Abstract
Social deprivation is known to trigger a variety of behavioral and physiological modifications in animal species but the underlying genetic and cellular mechanisms are not fully understood. As we described previously, adult female flies reared in isolation show increased frequency of aggressive behaviors than those reared in group. Here we report that isolated rearing also caused significantly altered nerve and muscle excitability and enhanced synaptic transmission at larval neuromuscular junctions. We found that mutations of two genes, Hyperkinetic (Hk) and glutathione S-transferase-S1 (gsts1) alter the response to social isolation in Drosophila. Hk and gsts1 mutations increased adult female aggression and larval neuromuscular hyperexcitability even when reared in group. Unlike wild type, these behavioral and electrophysiological phenotypes were not further enhanced in these mutants by isolated rearing. Products of these two genes have been implicated in reactive oxygen species (ROS) metabolism. We previously reported in these mutants increased signals from a ROS probe at larval neuromuscular junctions and this study revealed distinct effects of isolation rearing on these mutants compared to the control larvae in ROS-probe signals. Our data further demonstrated modified nerve and muscle excitability by a reducing agent, dithiothreitol. Our results suggest that altered cellular ROS regulation can exert pleiotropic effects on nerve, synapse, and muscle functions and may involve different redox mechanisms in different cell types to modify behavioral expressions. Therefore, ROS regulation may take part in the cellular responses to social isolation stress, underlying an important form of neural and behavioral plasticity.
Keywords: ROS, Drosophila, Hyperkinetic, glutathione S-transferase, synaptic transmission
INTRODUCTION
Animal behaviors can be significantly modified by social isolation. In mammals including humans, the outcome of social deprivation may range from hyperactivity (Sahakian et al., 1975; Gentsch et al., 1981) to anxiety, depression, aggression, and schizophrenia (Spitz 1945); Harlow & Harlow 1962; Hatch et al., 1965), suggesting profound effects on nervous system functions. The effects of social isolation are also evident in invertebrate species. For example, rearing in isolation increases aggressive behaviors in the crayfish (Yeh et al., 1996), cricket (Alexander, 1961), bee (Breed 1983), wasp (Pfennig & Reeve, 1989) and fruitfly (Hoffmann 1990), Ueda & Kidokoro 2002), and affects locomotive activity, aggregation, and dispersion in certain species of locusts (Ellis 1953); Pener & Yerushalmi, 1998) and butterflies (Long, 1953).
Despite its important consequences, our understanding of the genetic and cellular mechanisms mediating social isolation effects is still limited. Extensive studies have implicated important roles of hormonal and monoamine systems in these social isolation-induced behavioral phenotypes in both vertebrate (Schiller et al., 2006; Serra et al., 2007) and invertebrate (Edwards & Kravitz 1997; Pener & Yerushalmi, 1998; Anstey et al., 2009) species. Genetic studies have identified several genes related to the cAMP second messenger cascade in mediating social isolation-induced neuroanatomical modifications in Drosophila (Balling et al., 1989) and behavioral changes in mice (Barrot et al., 2005). So far, reports on electrophysiological alterations of the nervous system following social isolation have been limited to hippocampal long-term potentiation (Kehoe et al., 1995).
Modifications in nervous system development by social isolation have been documented in Drosophila. When larvae are grown at a low density throughout development, the motor axon terminal arbor becomes more elaborated and expanded (Stewart & McLean 2004). In addition, during the initial several days following eclosion, social isolation affects the number of axon fibers in the mushroom bodies, an important adult brain structure for behavioral plasticity (Technau 1984). Additionally, aggressive behaviors of adult males (Hoffman 1990) and females (Ueda & Kidokoro 2002) are enhanced by social isolation. However, little is known about whether social deprivation causes physiological changes and which genes regulate social isolation-induced behavioral modifications in Drosophila. In this study, we explored electrophysiological phenotypes associated with social deprivation, and mutational effects of identified genes on the corresponding physiological and behavioral modifications.
We found that social isolation exerts significant effects on behavioral and physiological traits at different developmental stages. In addition to the well documented enhancement of aggressive behaviors in adults, isolated rearing can increase nerve excitability, indicated by activity-dependent enhancement of synaptic transmission at the larval neuromuscular junction. Furthermore, our results identified two genes, Hyperkinetic (Hk) and glutathione S-transferase-S1 (gsts1) that may play important roles in physiological and behavioral responses to social isolation. Significantly, these two genes have been implicated in reactive oxygen species (ROS) metabolism. The gsts1 gene encodes a sigma class glutathione S-transferase (Beall et al., 1992), which conjugate glutathione to reactive oxygen intermediates (Singh et al., 2001). Hk codes for the auxiliary subunit for the Shaker (Sh) K+ channel (Chouinard et al., 1995) and modulates its gating and inactivation (Wang & Wu, 1996). However, the Hk polypeptide and its vertebrate orthologs Kvβ polypeptides (auxiliary subunits of the Sh ortholog, Kv1α, McCormack & McCormack 1994) share sequence similarities with aldo-keto reductase superfamily enzymes that catalyze NADP(H)-dependent reduction of a variety of substrates (Jin & Penning 2007). Recent studies have shown that vertebrate Kvβ1 and Kvβ2 not only modulate Kv1α– mediated K+ current but also catalyze reduction of aldehydes to alcohols in heterologous expression systems (Weng et al., 2006; Pan et al., 2008). Consistent with the above studies, Hk and gsts1 mutations have recently been shown to alter cellular ROS levels in larval motor terminals (Ueda & Wu 2008). Furthermore, Hk mutant flies are more sensitive to ingestion of the ROS generating agent, paraquat (Wang et al 2000).
Our results demonstrate a remarkable form of neural and behavioral plasticity induced by social deprivation. This paper presents an initial characterization of the wide-ranging effects of social isolation and manipulations of redox regulation on nerve, muscle, and synapse functions, in order to provide a basis for further investigations into the neural circuits relevant to associated physiological and behavioral changes.
MATERIALS AND METHODS
Drosophila Stocks
The Drosophila melanogaster mutant stocks used included Hk1, Hk2, gsts106253; ry506, and gsts104227; ry506. The Hk alleles were generated by EMS mutagenesis (Kaplan & Trout 1969). The gsts1 alleles were P-element insertion-induced and therefore carry ry506 as a marker (Spradling et al., 1999). The gsts1; ry506 and the ry506 lines were obtained from Bloomington Stock Center (Indiana University, Bloomington, IN). Second-site lethal mutations carried in the original gsts1; ry506 stocks were removed by backcrossing to the ry506 control line eight or more times (Ueda & Wu, 2008). The ry506 control and gsts104227; ry506 mutant lines were Cantonized 7–8 times further, and part of behavioral and physiological data were taken from these Cantonized stocks. These mutant alleles represent independent isolates, and thus provide a means for controlling possible effects from unidentified second-site mutations. The wild-type strain, CS, and ry506 served as control for Hk and gsts1, respectively.
Rearing larvae in isolation and in group
To rear larvae in isolation, we let adult flies lay eggs in a vial containing standard cornmeal food media with yeast paste for several hours to one day. Within a few hours after hatching, individual first instar larvae were transferred to a fresh vial containing yeast paste on the food surface. These larvae were allowed to grow individually until third instar wandering stage for physiological recordings. The control larvae were grown in the same vials in which parental flies laid the eggs with or without yeast paste. In both conditions, about 100 – 200 larvae pupated per vial and produced no significant differences in physiological phenotypes.
