from the U.S. Department of Energy (DOE), and a grant from the State of Wisconsin. However, any opinions, findings, conclusions, or recommendations expressed herein are those of the authors, and do not necessarily reflect the views of EPRI, U.S. EPA, NIEHS, NIH, or DOE.
References
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr., Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Brown SG, Frankel A, Raffuse SM, Roberts PT, Hafner HR, Anderson DJ. Source apportionment of fine particulate matter in Phoenix, AZ, using positive matrix factorization. J Air Waste Manag Assoc. 2007;57:741–52. doi: 10.3155/1047-3289.57.6.741. [DOI] [PubMed] [Google Scholar]
- Burnett RT, Dales R, Krewski D, Vincent R, Dann T, Brook JR. Associations between ambient particulate sulfate and admissions to Ontario hospitals for cardiac and respiratory diseases. Am J Epidemiol. 1995;142:15–22. doi: 10.1093/oxfordjournals.aje.a117540. [DOI] [PubMed] [Google Scholar]
- Chen LC, Lippmann M. Effects of metals within ambient air particulate matter (PM) on human health. Inhal Toxicol. 2009;21:1–31. doi: 10.1080/08958370802105405. [DOI] [PubMed] [Google Scholar]
- Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–6. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- Coull BA, Wellenius GA, Gonzalez-Flecha B, Diaz EA, Godleski JJ. Methods for the Statistical Analysis of TERESA Health Data. 2010.
- Devlin RB, Ghio AJ, Kehrl H, Sanders G, Cascio W. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur Respir J Suppl. 2003;40:76s–80s. doi: 10.1183/09031936.03.00402403. [DOI] [PubMed] [Google Scholar]
- Diaz EA, Lemos M, Long M, Coull BA, Ruiz PA, Gupta T, Kang CM, Vlassidis E, Godleski JJ. Toxicological Evaluation of Realistic Emission Source Aerosols (TERESA): Pulmonary Functional Health Effects Related to Power Plants. 2010.
- Diggle PJ, Heagerty PJ, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. 2nd Ed. University Press; Oxford: 2002. [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. Jama. 2006;295:1127–34. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall RM, Norris GA, Dailey LA, Burke JM, McGee JK, Gilmour MI, Gordon T, Devlin RB. Source apportionment of particulate matter in the U.S. and associations with lung inflammatory markers. Inhal Toxicol. 2008;20:671–83. doi: 10.1080/08958370801935117. [DOI] [PubMed] [Google Scholar]
- Finnerty K, Choi JE, Lau A, Davis-Gorman G, Diven C, Seaver N, Linak WP, Witten M, McDonagh PF. Instillation of coarse ash particulate matter and lipopolysaccharide produces a systemic inflammatory response in mice. J Toxicol Environ Health A. 2007;70:1957–66. doi: 10.1080/15287390701549229. [DOI] [PubMed] [Google Scholar]
- Gilmour MI, O'Connor S, Dick CA, Miller CA, Linak WP. Differential pulmonary inflammation and in vitro cytotoxicity of size-fractionated fly ash particles from pulverized coal combustion. J Air Waste Manag Assoc. 2004;54:286–95. doi: 10.1080/10473289.2004.10470906. [DOI] [PubMed] [Google Scholar]
- Godleski JJ, Diaz EA, Lemos M, Long M, Ruiz PA, Gupta T, Kang CM, Coull BA. Toxicological Evaluation of Realistic Emission Source Aerosols (TERESA): Assessment of Cellular Responses. 2010a. [DOI] [PMC free article] [PubMed]
- Godleski JJ, Koutrakis P, Kang CM, Diaz E, Rohr AC. Toxicological Evaluation of Realistic Emission Source Aerosols (TERESA): Introduction and Overview. 2010b. [DOI] [PMC free article] [PubMed]
- Godleski JJ, Verrier RL, Koutrakis P, Catalano P, Coull B, Reinisch U, Lovett EG, Lawrence J, Murthy GG, Wolfson JM, Clarke RW, Nearing BD, Killingsworth C. Mechanisms of morbidity and mortality from exposure to ambient air particles. Res Rep Health Eff Inst. 2000:5–88. discussion 89-103. [PubMed] [Google Scholar]
- Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, Allen G, Verrier M, Cherry R, Verrier R. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- Gurgueira SA, Lawrence J, Coull B, Murthy GG, Gonzalez-Flecha B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect. 2002;110:749–755. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Christensen WF, Eatough DJ, Henry RC, Kim E, Laden F, Lall R, Larson TV, Neas L, Hopke PK, Thurston GD. PM source apportionment and health effects: 2. An investigation of intermethod variability in associations between source-apportioned fine particle mass and daily mortality in Washington, DC. J Expo Sci Environ Epidemiol. 2006;16:300–10. doi: 10.1038/sj.jea.7500464. [DOI] [PubMed] [Google Scholar]
- Janse MJ, Opthof T, Kleber AG. Animal models of cardiac arrhythmias. Cardiovasc Res. 1998;39:165–77. [PubMed] [Google Scholar]
- Kang CM, Gupta T, Ruiz PA, Wolfson JM, Ferguson ST, Lawrence JE, Rohr AC, Godleski J, Koutrakis P. Aged particles derived from emissions of coal-fired power plants: The TERESA field results. Inhal Toxicol. 2010 doi: 10.3109/08958371003728040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ Health Perspect. 2000;108:941–7. doi: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D. Zero-inflated Poisson regression, with an application to defects in manufacturing. Technometrics. 1992;34:1–14. [Google Scholar]
- Lemos M, Diaz EA, Gupta T, Kang CM, Ruiz PA, Coull BA, Gonzalez-Flecha B. Cardiac and Pulmonary Oxidative Stress in Rats exposed to Realistic Emissions of Source Aerosols. 2010. [DOI] [PMC free article] [PubMed]
- Lippmann M, Hwang JS, Maciejczyk P, Chen LC. PM source apportionment for short-term cardiac function changes in ApoE-/- mice. Environ Health Perspect. 2005;113:1575–9. doi: 10.1289/ehp.8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttmann-Gibson H, Suh HH, Coull BA, Dockery DW, Sarnat SE, Schwartz J, Stone PH, Gold DR. Short-term effects of air pollution on heart rate variability in senior adults in Steubenville, Ohio. J Occup Environ Med. 2006;48:780–8. doi: 10.1097/01.jom.0000229781.27181.7d. [DOI] [PubMed] [Google Scholar]
- Mar TF, Ito K, Koenig JQ, Larson TV, Eatough DJ, Henry RC, Kim E, Laden F, Lall R, Neas L, Stolzel M, Paatero P, Hopke PK, Thurston GD. PM source apportionment and health effects. 3. Investigation of inter-method variations in associations between estimated source contributions of PM2.5 and daily mortality in Phoenix, AZ. J Expo Sci Environ Epidemiol. 2006;16:311–20. doi: 10.1038/sj.jea.7500465. [DOI] [PubMed] [Google Scholar]
- Maykut NN, Lewtas J, Kim E, Larson TV. Source apportionment of PM2.5 at an urban IMPROVE site in Seattle, Washington. Environ Sci Technol. 2003;37:5135–42. doi: 10.1021/es030370y. [DOI] [PubMed] [Google Scholar]
- O'Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, Horton ES, Schwartz J. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–20. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Peters A, Liu E, Verrier RL, Schwartz J, Gold DR, Mittleman M, Baliff J, Oh JA, Allen G, Monahan K, Dockery DW. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11:11–17. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Reiss R, Anderson EL, Cross CE, Hidy G, Hoel D, McClellan R, Moolgavkar S. Evidence of health impacts of sulfate-and nitrate-containing particles in ambient air. Inhal Toxicol. 2007;19:419–49. doi: 10.1080/08958370601174941. [DOI] [PubMed] [Google Scholar]
- Rhoden CR, Lawrence J, Godleski JJ, Gonzalez-Flecha B. N-acetylcysteine prevents lung inflammation after short-term inhalation exposure to concentrated ambient particles. Toxicol Sci. 2004;79:296–303. doi: 10.1093/toxsci/kfh122. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Schwartz J, Mittleman MA, Link M, Luttmann-Gibson H, Catalano PJ, Speizer FE, Dockery DW. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am J Epidemiol. 2005;161:1123–32. doi: 10.1093/aje/kwi143. [DOI] [PubMed] [Google Scholar]
