Abstract
Early life stress (ELS) is a common risk factor for psychopathology, but there are few functional neuroimaging studies investigating its effects. In this preliminary study, we investigated the correlates of ELS exposure on the default network (DN) through measurements of task-associated DN deactivation. Data were analyzed from 19 subjects without psychiatric illness (10 with ELS). Subjects performed the working memory (WM) N-back task (including a 2-back WM and 0-back control condition) while undergoing functional MRI. We compared brain responses in the two groups across 5 bilateral DN regions using an a priori region of interest (ROI) analysis. The ELS group demonstrated significantly greater DN deactivation, observed in the right posterior cingulate cortex PCC, bilateral medial prefrontal cortex, left middle/superior frontal gyrus and right middle temporal region. These preliminary results indicate subjects with ELS demonstrate greater DN deactivations to WM challenges compared to non-ELS controls, potentially reflecting a biomarker of long-term effects of ELS exposure.
Keywords: Early Life Stress, Default Network, Working Memory, Medial Prefrontal Cortex, FMRI
Introduction
There is extensive evidence that exposure to early life stress (ELS) confers a significant risk for psychiatric illness. ELS, often defined as child abuse, neglect, or parental loss, is strongly linked to post-traumatic stress disorder (PTSD) in addition to a wide variety of psychiatric disorders (Heim and Nemeroff, 2001; Heim et al., 2010; Kendler et al., 2000; Kendler et al., 2004). ELS is also highly prevalent; reports indicate that 63.9% of children experience at least one adverse childhood event, with 12.5% experiencing at least four of these events (U.S. Department of Health and Human Services, 2007). ELS is often under-reported (Briere and Elliott, 2003), and is associated with poorer response to treatment, increased chronicity of symptoms, and suicide risk (Brown and Moran, 1994; Dube et al., 2001; Zlotnick et al., 2001; Zlotnick et al., 1997).
ELS and Working Memory
The association between ELS and a wide range of cognitive impairments has been well documented. Children who have been exposed to ELS show poorer cognitive functioning in language, visual-spatial abilities, memory, attention, and executive functioning (DePrince et al., 2009), as well as lower academic performance and IQ (Kendall-Tackett and Eckenrode, 1996; Majer et al., 2010). In particular, ELS exposure has been associated with impaired working memory (WM). One group studied school-aged children with familial trauma, non-familial trauma, and no trauma exposure, with results indicating impaired WM performance in those with familial trauma (DePrince et al., 2009). Similar results have been found in WM studies of adults who experienced childhood abuse. Another study investigated reaction time and accuracy on a spatial WM task in a group of healthy adults with previous ELS exposure. The authors found that childhood exposure to emotional abuse and physical neglect was significantly associated with errors on the spatial WM task (Majer et al., 2010). Furthermore, the authors found that WM performance was more impaired with increased ELS exposure. While very few studies have utilized neuroimaging to evaluate WM in individuals with a history of ELS, in one study, whole-brain activation was compared during a visual and verbal memory task in a group of 23 adult males, ten of whom had experienced severe physical abuse during childhood. Participants with severe physical abuse were found to have reduced right hemisphere activation during the task (Raine et al., 2001). Decreased activation was found in the dorsolateral prefrontal cortex, anterior prefrontal cortex, inferior prefrontal gyrus, lateral temporal cortex, occipital cortex, and superior temporal gyrus. These findings support the notion that highly stressful experiences may result in changes in brain function that can be observed during WM tasks.
Working Memory and the Default Network
One way to evaluate WM-related brain functions is through task-related deactivations of the default network (DN). The DN is a network of brain regions that includes the posterior cingulate cortex (PCC), medial prefrontal cortex (MPFC), middle frontal regions, lateral parietal and medial temporal regions, and is thought to be involved in introspection and background processing (Andrews-Hanna et al., 2010; Fox et al., 2005; Raichle et al., 2001; Sheline et al., 2009; Sweet et al., 2008). One of the principal features of this network is its deactivation during cognitive challenges, relative to resting baseline. Moreover, as the difficulty of the challenge increases, DN deactivation (also called DN suppression) increases (Fransson, 2006; Fransson and Marrelec, 2008; McKiernan et al., 2003; Sweet et al., 2008).
Working Memory, Early Life Stress and the Default Network
To date, there have been few investigations of ELS on WM within the context of the DN. Most of the available WM literature has focused on PTSD, which has demonstrated impaired or inefficient WM in PTSD samples. In two studies, participants with severe PTSD and co-morbid depression demonstrated impaired WM compared to healthy controls (Moores et al., 2008; Shaw et al., 2009). Utilizing visual and verbal WM tasks, these studies found that PTSD subjects were inefficiently allocating cognitive resources to perform these challenges. In both studies, DN regions were consistently involved in their results, indicating a possible relationship between the DN, WM and past stress exposure. In a subsequent study, the effect of PTSD on the DN during WM tasks was examined, using connectivity data from the previously discussed Moores and Shaw experiments. The authors found that, compared to healthy controls, PTSD participants did not have the expected decrease in DN connectivity during WM tasks, nor the expected increased connectivity within executive networks (Daniels et al., 2010). Although this study did not evaluate changes in regional DN activity, their results could have been driven by a series of highly coordinated deactivations. This study of connectivity, supported a relationship between the DN and stress-related conditions, and suggested that further studies to describe WM-associated changes in brain activity are needed.