Electrophysiology
Postfeeding third instar larvae were used for electrophyiological recordings. Dissections were performed in HL3 saline containing (in mM) 70 NaCl, 5 KCl, 20 MgCl2, 10 NaHCO3, 5 treharose, 115 sucrose, and 5 HEPES, at pH 7.2 (Stewart et al., 1994). Excitatory junctional potentials (ejps) and nerve action potentials were recorded in a modified HL3 saline containing (in mM) 150 NaCl, 5 KCl, 4 MgCl2, 0.2~1.0 CaCl2, 10 NaHCO3, 5 treharose, 7.5 sucrose, and 5 HEPES, at pH 7.2. The increased Na+ and decreased Mg2+ concentrations in this modified saline enhanced the expression of neuronal hyperexcitability (cf. HL3.1 saline; Feng et al., 2004) and helped clear demonstration of the effects of isolated rearing and of Hk and gsts1 mutations. Unless otherwise stated, we used saline containing 0.2 mM Ca2+ to avoid muscle contraction during repetitive stimulation. In addition, the altered neuromuscular transmission was most striking at low Ca2+ concentrations, as previously shown in several hyperexcitable mutants (Ganetzky & Wu, 1982, 1983; Lee et al., 2008). To evoke nerve action potentials and ejps, we severed the segmental nerve from the ventral ganglion and stimulated through the cut end with a suction electrode (10 µm inner diameter). The stimulation voltage was adjusted to 2.5 times the threshold voltage to ensure action potential initiation. For ejp recordings, intracellular glass microelectrodes were filled with 3M KCl and had a resistance of ~60 MΩ. Ejps were recorded from muscles 1, 2, 6, 7, 9, 10, and 14 with direct current preamplifiers (model M701 microprobe system; WPI, Sarasota, FL, and an additional custom-built amplifier). Action potentials were recorded extracellularly from the segmental nerve en passant with a suction pipet (Wu et al., 1978). Signals were picked up by differential alternating-current amplifiers (DAM-5A; WPI, P15, and an additional custom-built amplifier). Both ejps and action potentials were stored on VCR tapes with a pulse code modulator (model Neuro-Corder DR-384; Neuro Data, New York, NY). Data was digitized with pClamp5 (Molecular Devices, Burlingame, CA) and analyzed with an IBM-compatible computer. To characterize the frequency-dependent profile of giant ejps induction, frequency response of giant ejps was tested from higher to lower frequency, i.e. from a more effective frequency (15 Hz) to progressively more stringent conditions (10- and 5 Hz, see Results). After initial 15-Hz stimulation, 10-Hz stimulation was tested only if 15 Hz stimulation successfully triggered giant ejps, and 5-Hz stimulation was tested only if 10-Hz stimulation triggered giant ejps. In this manner, the propensity of a NMJ to generate giant ejps can be determined without risking preparation run-down.
Dihydrorhodamine-123 (DHR) staining of the larval neuromuscular preparation
We followed the methods published before (Ueda & Wu, 2008). Briefly, larval neuromuscular preparations were incubated for 45 min with 1 µg/ml DHR in saline containing (in mM) 150 NaCl, 5 KCl, 4 MgCl2, 0.2 CaCl2, 10 NaCHO3, 5 Trehalose, 7.5 Sucrose, 5 HEPES, and 1 DMSO at pH 7.2. Subsequently, the preparation was washed with the same saline to remove external DHR. For initial trials, pluronic-F (1 ng/ml) was added to help disperse DHR. However, we observed comparable staining pattern and fluorescence intensities with or without pluronic-F. Data from the two protocols were combined. See Ueda & Wu (2008) for additional details.
Adult female aggressive behaviors
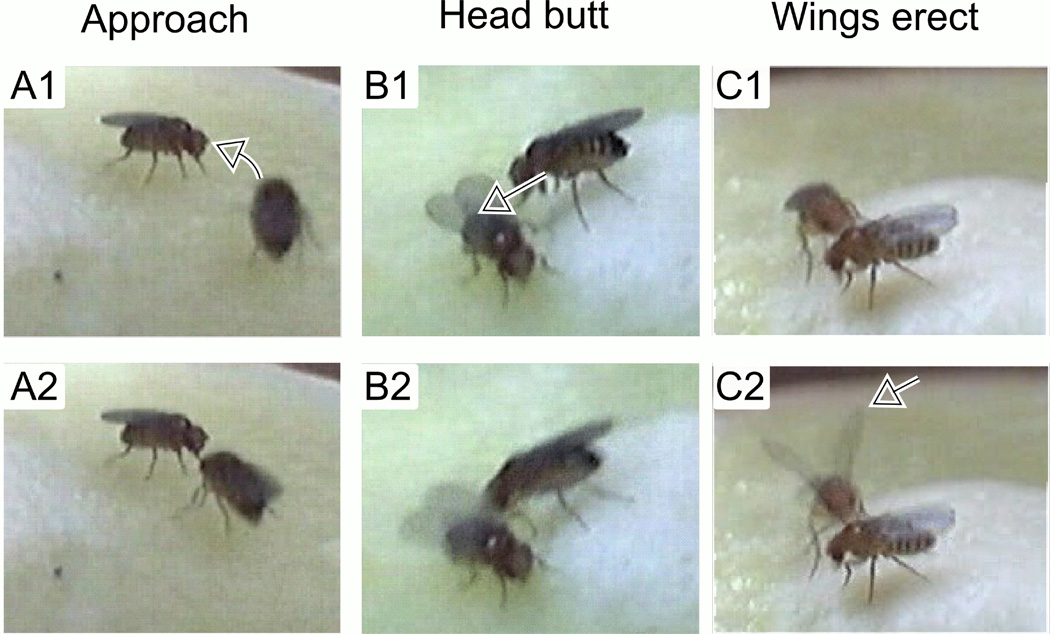
Virgin females were collected within 6 hours after eclosion and kept in isolation or in groups of 10 in food vials for 4 to 6 days. Before observation of aggressive behaviors, we starved flies by supplying only Kimwipe tissue paper moistened with distilled water for 6 – 8 hours. To observe aggressive behaviors, a pair of females was placed in an observation cell (φ= 1.45 cm, h = 0.95 cm, containing standard corn meal food medium covered with live yeast or dry yeast powder), and their behaviors were video taped for 10 min. Flies were allowed to acclimate up to 5 min before scoring of aggressive behaviors. We discarded fly pairs which remained agitated beyond 5 min. Occurrences of three types of behaviors were counted (Ueda & Kidokoro 2002) for 5 min. ‘Approach’ – a directed movement of a female toward the other (from a location more than one-body length away). Movements outside the arena surface (e.g. on the observation chamber wall) was not considered as ‘approach’. ‘Head butt’ (Nilsen et al., 2004; ‘lunge’ in Ueda & Kidokoro, 2002) – a female head-butted at another female (Figure 1). ‘Wings erect’ – one or both wings were briefly erected against the other female (Figure 1). This was frequently coupled with head butt. Behavioral scoring was performed either double blind or single blind.
Figure 1.
Behavioral components of female aggression. A1-2. Approach: a directed movement of a fly toward the opponent (arrow), often followed by “head butt” and/or “wings erect”. B1-2. Head butt: a fly dashing at the opponent (arrow). C1-2. Wings erect: a fly erecting both wings (arrow), facing the opponent. Consecutive frames (67-ms interval) are shown for each behavior from video recordings of group-reared Hk female pairs on corn meal medium surface supplemented with live yeast.
Statistical Analysis
Unless otherwise stated, X2-test was carried out for comparing the frequency of aggressive behaviors, which is not normally distributed.