- Ruiz PA, Gupta T, Kang CM, Lawrence JE, Ferguson ST, Wolfson JM, Rohr AC, Koutrakis P. Development of an exposure system for the toxicological evaluation of particles derived from coal-fired power plants. Inhal Toxicol. 2007a;19:607–19. doi: 10.1080/08958370701353148. [DOI] [PubMed] [Google Scholar]
- Ruiz PA, Lawrence JE, Ferguson ST, Wolfson JM, Koutrakis P. A counter-current parallel-plate membrane denuder for the non-specific removal of trace gases. Environ Sci Technol. 2006;40:5058–63. doi: 10.1021/es060563w. [DOI] [PubMed] [Google Scholar]
- Ruiz PA, Lawrence JE, Wolfson JM, Ferguson ST, Gupta T, Kang CM, Koutrakis P. Development and evaluation of a photochemical chamber to examine the toxicity of coal-fired power plant emissions. Inhal Toxicol. 2007b;19:597–606. doi: 10.1080/08958370701353361. [DOI] [PubMed] [Google Scholar]
- Sarnat JA, Marmur A, Klein M, Kim E, Russell AG, Sarnat SE, Mulholland JA, Hopke PK, Tolbert PE. Fine particle sources and cardiorespiratory morbidity: an application of chemical mass balance and factor analytical source-apportionment methods. Environ Health Perspect. 2008;116:459–66. doi: 10.1289/ehp.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat SE, Suh HH, Coull BA, Schwartz J, Stone PH, Gold DR. Ambient particulate air pollution and cardiac arrhythmia in a panel of older adults in Steubenville, Ohio. Occup Environ Med. 2006;63:700–6. doi: 10.1136/oem.2006.027292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger RB, Cassee F. Atmospheric secondary inorganic particulate matter: the toxicological perspective as a basis for health effects risk assessment. Inhal Toxicol. 2003;15:197–235. doi: 10.1080/08958370304503. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Morris R. Air pollution and hospital admissions for cardiovascular disease in Detroit, Michigan. Am J Epidemiol. 1995;142:23–35. doi: 10.1093/oxfordjournals.aje.a117541. [DOI] [PubMed] [Google Scholar]
- Smith KR, Veranth JM, Kodavanti UP, Aust AE, Pinkerton KE. Acute pulmonary and systemic effects of inhaled coal fly ash in rats: comparison to ambient environmental particles. Toxicol Sci. 2006;93:390–9. doi: 10.1093/toxsci/kfl062. [DOI] [PubMed] [Google Scholar]
- Tsai SS, Goggins WB, Chiu HF, Yang CY. Evidence for an Association Between Air Pollution and Daily Stroke Admissions in Kaohsiung, Taiwan. Stroke. 2003;34:2612–2616. doi: 10.1161/01.STR.0000095564.33543.64. [DOI] [PubMed] [Google Scholar]
- US Energy Information Administration 2009. Annual Energy Review, 2008. DOE/EIA-0384(2008)
- Wellenius GA, Batalha JR, Diaz EA, Lawrence J, Coull BA, Katz T, Verrier RL, Godleski JJ. Cardiac Effects of Carbon Monoxide and Ambient Particles in a Rat Model of Myocardial Infarction. Toxicol Sci. 2004;80:367–376. doi: 10.1093/toxsci/kfh161. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Saldiva PH, Batalha JR, Krishna Murthy GG, Coull BA, Verrier RL, Godleski JJ. Electrocardiographic changes during exposure to residual oil fly ash (ROFA) particles in a rat model of myocardial infarction. Toxicol Sci. 2002;66:327–335. doi: 10.1093/toxsci/66.2.327. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among medicare beneficiaries. Stroke. 2005;36:2549–53. doi: 10.1161/01.STR.0000189687.78760.47. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Schwartz J, Mittleman MA. Particulate air pollution and hospital admissions for congestive heart failure in seven United States cities. Am J Cardiol. 2006;97:404–8. doi: 10.1016/j.amjcard.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case-crossover analysis. Environ Health Perspect. 2005;113:978–82. doi: 10.1289/ehp.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]