Objectives
Since there are no previous studies investigating activity patterns of the DN during WM tasks in this population, we sought to explore this topic in this pilot study. We hypothesized that the prior findings of altered connectivity patterns in trauma-exposed populations were driven by changes in regional DN activity. As such, we hypothesized that ELS-exposed individuals would demonstrate greater DN deactivation during WM tasks, compared to non-exposed individuals, perhaps reflecting a reallocation of cognitive resources to perform WM tasks.
To our knowledge, there have been no studies investigating differential DN response to WM challenges during functional magnetic resonance imaging (FMRI) based on ELS exposure. In order to isolate the effects of ELS exposure and eliminate confounding effects of medications and associated psychiatric morbidity, we studied healthy participants with no psychiatric or significant medical illness. We hypothesized that ELS subjects would exhibit greater DN deactivation during WM challenges, compared to controls.
Materials and Methods
Participants
Twenty-one healthy subjects were identified from the control sample of a larger neuroimaging study of nicotine dependence and WM (Sweet et al., 2010). All participants were nonsmokers and without psychiatric or significant medical illness. The Early Life Stress Questionnaire (ELSQ) self-report was used to quantify ELS events occurring prior to the age of 18 years. The ELSQ is based upon the Child Abuse and Trauma scale (Sanders and Becker-Lausen, 1995) and includes 19 adverse childhood events shown to be traumatic or stressful, such as physical and sexual abuse, deaths in the family, divorce, etc. ELS exposure was dichotomized using a median split into low (0–3 adverse childhood events) vs. high (≥ 4), which we operationally defined as non-ELS and ELS groups, respectively. All participants provided written, informed consent consistent with the Helsinki Declaration.
Working Memory Paradigm
The N-back task was administered to participants during functional imaging runs to challenge the WM system. This task has been widely used in FMRI research (Owen et al., 2005) and generates reliable deactivation of the DN (Bluhm et al., 2011; Newton et al., 2011; Sweet et al., 2008). In this block design study, the N-back task consisted of two six-minute imaging runs, and each run included three cycles of baseline (i.e., three 30 seconds of crosshair fixation) followed by the 0-back control task of sustained attention and 2-back WM task (Figure 1A + B) (Chen et al., 2012; Owen et al., 2005; Sweet, 2010; Sweet et al., 2008).
Figure 1.
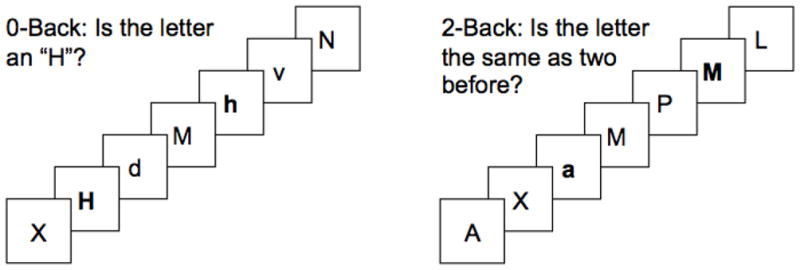
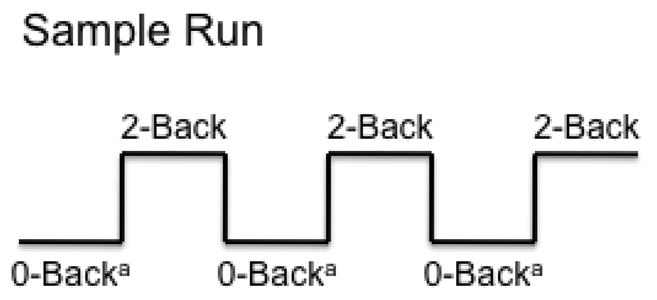
Figure 1A + B. N-back Task of Verbal Working Memory and Sample Run
Stimulus duration: 500 milliseconds
Inter-stimulus interval: 2500 milliseconds
a preceded by 30 second rest block
During the 2-back blocks, six 45-second series of 15 consonants were visually presented for 500 ms each, with an inter-stimulus interval of 2500 ms. Participants were asked to indicate whether the displayed consonant was the same as the consonant presented two previously. Subjects then made a yes or no decision using a response box held in their dominant hand. The six 27-second 0-back control blocks consisted of 9 consonants that were presented and subjects were instructed to respond in the same fashion. Every consonant list in both N-back blocks contained 33% targets (i.e., correct “yes” responses), and used randomized capitalization to limit visual matching. Runs in which participants performed at less than 60% accuracy during the 2-back were excluded from subsequent data analysis.