RESULTS
Effects of social isolation on adult female aggression in wild-type flies
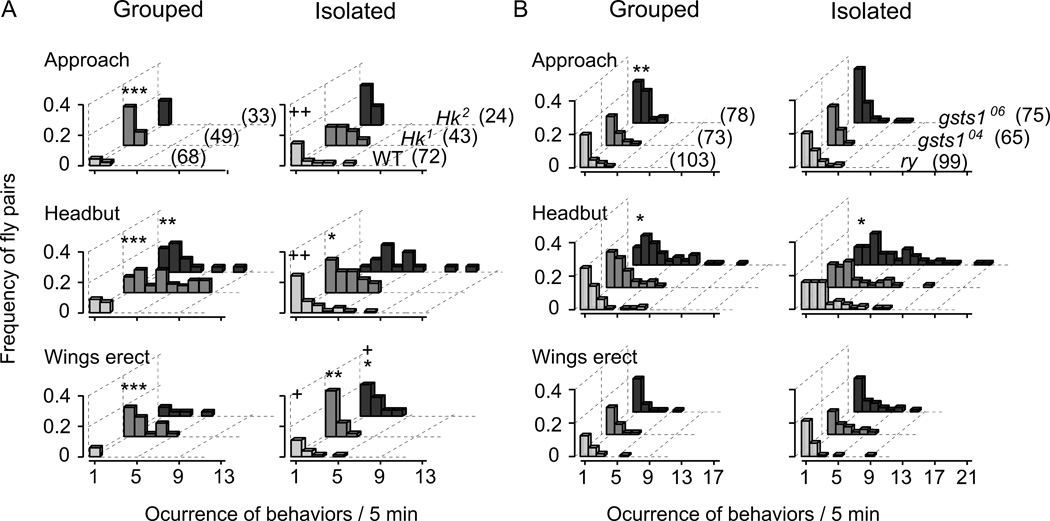
To score aggression behaviors, we placed a pair of female flies in an observation chamber, and recorded the frequency of aggressive behaviors in a 5 min session. These included “approach”, “head butt”, and “wings erect” (Figure 1). We compared female flies socially isolated for 3 to 6 days after eclosion with control flies maintained in a group of 10 for the same time period (Figure 2 and Table 1). As reported previously (Ueda & Kidokoro 2002), when reared individually, the wild-type (WT) strain, CS, female flies more frequently displayed the stereotypic aggressive behaviors. They often approached each other and performed head butt against the opponent. The head-butt behavior was occasionally coupled with erecting one or both wings (Figure 1). In some incidents, one fly chased the other after a head-butt event. These behaviors were significantly different between socially isolated and control flies, with head butt showing the greatest, and wings erect the least, difference in the frequency of occurrence. These observations are summarized in Figure 2, which displays the frequency distribution of the number of occurrence of different aggressive behaviors for the observed fly pairs, and in Table 1, which presents the mean frequency of occurrence per fly pair and the percentage of fly pairs displaying repeated incidents (counts ≥ 2) of each aggressive behavior within an observation session.
Figure 2.
Effects of social isolation and Hk and gsts1 mutations on female aggressive behaviors. Frequency distributions of different aggressive behaviors during the 5-min observation period are shown for Hk and gsts1 mutant alleles and control lines grown in isolation or in group. A. Isolation rearing increased frequencies of all three aggressive behaviors (approach, head butt, and wings erect) in WT flies. Group-reared Hk1 flies displayed aggressive behaviors at higher frequencies than group-reared WT flies. Hk2 flies exhibited a weaker tendency than Hk1. However, isolation caused little increase in aggression of both Hk alleles, except for wings erect in Hk2. B. Group-reared gsts106253 (gsts106) flies showed increased frequencies of approach and head butt compared to ry control while a tendency of increased head butt was seen in gsts104227 (gsts104). (See Table 1 for an assessment of isolation-rearing effects in gsts1 and ry control, as well as in Hk and WT.) X2-test based on the proportions of fly pairs with and without incidents (non-0 vs. 0) of aggressive behaviors in the observation session: +, p < 0.05; ++, p < 0.01 for group- vs. isolation-reared flies within the same genotype; *, p < 0.05; **, p < 0.01; ***, p < 0.001 for mutant vs. control lines within the same rearing condition. Numbers of fly pairs examined are shown in parenthesis.
Table 1.
Total and mean incidents of aggressive behaviors per fly pair and percentage of multiple incidents during 5-min sessions.
| CS control | Hk1 | Hk2 | ry control | gsts1 04;ry | gstsi 06; ry | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grouped | Isolated | Grouped | Isolated | Grouped | Isolated | Grouped | Isolated | Grouped | Isolated | Grouped | Isolated | |
| Total fly pairs | 68 | 72 | 49 | 43 | 33 | 24 | 103 | 99 | 73 | 65 | 78 | 75 |
| Approach | ||||||||||||
| Total counts | 5 | 27 | 20 | 35 | 5 | 12 | 45 | 70 | 36 | 30 | 71 | 65 |
| Mean counts per fly pair |
0.07 | 0.38 | 0.41 | 0.81 | 0.15 | 0.50 | 0.44 | 0.71 | 0.49 | 0.46 | 0.91 | 0.87 |
| % fly pairs w/ counts ≥ 2 |
1.5 | 6.9 | 8.2 | 26+** | 0 | 13 | 8.7 | 18+ | 12 | 11 | 27** | 19 |
| Headbut | ||||||||||||
| Total counts | 16 | 74 | 140 | 70 | 44 | 62 | 114 | 215 | 116 | 143 | 218 | 312 |
| Mean counts per fly pair |
0.24 | 1.0 | 2.9 | 1.6 | 1.3 | 2.6 | 1.1 | 2.2 | 1.6 | 2.2 | 2.8 | 4.2 |
| % fly pairs w/ counts ≥ 2 |
7.4 | 22+ | 59*** | 44* | 36*** | 54** | 27 | 51+++ | 42* | 48 | 62*** | 72** |
| Wings-erect | ||||||||||||
| Total counts | 4 | 22 | 45 | 24 | 12 | 18 | 38 | 54 | 30 | 46 | 38 | 70 |
| Mean counts per fly pair |
0.06 | 0.31 | 0.92 | 0.56 | 0.36 | 0.75 | 0.37 | 0.55 | 0.41 | 0.71 | 0.49 | 0.93 |
| % fly pairs w/ counts ≥ 2 |
0 | 6.9+ | 24*** | 12 | 9.1*** | 21 | 8.7 | 11 | 10 | 17 | 9.0 | 20 |
X2-test based on the proportion of fly pairs with multiple aggression incidents (equal or greater than 2):
p < 0.05;
p < 0.01 for group- vs. isolation-reared flies within the same genotype;
p < 0.05;
p < 0.01;
p < 0.001 for mutant vs. control lines under the same rearing condition. The frequency of aggressive behaviors was not normally distributed among pairs and statistical tests between mean counts per fly pair were not conducted between genotypes or rearing conditions.
Increased adult female aggression in Hk and gsts1
Interestingly, we found that Hk and gsts1 flies showed higher frequencies of aggressive behaviors, even when they were reared in groups of 10 or more. The Hk and gsts1 mutants exhibited stereotypic aggressive patterns, including approach, head butt, and wings erect, similar to the aggressive behaviors observed in isolated WT and ry control females described above.
For Hk mutant flies, there was an overall increase in the frequencies of these aggressive behaviors compared to group-reared WT control, with a more pronounced increase in head-butting (Figure 2 and Table 1). In addition, Hk flies sometimes chased around the opponents while chasing was rarely observed in WT control even when reared in isolation (data not shown). There were noticeable allele-dependent effects of social isolation on specific aggressive behaviors in Hk flies. Hk1 showed higher frequencies than Hk2 in all categories of aggressive behaviors and social isolation apparently caused increase in Hk2, but not Hk1, in head-butt and wings-erect behaviors (Figure 2 and Table 1).
In comparison, the enhanced aggressive phenotype of gsts1 mutant flies was less pronounced and more allele dependent when compared to ry control flies (Figure 2 and Table 1). In group-reared flies, the allele 06253 (06 hereafter) displayed significantly higher occurrences of approach and head butt, but 04227 (04 hereafter) displayed only a trend of increased head-butt behavior. Social isolation increased the occurrence of approach and head butt in ry control, but exerted no significant effect on aggressive behaviors in gsts1 mutant flies (Table 2).
Table 2.
Effects of rearing condition on the dihydrorhodamine-123 (DHR) staining of presynaptic boutons.
| Genotype | CS control | Hk | ry control | gstsi; ry | ||||
|---|---|---|---|---|---|---|---|---|
| Grouped | Isolated | Group | Isolated | Group | Isolated | Group | Isolated | |
| number of NMJs | 24 | 16 | 20 | 15 | 8 | 6 | 6 | 10 |
| F45-0 | 388 ± 29 | 385 ± 46 | 574 ± 38 | 398 ± 47 +++ | 317 ± 54 | 259 ± 15 | 537 ± 78 | 428 ± 45 |
| F75-45 | 129 ± 14 | 95 ± 18++ | 81 ± 23 | 97 ± 22 | 106 ± 20 | 43 ± 44+ | 111 ± 26 | 75 ± 15 |
| F75-45 | 0.33 | 0.25 | 0.14 | 0.24 | 0.33 | 0.17 | 0.21 | 0.18 |
| F45-0 | ||||||||
NMJs were stained with DHR (1 µg / ml, 45-min, Ueda & Wu, 2008). After the removal of DHR, the larval preparation was incubated for additional 30 min without DHR. Oxidized DHR fluorescence signals were measured at the end of the 45-min incubation period (F45-0) and the subsequent increment of fluorescence was collected at 30 min after DHR wash out (F75-45). Fluorescent intensity readings are in arbitrary unit.