Image Acquisition
Whole brain echoplanar blood-oxygen-dependent (BOLD) FMRI images were acquired using a Siemens (Erlangen, Germany) TIM TRIO 3 Tesla scanner (TR = 2500 mg, TE = 28 ms, FOV = 1922 mm, and matrix size 642 in 3-mm axial slices). This sequence yielded 147 whole brain volumes for each of the two six-minute functional imaging runs, with spatial resolution of 3 mm3 per voxel. Whole brain high-resolution (1 mm3) T1 images were acquired prior to other scans for anatomic reference.
Preprocessing and Individual Dataset Analyses
All FMRI dataset processing and statistical analyses were conducted using the Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). After the raw data were reconstructed into 3D + time datasets, they were concatenated and registered to the fifth volume of the first series to minimize movement artifact. This procedure yielded movement correction parameters for subsequent use as covariates in voxel-based general linear model (GLM) analyses. A band pass filter (0.009–0.08 Hz) was applied, and voxel-based GLM was used to quantify task-specific activity in each brain voxel of the individual datasets. Independent variables in the GLM were the temporal course of the 0- and 2-back task presentations (including hemodynamic transitions modeled as a gamma function) and covariates (linear drift and observed movement), with the BOLD signal over time as the dependent variable. Resulting individual activation maps reflecting the unique activity of 0- and 2-back compared to resting baseline were co-registered to the high-resolution T1 anatomic images and transformed into standard stereotaxic space (Talairach, 1988). Resulting beta weights from individual level datasets of brain response to 0- and 2-back (versus baseline) served as the basic measure of brain activity in group-level statistical analyses.
Regions of Interest and Analyses
A region of interest (ROI) approach was used to examine the effects of ELS. Separate 0- and 2-back response was averaged in each ROI of each individual for use as the dependent measure for group contrasts. A priori ROIs were defined using seed region functional connectivity analyses of a separate sample of 25 healthy adults during a 4-minute resting state (Sweet et al., 2010). Sweet and colleagues identified DN ROIs by conducting the following analyses, described here in brief. Mean BOLD signal over time was extracted from a 5-mm radius within the PCC (Greicius et al., 2003) and individual correlation maps reflecting the strength of association between the time course of the PCC region and the time course from all other brain voxels were computed using a voxel-wise multiple regression (Philip et al., 2012; Sweet et al., 2010) The results revealed 5 bilateral ROIs, including bilateral MPFC, middle/superior frontal gyrus (MFG), PCC, lateral parietal (LP) and medial temporal regions (MTR) (Figure 2A+B). These ROIs were consistent with regions identified in prior DN literature (Broyd et al., 2009; Buckner et al., 2008). Hence, in the present study, mean BOLD responses within each of these a priori ROIs were extracted to examine the relationship between ELS and DN activity during the N-back in the current sample. Advantages of this ROI analysis include the use of validated spatial extents to test our hypothesis, and avoidance of Type I errors associated with multiple comparisons (Smith et al., 2010).
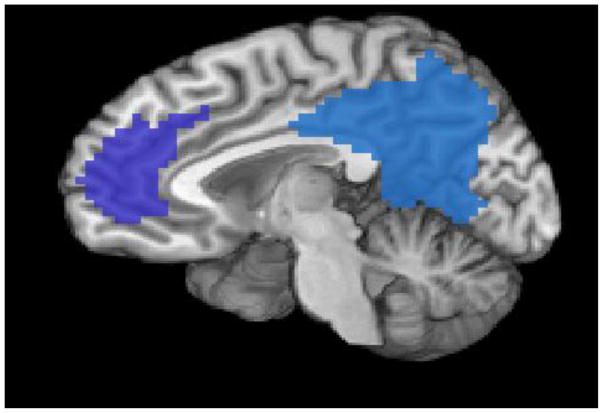
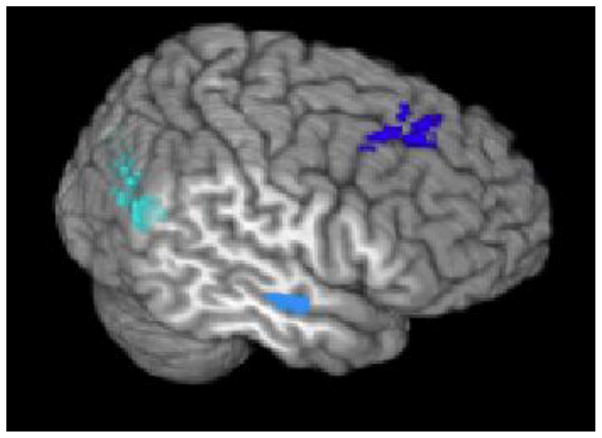
Figure 2.