+, ++, and +++ indicate p < 0.05, 0.02, and 0.01 (Mann-Whitney U test) for comparisons between NMJs in group-reared and isolation-reared larvae.
Effects of isolated rearing on larval neuromuscular transmission in WT
To obtain insights into the cellular effects of prolonged isolation on the nervous system, we took advantage of the well-established physiological protocols for the larval neuromuscular preparation to examine nerve excitability and synaptic transmission (Jan & Jan 1976; Wu et al., 1978). Larvae were grown either individually (one larvae/vial) or in a crowded condition (typically 100 – 200 larvae per vial, see Methods) from first instar until late third instar. Notably, at wandering stage, isolation-reared larvae were less mobile and stayed on food surface or on the glass wall immediate above food surface, in contrast to higher pupation sites observed in crowded vials. Since these larvae were raised in the same food vial until the wandering stage, rearing in groups softened the food, while isolated larvae experienced harder food medium. These rearing conditions might produce differences in nutritional and sensory factors, which could contribute to behavioral and physiological changes in larval development. Nevertheless, isolated rearing did not seem to prolong the wandering stage because these larvae pupated within several hours, about the same time as the group-reared larvae did.
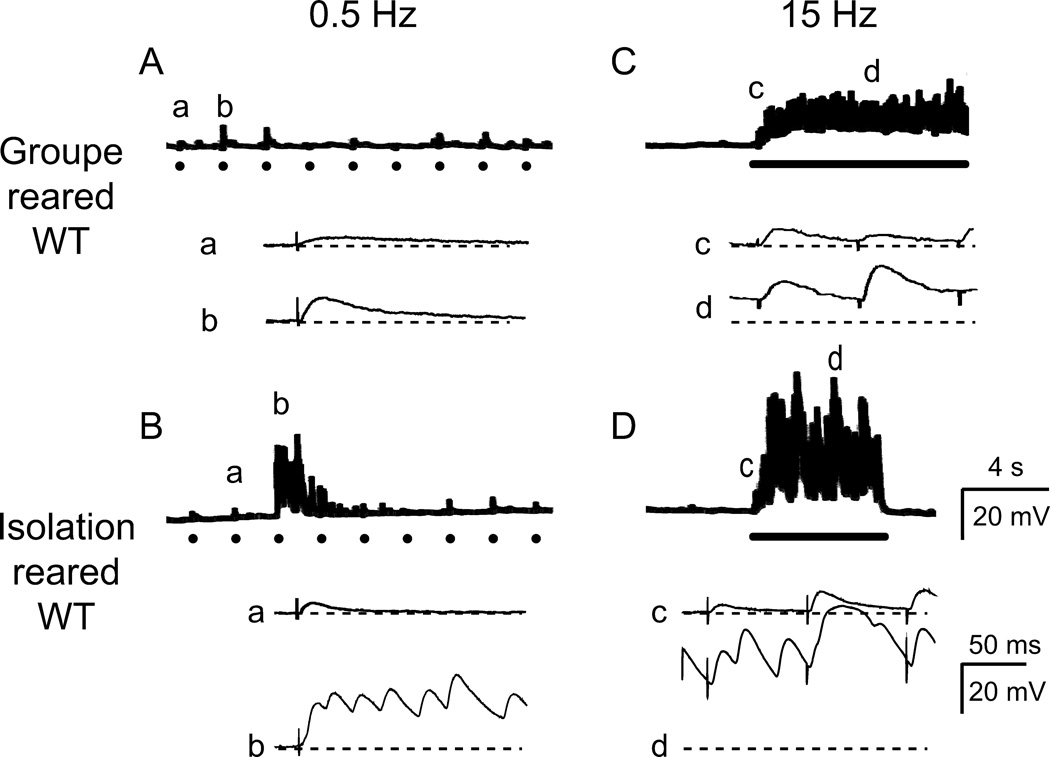
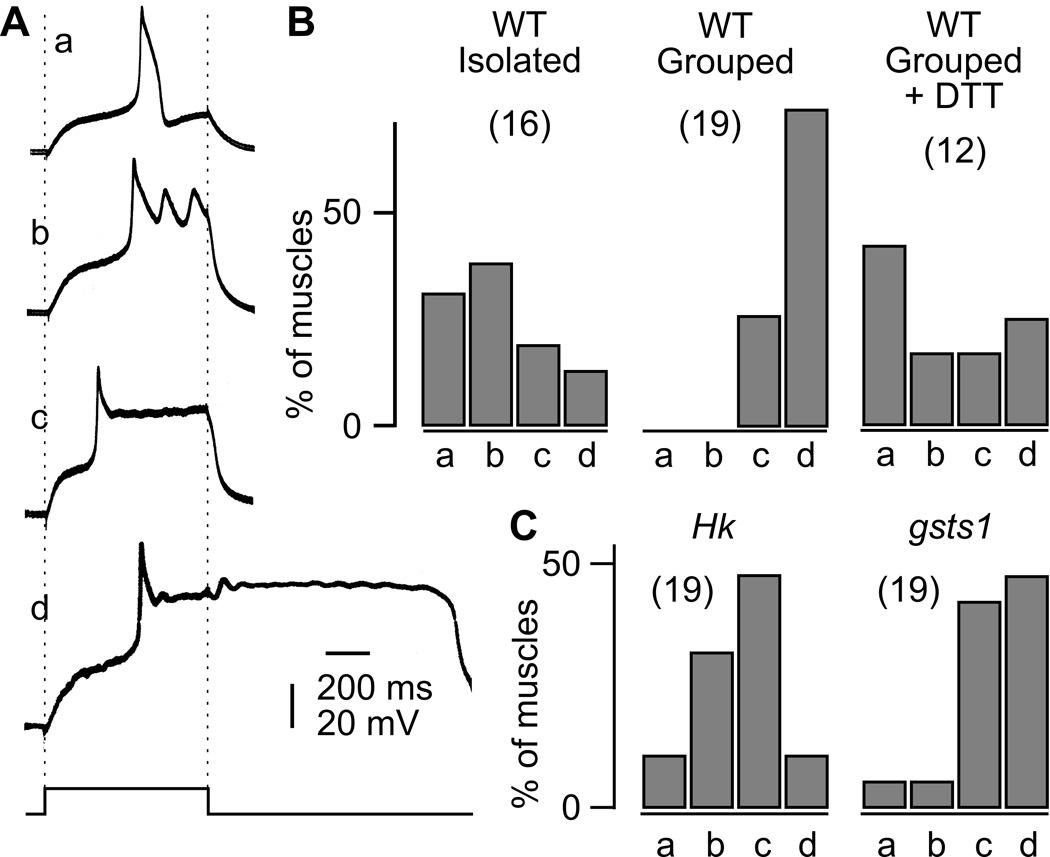
The increased Na+ and low Ca2+ and Mg2+ concentrations in the recording saline (see Methods) enhanced hyperexcitability expression and facilitated the detection of the effects of isolated rearing. We found that isolation rearing affected mainly activity-dependent enhancement of synaptic transmission upon repetitive stimulation at low Ca2+ levels. During initial low-frequency stimulation (≤ 0.5 Hz), neuromuscular transmission of the isolation-reared WT larvae appeared similar to those of group-reared larvae (‘a’ and ‘b’ in Figure 3Aand ‘a’ in 3B), consistent with a previous report of unaltered ejp sizes from larvae grown at low densities (10 larvae/vial, Stewart & McLean 2004). At 0.2 mM Ca2+, the average amplitude and the failure rate of ejps in isolation-reared larvae (5.1 ± 3.6 mV and 24 ± 38 % for muscles 6, 7 and 14, n = 7) were similar to those of group-reared larvae (8.0 ± 5.1 mV and 20 ± 33 %, n=6). However, after tens of seconds of repetitive nerve stimulation (0.5 Hz), bursts of transmitter release were recruited in isolation-reared larvae more frequently than group-reared larvae (3 out of 15 NMJs compared to 1 out of 14). Summation of these recurrent, supernumerary ejps could sometimes amount to a few tens of millivolts (multiple-peak giant ejps following a single stimulus, ‘b’ in Figure 3B).
Figure 3.
Greatly enhanced larval neuromuscular transmission by isolation rearing. A. Group-reared WT larvae displayed low levels of basal release at low external Ca2+ (0.2 mM) at neuromuscular junctions (NMJs). In a faster time scale, excitatory junctional potentials (ejps) of small amplitudes were time-locked to individual nerve stimulation (a, b). ● indicates 0.5-Hz nerve stimuli. B. Isolation-reared WT displayed similar basal-level of ejps (a), but following repeated 0.5-Hz stimulation, burst of transmitter release could occur, resulting in giant ejps (b). In faster time scale, giant ejps are resolved into supernumerary events of transmitter release. C. In group-reared WT, high-frequency (15-Hz, bar) stimulation resulted in only synaptic facilitation at low Ca2+, producing gradual increase in ejp amplitudes (compare c and d). D. In isolation-reared WT, 15-Hz stimulation (bar) triggered recurrent giant ejps that persisted for seconds (d).
In comparison, for a majority of isolation-reared larvae, high-frequency nerve stimulation (15 Hz, 10 s) rapidly evoked bursting activities of giant ejps within a few seconds. These recurrent giant ejps persisted up to tens of seconds and regularly reached a few tens of millivolts in amplitudes (Figure 3D). Giant ejp induction was scarce in group-reared WT larvae compared to isolation-reared larvae (1 out of 12 vs. 8 out of 15). In general, high-frequency repetitive stimulation in group-reared larvae induced only synaptic facilitation with a gradual increase in ejps amplitude within 10 seconds of stimulation (Figure 3C). For both isolation- or group-reared larvae, some of the NMJs that failed to express giant ejps could still be recruited to do so by extending the stimulus period from 10 to 50 seconds (10 out of 15 vs. 3 out of 12, isolation-reared vs. group-reared larvae).
The effect of isolated rearing appeared to be common among different NMJs within the larval body wall musculature. A dorso-ventral difference in synaptic strength has been reported for NMJs within the abdominal segment (Lee et al., 2008). We found that giant ejps occurred in both ventral and dorsal muscle fields (4 out of 8 in dorsal vs. 4 out of 7 in ventral NMJs). Therefore, we present combined data from different muscles from both group- and isolation-reared larvae of different genotypes hereafter.
Increased neuromuscular transmission and lack of isolation-rearing effects at Hk and gsts1 larval NMJs
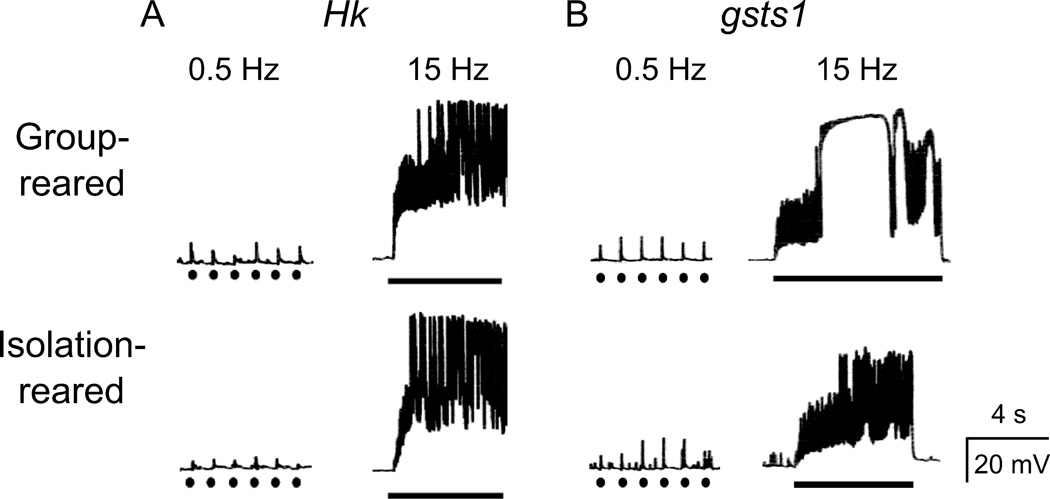
Behavioral observations indicated allele differences in female aggression between Hk mutants, with Hk1 more extreme than Hk2 following group rearing (Figure 2). Such allele differences were even more evident for neuromuscular transmission, with Hk1 far more extreme in activity-dependent hyperexcitability. During 10-s high-frequency (15 Hz) nerve stimulation at 0.2 mM Ca2+, we observed giant ejps far more frequently in the group-reared Hk1 mutant larvae (Figure 4A) than in WT larvae, consistent with a previous report (Stern & Ganetzky, 1989). However, this phenotype occurred only slightly more frequently in Hk2 than in WT (Figure 5A). In contrast to Hk, allele differences of gsts1 mutations were less pronounced in neuromuscular transmission. Upon 15-Hz stimulation, both mutant alleles of gsts1 displayed giant ejps more frequently than the ry control line (Figures 4B and 5B).
Figure 4.
Giant ejps and lack of isolation rearing effects in Hk and gsts1 mutants. In Hk (A) and gsts1 (B) mutant larvae, low frequency stimulation (0.5 Hz, ●) triggered basal transmitter release of small amplitudes, but high-frequency stimulation (15-Hz, bar) readily evoked recurrent giant ejps that persisted for seconds, regardless of rearing condition. Ca2+ = 0.2 mM
Figure 5.
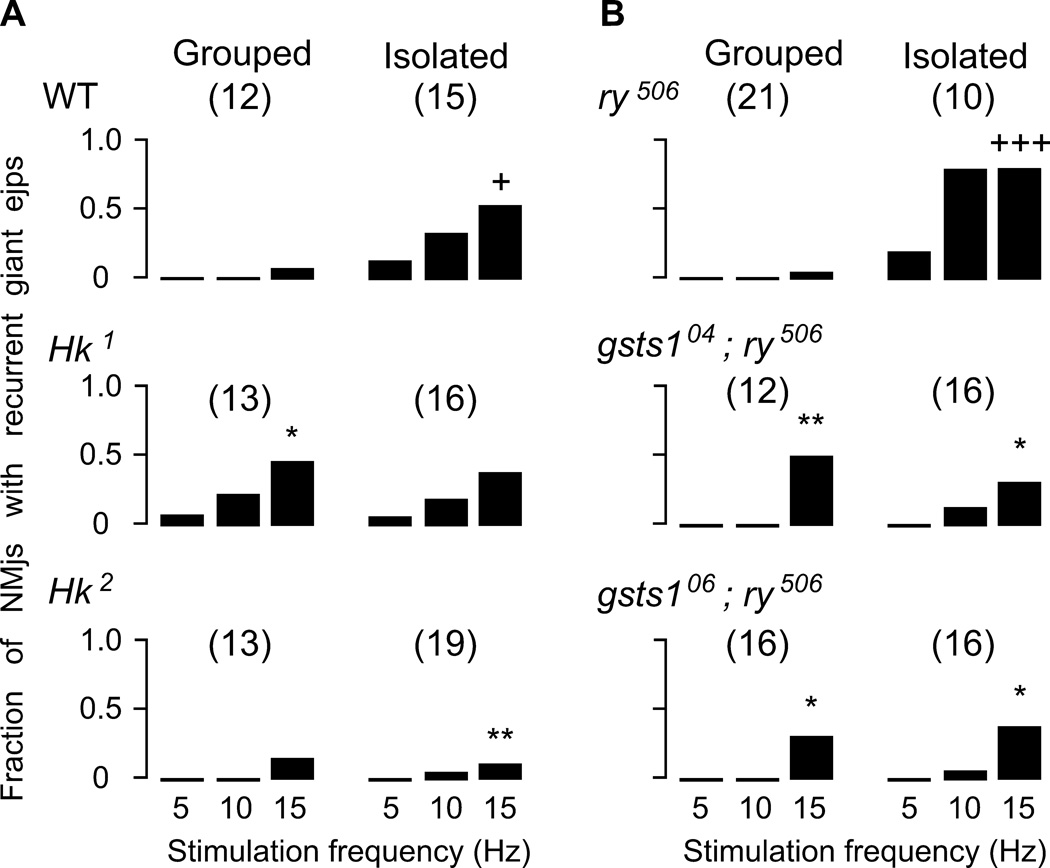
Stimulus-frequency dependent profile of persistent giant ejp induction. The fraction of NMJs displaying recurrent giant ejps during a 10-s stimulation period is plotted against the nerve stimulation frequency (see Methods). Note that WT and ry control line displayed clear effects of isolation rearing to increase the propensity of giant ejp induction (+, p < 0.05 and +++, p < 0.001. X2-test for 15-Hz nerve stimulation). Group-reared Hk1 and gsts1 mutant alleles displayed higher neuromuscular transmission than group-reared WT and ry control (*, p < 0.05 and **, p < 0.01). However, isolation rearing did not further enhance the excitability level in these mutants. Note that isolation-reared WT and ry displayed more frequent recurrent giant ejps than Hk2 and gsts1 alleles. Total numbers of NMJs tested for each genotype are shown in parenthesis.