Figure 2A + B. Default Network Regions of Interest
Figure 2A. Sagittal view of medial prefrontal cortex (dark blue) and posterior cingulate cortex (blue) regions of interest.
Figure 2B. Temporal view of middle/superior frontal gyrus (deep blue), medial temporal (light blue), and lateral parietal (cyan) regions of interest. 55mm cut used to visualize the medial temporal region.
To evaluate the validity of our a priori ROI selections in the current sample, we performed a whole-brain voxel-wise analysis in AFNI that showed task-associated activations in expected fronto-parietal and other WM regions (Owen et al., 2005), and task-associated deactivations in DN regions (uncorrected p< 0.005). Furthermore, to determine whether our a priori DN regions exhibited significant responses (i.e., changes in BOLD signal) during the N-back task, all regions were initially evaluated using a one-sample t-test conducted in SPSS Statistics 19 (IBM Corporation, Armonk, New York), with the null hypothesis that activity within each ROI would not significantly differ from zero (i.e., no difference compared to baseline). DN regions that did not differ significantly from zero during either the 0- or 2-back were excluded from subsequent analyses.
To test our hypotheses about differences between ELS groups, we conducted a repeated measures analysis of variance (ANOVA) for each ROI, with N-back level (i.e., 0- and 2-back) as the repeated measure and ELS group as a fixed factor. Results from this ANOVA were corrected for multiple comparisons using established methods to control the false discovery rate (FDR) (Benajmini and Hochberg, 1995). This was followed by planned independent samples t-tests, used to examine group differences in task-related activity. The significance level for these contrasts was set at a p < 0.05. Task-associated accuracy and response time were compared between groups using independent samples t-tests. SPSS was used for all statistical hypothesis testing.
Results
Two subjects were excluded from analysis due to performance (< 60% accuracy) on the N-back task, resulting in a final sample size of 19, which included 10 subjects with ELS and 9 without ELS. Demographic, ELS, and performance data are presented in Table 1. Groups did not significantly differ in age, gender distribution or ethnicity. There was no significant difference between groups in accuracy or response time during the N-back. Mean number of adverse childhood events was 5 (median = 4), and the ELS group reported significantly more ELS exposure than the non-ELS group. Over one third of ELS participants reported witnessing abuse or being physically threatened by adults in the home, and one quarter of this group reported more serious exposure such as childhood parental loss, physical abuse or sexual abuse.
Table 1.
Demographics, Task Performance and Early Life Stress
| Non-ELS (n = 9) | ELS (n = 10) | p-value | |
|---|---|---|---|
| Age (SD) | 39.7 (15) | 37.4 (12) | 0.70 |
| Female (%) | 4 (44) | 7 (70) | 0.28 |
| White (%) | 6 (66) | 8 (80) | 0.70 |
| Task Performance | |||
| 0-back Percent Accuracy (SEM) | 94 (2.6) | 96 (2) | 0.52 |
| 0-back Response Time1 (SEM) | 0.60 (0.04) | 0.55 (0.04) | 0.34 |
| 2-back Percent Accuracy (SEM) | 69 (1.4) | 70 (0.8) | 0.65 |
| 2-back Response Time1 (SEM) | 0.62 (0.05) | 0.55 (0.07) | 0.25 |
| Adverse Childhood Events, Mean (SD) | 1.8 (1.3) | 7.8 (4.5) | < 0.01 |
SD = standard deviation, SEM = standard error of the mean
units in seconds
One sample t-tests indicated no changes from baseline activity in bilateral LP regions, and these regions were excluded from further analysis, leaving 4 remaining bilateral ROIs (Table 2). Results from repeated measures ANOVA are shown on Table 3. After correction for multiple comparisons, there was a significant main effect of ELS on DN deactivations in the right PCC, bilateral MPFC, left MFG and right MTR. There were significant main effects of the N-back in the right PCC and left MTR; this effect did not survive multiple comparison correction. There were no statistically significant interactions between task difficulty and ELS, although trend level effects were observed for interactions in the right PCC (p = .06).
Table 2.
Default Network Regions of Interest
| Default Network Region | Cluster Size (mm3) | Talairach Coordinates (x, y, z) |
|---|---|---|
| PCC | ||
| Left | 800 | 6, 51.9, 27.1 |
| Right | 496 | −7.9, 50.2, −7.5 |
|
| ||
| MPFC | ||
| Left | 271 | 4.8, −44.1, 16.3 |
| Right | 111 | −5.7, −45.9, 16.8 |
|
| ||
| MFG | ||
| Left | 127 | 24.6, −22.3, 44 |
| Right | 100 | −22.9, −25, 45.8 |
|
| ||
| MTR | ||
| Left | 46 | 56.3, 16.4, −5.8 |
| Right | 31 | −54.8, 9.7, −8 |
PCC = posterior cingulate cortex, MPFC = medial prefrontal cortex, MFG = middle/superior frontal gyrus, MTR = medial temporal region.