In addition to the recurrent giant ejps described above during the course of 10-s, 15-Hz stimulation, we also observed isolated giant ejps that sporadically occurred at intervals of seconds. Such sporadic giant ejps appeared as a prelude to the recurrent giant ejps that occurred when stimulation persisted beyond 10 s. For example, in group-reared Hk1 and Hk2 larvae, as well as isolation-reared WT, sporadic giant ejps were all associated with ensuing events of recurrent giant ejps (4 out of 4, 1 out of 1, and 2 out of 2 for Hk1, Hk2, and WT). In contrast, a smaller portion of group-reared WT larvae that displayed sporadic giant ejps eventually produced recurrent giant ejps (2 out of 7).
The occurrence of recurrent giant ejps was stimulus-frequency dependent. When stimulus frequency was lowered from 15 to 10 Hz, their occurrence was significantly reduced in group-reared mutant larvae. If the stimulus frequency was further reduced to 5 Hz, many NMJs across all genotypes ceased to display giant ejps in the 10-s stimulation bouts (Figure 5).
To characterize basal transmitter release in Hk anf gsts1 mutant larvae, we monitored synaptic responses to single nerve stimuli (delivered at a low frequency, e.g. 0.5 Hz) to avoid complications introduced by frequency-dependent synaptic facilitation. We found that basal release at 0.2 mM Ca2+, as measured by ejp amplitude, was not different between group-reared Hk and WT larvae (7.8 ± 3.4mV, 3.9 ± 1.9 mV, and 8.0 ± 5.1mV for Hk1, Hk2, and WT; ventral muscles 6, 7, and 14; n = 6 each). Basal transmitter release was also similar among gsts1 mutants and ry control (3.4 ± 4.4 mV, n=6 for gsts104; 4.3 ± 5.6 mV, n = 12 for gsts106; 4.0 ± 2.8 mV, n = 9 for ry; ventral muscles).
Interestingly, unlike WT and ry control larvae, the fraction of NMJs in Hk and gsts1 larvae that exhibited giant ejps did not further increase when reared in social isolation. The fraction of NMJs with giant ejps in isolation-reared Hk and gsts1 larvae were directly comparable to those of group-reared mutant larvae at 5-, 10-, or 15-Hz stimulation, and were in fact, less than those in isolation-reared WT and ry control (Figure 5). In contrast, isolation-reared WT and ry larvae displayed recurrent giant ejps at least 5 times more frequently than group-reared larvae upon either 5-, 10-, or 15-Hz stimulation (Figure 5).
Increased neuronal excitability by isolation rearing and by Hk and gsts1 mutations
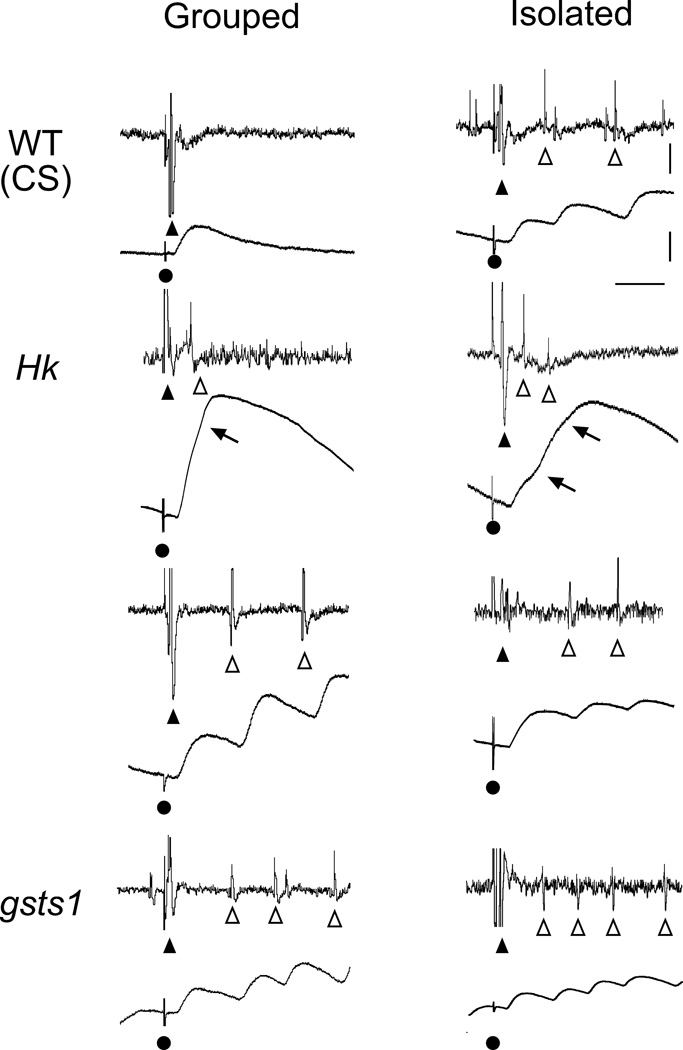
The above observations raise the possibility that Hk and gsts1 mutations affect mainly activity-dependent nerve excitability rather than facilitation of synaptic transmission. It is also important to determine whether the same underlying cellular mechanisms are responsible for the giant ejps in isolation-reared WT larvae. To investigate the source of excitability leading to these giant ejps, we performed simultaneous recordings of nerve action potentials and muscle ejps (Ganetzky & Wu 1982, 1983; Ueda & Wu, 2006). We found that each nerve stimulus evoked repetitive firing in motor axons, with each action potential occurring a few milliseconds prior to a corresponding supernumerary ejp within the recurrent giant ejps (Figure 6). The repetitive firing of motor axons was observed whenever giant ejps appeared in WT, Hk, ry, and gsts1 alleles, regardless of the stimulus frequencies (0.5, 5, 10, and 15 Hz). This observation suggests that the bursting giant ejp activity was caused by motor neuron spike trains, and that isolated rearing lead to hyperexcitability of larval motor neurons, a neural plasticity phenomenon that is altered in Hk and gsts1 mutants.
Figure 6.
Increased motor neuron excitability by mutations in Hk and gsts1 and by isolation rearing. During 15-Hz nerve stimulation, group-reared WT displayed only a compound action potential (▲) and a single ejp following nerve stimulation (●). However, isolation-reared WT displayed supernumerary spikes (Δ) coupled with increased ejp amplitudes. Note that Hk and gsts1 mutants displayed supernumerary spikes and giant ejps in both isolation- and group-rearing condition. Two types of supernumerary firing are shown for Hk, one with shorter (< 15 ms) and the other with longer (> 20 ms) inter-spike intervals. They were associated with giant ejps exhibiting notches (arrows) in the rising phase or multiple peaks, respectively. Both types of supernumerary spikes were observed in gsts1 and isolated WT larvae as well.
Neuronal excitability at physiological concentrations of Ca2+
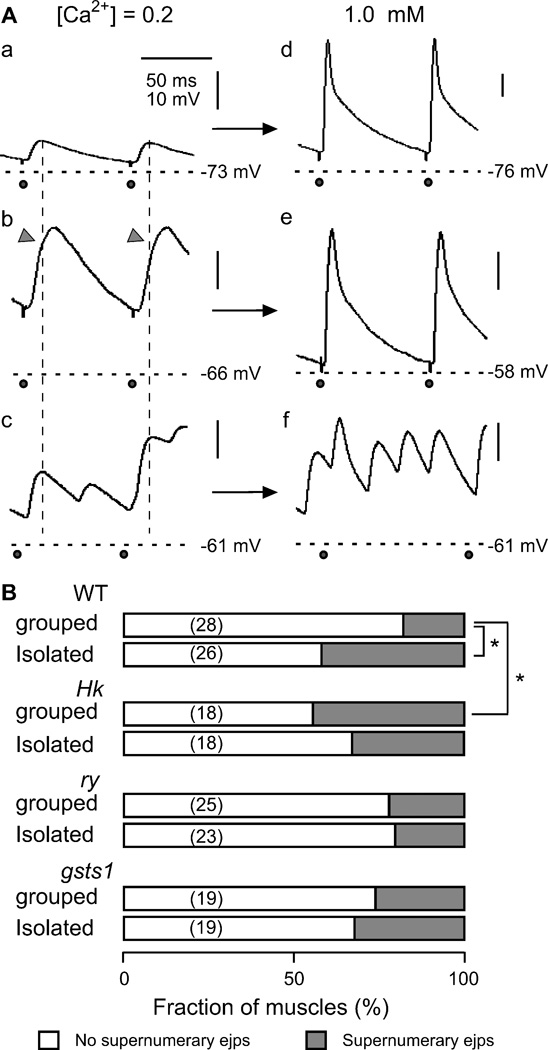
We confirmed that the enhanced nerve excitability described above could still be detected in isolation-reared WT larvae when the concentration of Ca2+ was increased to physiological Ca2+ levels. Even though the hyperexcitability phenotype became less striking with Ca2+ in saline increased to 0.5 or 1.0 mM, it was evident that isolated WT larvae more frequently displayed supernumerary ejps (extra responses occurring between stimuli). At physiological Ca2+ levels, with ejps approaching the saturation amplitude and with more effective repolarization by Ca2+-activated K+ currents, hyperexcitability is more readily revealed by supernumerary ejps rather than enhanced ejp amplitudes. Upon increasing Ca2+ from 0.2 to 1.0 mM, we observed two different types of transitions of giant ejps. The NMJs exhibiting supernumerary ejps at 0.2 mM Ca2+ retained extra synaptic responses between stimuli during the 10- or 15-Hz repetitive stimulus train at 1.0 mM Ca2+ (Figure 7A, c and e). However, the giant ejps produced by summation of closely spaced repetitive transmitter release, as indicated by notches in the ejp rising phase at 0.2 mM Ca2+ (Figure 7A, b, arrowhead), turned into single ejps (Figure 7A, d). Their amplitudes were indistinguishable from those of NMJs displaying normal sized ejps at 0.2 mM Ca2+ (Figure 7A, a and d).
Figure 7.
Supernumerary ejps at physiological concentrations of Ca2+. A. Different types of ejps triggered by high-frequency nerve stimulation (10 and 15 Hz) in saline containing low (0.2 mM, left column) or physiological (1.0 mM, right column) concentrations of Ca2+ in WT larvae. NMJs displaying normal ejps (a, group-reared larvae) or giant ejps with a notch in the rising phase (arrow head in b, isolation-reared larvae) in low-Ca2+ saline produced large ejps with a normal waveform when Ca2+ was increased to 1.0 mM (d, e). NMJs displaying multi-peak ejps in low-Ca2+ saline (c, isolation-reared larvae) retained supernumerary discharges when Ca2+ was raised to a physiological concentration (f). Horizontal dashed lines indicate resting membrane potential and vertical dotted lines mark the peaking time of normal ejps.  , nerve stimulation. B. Fraction of NMJs displaying supernumerary ejps at 1 mM Ca2+ associated with different genotypes and rearing conditions. *, p < 0.05 (X2-test). Number of NMJs in parentheses.
, nerve stimulation. B. Fraction of NMJs displaying supernumerary ejps at 1 mM Ca2+ associated with different genotypes and rearing conditions. *, p < 0.05 (X2-test). Number of NMJs in parentheses.
These two types of giant ejps at 0.2 mM Ca2+ reflected different patterns of repetitive firing of the motor axon, the one with a few closely spaced extra spikes (< 15 ms, Figure 6, Hk upper panels) and the other one longer inter-spike intervals (> 20 ms, Figure 6, the rest). These two types of giant ejps were found across different genotypes and rearing conditions but with different frequencies of occurrences. They resembled the typical ejps at low Ca2+ levels in the K+ channel single mutant eag (with supernumerary peaks) and double mutant Sh; Shab (with notches in the rising phase), in which transient Sh and sustained eag or Shab K+ currents are eliminated, resulting in extreme hyperexcitability (Ganetzky & Wu, 1983; Ueda & Wu, 2006).
We confirmed with simultaneous recordings that the supernumerary ejps at physiological Ca2+ also followed motor axon repetitive firing (data not shown) and the frequency of their occurrence at 1.0 mM Ca2+ is summarize in Figure 7B. In WT, they were significantly more frequent in isolated larvae than in grouped larvae (Figure 7B). However, the difference between isolation-reared and group-reared larvae in Hk and gsts1 was not statistically significant. Again, supernumerary ejps were significantly more frequent in grouped Hk compared to grouped WT control larvae (Figure 7B).
It should be noted that for gsts1 and ry control, there were no clear difference between isolation- and group-reared larvae and between the two genotypes at 1.0 mM Ca2+, unlike the clear differences at 0.2 mM Ca2+ (compare Figures 5B and 7B). One possible explanation is suggested by our preliminary results that at low Ca2+ levels, a higher proportion of giant ejps in isolated ry larvae resulted from motor axon multiple firing with closely spaced inter-spike intervals (see example in Figure 6). This type of giant ejps (with notches in the ejp rising phase, data not shown) typically turn into single ejps in response to individual stimuli at 1.0 mM Ca2+ (see example in Figure 7A, b and d).