Table 3.
Default Network Regions, Early Life Stress, and N-back Results
| Default Network Region | Effect of ELS a (F, df, p) | Effect of N- back b (F, df, p) | 0-Back c (t, df, p) | 2-Back c (t, df, p) |
|---|---|---|---|---|
| PCC | ||||
| Left | 3.87, 1, 0.07 | 4.19, 1, 0.06 | - | - |
| Right | 5.70, 1, 0.03 | 4.62, 1, 0.05 | 2.01, 17, 0.06 | 2.34, 17, 0.03 |
|
| ||||
| MPFC | ||||
| Left | 9.60, 1, 0.01 | 0.29, 1, 0.60 | 2.75, 17, 0.01 | 2.96, 17, 0.01 |
| Right | 7.37, 1, 0.02 | 0.12, 1, 0.73 | 2.16, 17, 0.05 | 2.78, 17, 0.01 |
|
| ||||
| MFG | ||||
| Left | 8.18, 1, 0.01 | 3.41, 1, 0.08 | 2.32, 17, 0.03 | 2.53, 17, 0.02 |
| Right | 3.12, 1, 0.09 | 3.63, 1, 0.07 | - | - |
|
| ||||
| MTR | ||||
| Left | 0.31, 1, 0.58 | 5.37, 1, 0.03 | - | - |
| Right | 6.60, 1, 0.02 | 3.67, 1, 0.07 | 2.34, 17, 0.03 | 2.26, 17, 0.04 |
ELS = early life stress, PCC = posterior cingulate cortex, MPFC = medial prefrontal cortex, MFG = middle/superior frontal gyrus, MTR = medial temporal region.
Results from repeated measures analysis of variance for significant effect of ELS on brain response by region of interest (corrected for multiple comparisons)
Results from repeated measures analysis of variance for significant effect of N-back on brain response by region of interest
Independent samples t-test of brain response comparing ELS vs. non-ELS groups, shown only in the case of a significant main effect of ELS.
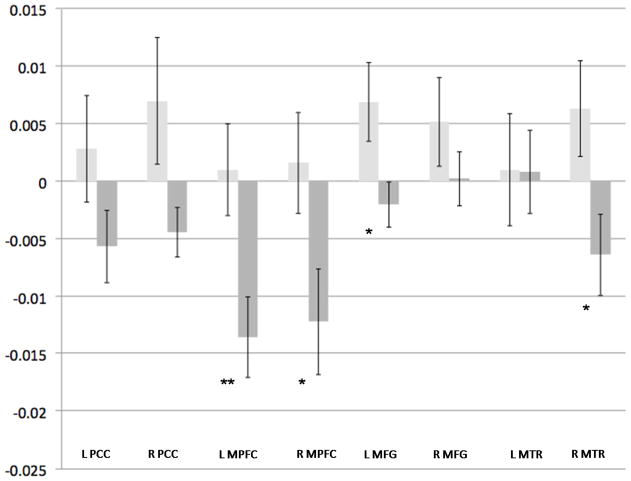
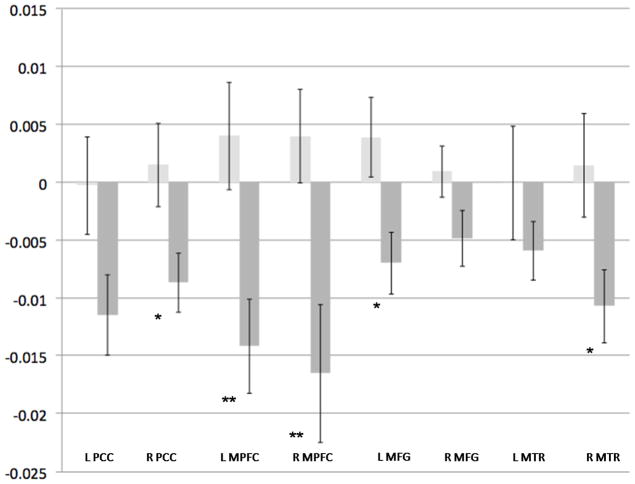
To further evaluate the main effect of ELS, planned independent samples t-tests were conducted for the 0- and 2-back conditions; results are presented in Table 3 and depicted in Figures 3 and 4. During the control condition, ELS participants demonstrated significantly greater DN deactivation in the right PCC, bilateral MPFC, left MFG and right MTR. During the 2-back WM task there was significant deactivation in the ELS group, compared to controls, in the right PCC, bilateral MPFC left MFG and right MTR.
Figure 3. Effect of ELS on 0-Back Associated Activity.
Light-gray bars indicate the non-ELS group, and dark-gray bars indicate the ELS group. Y-axis indicates beta weight, as an estimation of percent signal change. Error bars indicate standard error of the mean. X-axis indicates Default Network Regions of Interest.
* p = 0.05, ** p < 0.01, corrected for multiple comparisons; ELS = early life stress, PCC = posterior cingulate cortex, MPFC = medial prefrontal cortex, MFG = middle/superior frontal gyrus, MTR = middle temporal region
Figure 4. Effect of ELS on 2-Back Associated Activity.
Light-grey bars indicate the non-ELS group, and dark-gray bars indicate the ELS group. Y-axis indicates beta weight, as an estimation of percent signal change. Error bars indicate standard error of the mean. X-axis indicates Default Network Regions of Interest.
* p < 0.05, ** p < 0.01; ELS = early life stress, PCC = posterior cingulate cortex, MPFC = medial prefrontal cortex, MFG = middle/superior frontal gyrus, MTR = middle temporal region
In summary, there was greater task-associated DN deactivations in ELS-exposed participants compared to those without ELS exposure. This difference was observed during both the 0-back control and 2-back WM conditions, and included prominent involvement of the MPFC.
Discussion
We observed greater deactivation of the DN during a WM challenge in adults with a self-reported history of ELS compared to those in the non-ELS group. This suggests that ELS is associated with greater suppression of baseline DN processes during WM. These results are similar to previous studies examining changes in DN activity during WM tasks (Fransson, 2006; Fransson and Marrelec, 2008; McKiernan et al., 2003; Sweet et al., 2008).
Most DN regions demonstrated greater deactivation during WM tasks in the ELS group, compared to non-exposed controls. The pattern of this response indicates robust involvement of bilateral MPFC regardless of difficulty level. ELS-related volume reductions have been observed previously in the MPFC (Cohen et al., 2006), and this region is consistently implicated in the PTSD imaging literature as a component of the stress-induced fear circuitry loop, encompassing the MPFC, hippocampus and amygdala (Bryant et al., 2008; Shin and Handwerger, 2009). MPFC and hippocampal activation are required for extinction of fear conditioning in healthy individuals (Milad et al., 2007), whereas in PTSD subjects, hypo-activation of the MPFC has been reported (Rougemont-Bucking et al., 2011). Interestingly, restoring MPFC activity has been associated with improvement in clinical symptomatology. In recently traumatized police officers receiving exposure psychotherapy, increased MPFC activity was associated with decreased amygdala activity during traumatic memory retrieval (Peres et al., 2011), lending support to the hypothesis that abnormal MPFC activity (e.g., inadequate MPFC inhibition of amygdala activity) may contribute to the clinical symptoms of PTSD (Koenigs and Grafman, 2009). Our findings indicate that even in subjects without current psychopathology, individuals with ELS exposure demonstrate reduced MPFC activity. Taken in context, our results raise the possibility that reduced MPFC activity evaluated during WM tasks could serve as a neural marker of risk for developing psychological abnormalities in later life. Prospective studies evaluating changes in brain activity within DN regions in at-risk samples are needed to evaluate this hypothesis.
An alternate explanation to our results is that they reflect a neuroimaging correlate of resilience. DN deactivation was first identified from regions that deactivated during working memory tasks (Raichle et al., 2001), which has been traditionally understood as a compensatory re-allocation of cognitive resources (Fransson, 2006). Considered in that context, the increased deactivation observed in the study may reflect an adaptive response to the WM challenge. This is further supported in these results by the lack of differences in performance (i.e., response time and accuracy) between the two groups, indicating successful function of compensatory mechanisms.
Interestingly, participants with ELS demonstrated significant deactivation during the 0-back condition. One interpretation of this finding is that maintaining sustained attention during the 0-back results in attenuation of the DN in this population of otherwise healthy adults, which perhaps reflects greater cognitive effort being exerted by this group. If replicated, this raises important issues of experimental design, as the 0-back task is often used as a control condition.
It is possible that our results reflect an interaction between childhood ELS and the acute stress of performing a cognitive challenge. Previous research indicates that cognitive challenges can be experienced as stressors (Pruessner et al., 2010; Qin et al., 2009). This interpretation would suggest that performing cognitive challenges within an MRI scanner might be akin to a psychiatric “stress test,” in which participants can appear to be “normal” until challenged. This approach has been suggested as a possible method to evaluate and predict treatment response to antidepressant pharmacology (Salvadore et al., 2009; Salvadore et al., 2010). Under these circumstances, the N-back task might be considered a form of acute stress, which, for the ELS group, resulted in diminished MPFC activity that might be associated with reduced stress-tolerance, despite the fact that these individuals were psychologically healthy and denied any symptoms of clinical psychopathology. These findings give further support to the hypothesis that ELS is associated with latent neural abnormalities that may increase the risk of developing psychiatric illness secondary to stressful life events.
We did not find statistically significant group differences in behavioral performance, in contrast with group differences in BOLD response. This is consistent with previous findings indicating little difference in response time and accuracy during WM in PTSD vs. healthy control participants (Moores et al., 2008), and suggests that BOLD signal change may be a more sensitive measure of cognitive effort than are behavioral data.
Limitations and Conclusions
Our study had several limitations. The sample size was small; however, we sought to increase our power by employing an ROI analysis, and despite our sample size we found significant effects. Another limitation was the retrospective quantification of ELS. The ELSQ is a self-report scale which focuses on abuse and neglect during early developing years (Sanders and Becker-Lausen, 1995). With any self-report measure, it is difficult to assess both the veracity and the intensity of ELS retrospectively, although this problem is not limited to our study. Retrospective reporting of ELS may be subject to recall bias, although false negatives are more common than false positives (Hardt and Rutter, 2004). Such a bias could lead to mis-classification of some individuals in the control group and therefore group differences associated with ELS would be more difficult to detect. Thus, our results might underestimate the differences between individuals with and without a history of ELS. Since ELS is under-reported, and because we used a median split to group subjects, it is reasonable to assume that there was some low-level ELS exposure in the non-ELS group. However, since subjects had no psychiatric disorders that might act as a proxy for severe, undetected ELS, we could be reasonably confident in the ELS grouping. Future studies would benefit from utilization of more detailed ELS assessment and prospective data.
Another limitation involves the issue of relative baselines inherent in FMRI research. Although the ELS group clearly exhibited a greater decline from baseline in DN activity compared to controls, it is not certain whether this reflects attenuation from expected levels or attenuation of a potentially over-active DN at rest. The former suggests a compensatory mechanism engaged to successfully perform a cognitive task, while the latter suggests a trait maker that is not necessarily related to the cognitive challenge. Future studies designed to quantify absolute measures of activity during the resting state are needed to address this issue (e.g., perfusion imaging or positron emission tomography). Additionally, although we found a main effect of N-back difficulty, this did not survive multiple comparison correction, and planned contrasts found little DN deactivation in the non-ELS group. This suggests that N-back associated DN deactivation was driven by the changes in the ELS group. Further studies are needed to confirm this finding.
In summary, we found that subjects with ELS, but without psychiatric illness, displayed significantly greater task-dependent DN deactivation during WM tasks. These results have several important implications. They suggest that functional imaging may be used to detect latent neurodevelopmental effects of ELS exposure in adults, facilitating a better understanding of the pathophysiology and treatment of ELS-related conditions such as major depression and PTSD. This is an emerging area of research, as preliminary data suggest that the DN may have a role in predicting who will develop PTSD in the future (Lanius et al., 2010). Finally, our results raise important design considerations for future studies. We recruited from a sample of healthy controls, yet demonstrated significant differences within this group when stratifying for ELS. We found that ELS can influence regional brain response even in the 0-back, a test of sustained attention which is often used as a control task during WM paradigms. Since ELS is highly prevalent and under-reported, these findings highlight the importance of careful ELS screening in future functional neuroimaging studies.
Acknowledgments
Supported in part by NIH T32MH067553, R01HL084178, and P50CA084719. The authors wish to thank Denise M. Cote, M.S., for technical assistance, and to Jason Hassenstab, Ph.D., for methodological assistance. This data was presented in part at the 2011 Annual Meeting of the Society of Biological Psychiatry.
References
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- Briere J, Elliott DM. Prevalence and psychological sequelae of self-reported childhood physical and sexual abuse in a general population sample of men and women. Child Abuse Negl. 2003;27(10):1205–1222. doi: 10.1016/j.chiabu.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Brown GW, Moran P. Clinical and psychosocial origins of chronic depressive episodes. I: A community survey. Br J Psychiatry. 1994;165(4):447–456. doi: 10.1192/bjp.165.4.447. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, Gordon E, Williams LM. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum Brain Mapp. 2008;29(5):517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Wu CH, Liao YP, Hsu HL, Tseng YC, Liu HL, Chiu WT. Working memory in patients with mild traumatic brain injury: functional MR imaging analysis. Radiology. 2012;264(3):844–851. doi: 10.1148/radiol.12112154. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, McCaffery J, Hitsman B, Niaura R, Clark CR, McFarlane A, Bryant R, Gordon E, Williams LM. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59(10):975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Cox R. AFNI: software for analysis and visualization of functioanl magnetic resonance images. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Daniels JK, McFarlane AC, Bluhm RL, Moores KA, Clark CR, Shaw ME, Williamson PC, Densmore M, Lanius RA. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. J Psychiatry Neurosci. 2010;35(4):258–266. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePrince AP, Weinzierl KM, Combs MD. Executive function performance and trauma exposure in a community sample of children. Child Abuse Negl. 2009;33(6):353–361. doi: 10.1016/j.chiabu.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. JAMA. 2001;286(24):3089–3096. doi: 10.1001/jama.286.24.3089. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44(14):2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry. 2004;45(2):260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52(7):671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- Kendall-Tackett KA, Eckenrode J. The effects of neglect on academic achievement and disciplinary problems: a developmental perspective. Child Abuse Negl. 1996;20(3):161–169. doi: 10.1016/s0145-2134(95)00139-5. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med. 2004;34(8):1475–1482. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15(5):540–548. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, Neufeld RW, Williamson PC, Brimson M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121(1):33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Majer M, Nater UM, Lin JM, Capuron L, Reeves WC. Association of childhood trauma with cognitive function in healthy adults: a pilot study. BMC Neurol. 2010;10:61. doi: 10.1186/1471-2377-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Moores KA, Clark CR, McFarlane AC, Brown GC, Puce A, Taylor DJ. Abnormal recruitment of working memory updating networks during maintenance of trauma-neutral information in post-traumatic stress disorder. Psychiatry Res. 2008;163(2):156–170. doi: 10.1016/j.pscychresns.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres JF, Foerster B, Santana LG, Fereira MD, Nasello AG, Savoia M, Moreira-Almeida A, Lederman H. Police officers under attack: resilience implications of an fMRI study. J Psychiatr Res. 2011;45(6):727–734. doi: 10.1016/j.jpsychires.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Philip NS, Sweet LH, Tyrka AR, Price LH, Bloom RF, Carpenter LL. Decreased default network connectivity is associated with early life stress in medication-free healthy adults. Eur Neuropsychopharmacol. 2012;2012 doi: 10.1016/j.euroneuro.2012.10.008. (Epub Ahead of Print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, Dagher A, Lupien SJ. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35(1):179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJ, Luo J, Fernandez G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry. 2009;66(1):25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Park S, T L, Bihrle S, LaCasse L, Spatz-Widom C, Dayeh LA, Singh M. Reduced Right Hemisphere Activation in Severely Abused Violent Offenders During a Working Memory Task: An fMRI Study. Aggressive Behavior. 2001;27:111–129. [Google Scholar]
- Rougemont-Bucking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Rauch SL, Pitman RK, Milad MR. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci Ther. 2011;17(4):227–236. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr, Manji HK. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65(4):289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, Holroyd T, DiazGranados N, Machado-Vieira R, Grillon C, Drevets WC, Zarate CA., Jr Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35(7):1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders B, Becker-Lausen E. The measurement of psychological maltreatment: early data on the Child Abuse and Trauma Scale. Child Abuse Negl. 1995;19(3):315–323. doi: 10.1016/s0145-2134(94)00131-6. [DOI] [PubMed] [Google Scholar]
- Shaw ME, Moores KA, Clark RC, McFarlane AC, Strother SC, Bryant RA, Brown GC, Taylor JD. Functional connectivity reveals inefficient working memory systems in post-traumatic stress disorder. Psychiatry Res. 2009;172(3):235–241. doi: 10.1016/j.pscychresns.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Handwerger K. Is posttraumatic stress disorder a stress-induced fear circuitry disorder? J Trauma Stress. 2009;22(5):409–415. doi: 10.1002/jts.20442. [DOI] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Salimi-Khorshidi G, Webster M, Beckmann CF, Nichols TE, Ramsey JD, Woolrich MW. Network modelling methods for FMRI. Neuroimage. 2010;54(2):875–891. doi: 10.1016/j.neuroimage.2010.08.063. [DOI] [PubMed] [Google Scholar]
- Sweet L, Jerskey BA, Hassenstab JJ, Cote DM, Clark US. Attenuation of default network processing during working memory. 40th Annual meeting of the Society for Neuroscience; San Diego, CA. 2010. [Google Scholar]
- Sweet LH, Mulligan RC, Finnerty CE, Jerskey BA, David SP, Cohen RA, Niaura RS. Effects of nicotine withdrawal on verbal working memory and associated brain response. Psychiatry Res. 2010;183(1):69–74. doi: 10.1016/j.pscychresns.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet LH, Paskavitz JF, Haley AP, Gunstad JJ, Mulligan RC, Nyalakanti PK, Cohen RA. Imaging phonological similarity effects on verbal working memory. Neuropsychologia. 2008;46(4):1114–1123. doi: 10.1016/j.neuropsychologia.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar sterotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Thieme Medical Publishers, Inc; Stuttgart, Germany: 1988. [Google Scholar]
- U.S. Department of Health and Human Services, Aoc, youth and families. Child maltreatment 2007. Washington, DC: U.S. Government Printing Office; 2007. [Google Scholar]
- Zlotnick C, Mattia J, Zimmerman M. Clinical features of survivors of sexual abuse with major depression. Child Abuse Negl. 2001;25(3):357–367. doi: 10.1016/s0145-2134(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Zlotnick C, Warshaw M, Shea MT, Keller MB. Trauma and chronic depression among patients with anxiety disorders. J Consult Clin Psychol. 1997;65(2):333–336. doi: 10.1037//0022-006x.65.2.333. [DOI] [PubMed] [Google Scholar]