Acute effects of a reducing agent on neuromuscular excitability
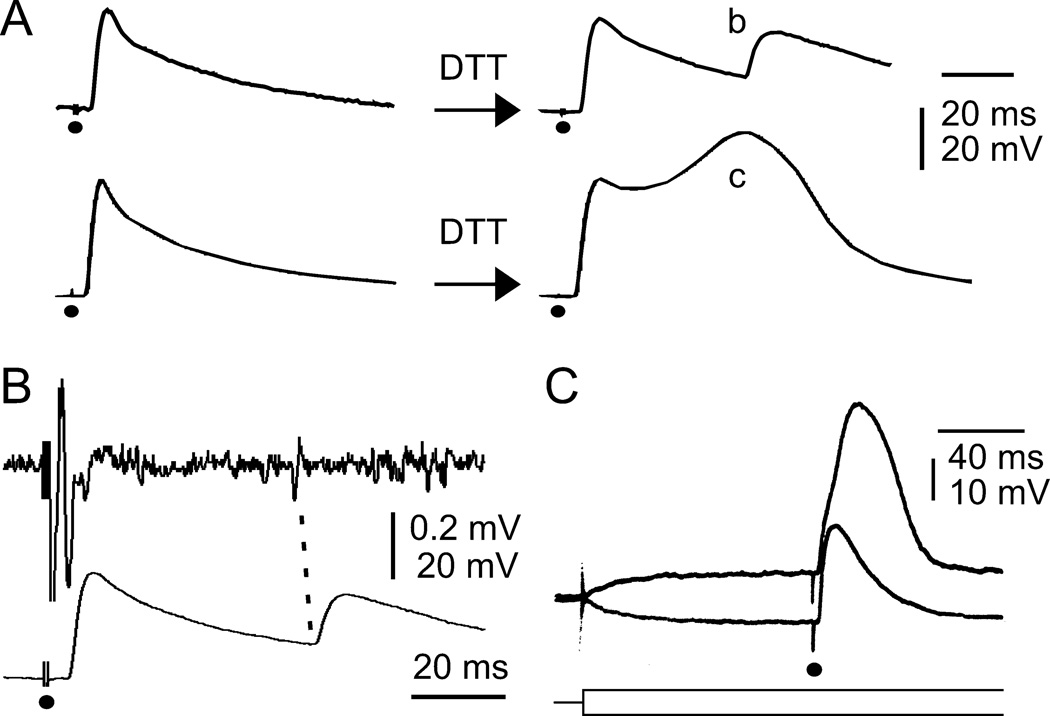
The Hk and gsts1 genes have been implicated in cellular redox regulation and increased ROS levels have been documented in the motor terminals of Hk and gsts1 (Ueda & Wu, 2008), raising the possibility that redox mechanisms may play a role in the regulation of membrane excitability. We employed dithiothreitol (DTT), a widely used agent to reduce disulfide bonds in physiological investigations (e.g., Del Castillo et al., 1971). After 30 min incubation with DTT, about 30% of WT NMJs showed a transition from single responses to supernumerary ejps upon nerve stimulation at 1.0 mM Ca2+ (4 out of 14 larvae, Figure 8A, b). Simultaneous recordings of nerve action potentials and ejps demonstrated that supernumerary ejps followed repetitive motor axon firing (Figure 8B), indicating increased nerve excitability.
Figure 8.
Acute effects of a reducing agent, dithiothreitol (DTT), on WT ejps at a physiological concentration of Ca2+ (1 mM). A. Acute DTT treatment (~30 min) resulted in supernumerary ejps (b) or ejps with a hump (c). B. Supernumerary ejps (bottom trace) temporarily correlated with motor axon repetitive firing (top trace) indicates increased nerve excitability induced by DTT treatment. C. Muscle regenerative potential manifested as a hump superimposing on the ejp. The hump was inhibited by hyperpolarizing current injection and promoted by depolarizing current injection (superimposed traces). Current injection onset is indicated by the superimposed schematic traces (bottom). ●, nerve stimulation. The DTT effects were similar in both isolation- and group-reared WT larvae.
Acute application of DTT also altered membrane excitability in muscle cells. A characteristic regenerative hump, with a waveform distinct from supernumerary ejps (Figure 8A, b and c), was seen superimposed on the ejp in about 50 % of NMJs (11 out of 20 muscles). We confirmed that this regenerative potential was muscle in origin because current injection causing depolarizing or hyperpolarizing muscle membrane could enhance or suppress, respectively, this regenerative event (Figure 8C).
Effects of isolation rearing and mutations on muscle membrane excitability
The above finding prompted us to examine potential modifications in muscle membrane excitability by depolarizing current injection. The results established a wide-ranging isolation-rearing effect on different excitable tissues. The action potential in larval body-wall muscles is mediated by Ca2+ (rather than Na+) currents, which is normally suppressed by K+ channel activation. Full-blown Ca2+ action potentials are evoked when K+ channel blockers are applied (Singh & Wu, 1990). All-or-none overshooting action potentials could also be produced by replacing external Ca2+ with Sr2+, a more effective charge carrier through Ca2+ channels and an inhibitor of K+ channels (Hille 2001). Such Sr2+ spikes displayed different waveforms during current injection. As shown in Figure 9A, they ranged from a single spike of about 100 ms duration to sustained oscillation or prolonged plateau potential which could outlast the current injection period for seconds. Interestingly, the distribution among these Sr2+ spike waveforms was distinct between grouped and isolated larvae (Figure 9B). In WT larvae, group rearing lead to exclusively prolonged plateau potentials (c and d) while isolation rearing produced a higher proportion of short-duration single spikes or oscillations (a and b in Figure 9B). Furthermore, when grouped larvae were treated with DTT, a shift from exclusively patterns c and d toward a more even distribution of patterns a, b, c, and d was observed (Figure 9B). Muscle Sr2+ spikes in group-reared Hk showed a distribution intermediate between those of isolated and grouped WT larvae (Figure 9C).
Figure 9.
Effects of rearing condition and mutations on muscle action potentials evoked in saline containing 2 mM Sr2+ and 0 mM Ca2+. Sr2+ replaced Ca2+ as the charge carrier to facilitate all-or-none action potential generation (see text). A. Classification of muscle Sr2+ action potentials. (a) Single action potentials (~100 ms in duration). (b, c) Action potentials that terminated at the cessation of depolarizing current with (b) or without (c) oscillation ripples. (d) Sustained action potentials that outlasted the current injection. Muscle action potentials were collected at near-threshold levels of 700-ms current injection (bottom trace). B. Effects of isolation rearing and acute DTT treatment (~ 30 min, group-reared) on muscle action potentials in WT larvae. Note that types a and b action potentials were absent in group-reared larvae. C. Group-reared Hk and gsts1. The distribution of muscle action potential types in group-reared Hk was significantly different from that of group-reared WT (p < 0.01, Fisher’s exact test after regrouping to a+b and c+d), whereas group-reared gsts1 and ry control (data not shown, n = 12) were similar to group-reared WT control. Number of muscles in parenthesis.
It should be noted that Hk1 and Hk2 showed detectable differences in Sr2+ spike waveforms. The initial peaks and the following oscillation in Hk2 tended to be less pronounced and sometimes, did not overshoot. This represents another allele-dependent mutational effects of Hk, in addition to the cases of aggression (Table 1) and motor axon excitability (Figure 5). Compared to Hk mutations, gsts1 mutations showed minimal effects on muscle excitability (Figure 9C), consistent with their milder effects on motor axon spikes and ejps (Figires 5 and 7B). The Sr2+ spike waveform distribution in group-reared gsts1 were only minimally different from ry control (n = 12), which were nearly identical to group-reared WT in the waveform distribution (data not shown).
Effects of rearing conditions on presynaptic boutons in the staining with a ROS-sensitive dye, dihydrorhodamine-123 (DHR)
Dihydrorhodamine-123 (DHR) is a fluorescent probe sensitive to oxidation by ROS (Kinsey et al., 1987). Upon oxidation by ROS in the cytosol, non-fluorescent DHR is converted to positively charged, fluorescent rhodamine-123 (Rothe et al., 1988; Emmendorffer et al., 1990), which is subsequently trapped in mitochondria due to their extremely low membrane potential (Johnson et al., 1980; Emaus et al., 1986). We employed DHR staining to investigate the possible effects of isolation rearing on cytosolic ROS levels in Hk and gsts1 mutants and their controls. The fluorescent intensities increased progressively during the 45-min staining period, leading to strong fluorescent puncta in type Ib presynaptic boutons, as previously described (Ueda & Wu, 2008). After washout of external DHR, the fluorescence signal continued to rise albeit at a slower rate, reflecting oxidation of the remaining cytosolic DHR by ROS.
As reported previously, under the group-rearing condition, the fluorescent signal was stronger in Hk and gsts1, compared to WT and ry control (p < 0.001 and 0.05 for Hk and gsts1, respectively, F45-0 in Table 2), suggesting higher cytosolic ROS levels in presynaptic boutons of these mutants (Ueda & Wu, 2008). We subsequently examined isolation-reared larvae using the same protocol and found a similar pattern of isolation effect on fluorescent accumulation in WT and ry control larvae. Although isolation rearing caused no significant changes in fluorescence accumulation following 45-min staining compared to group rearing (F45-0, Table 2), further fluorescence increase in 30 min after washout (F75-45) was proportionally less in isolated larvae.
Importantly, application of this staining protocol on Hk and gsts1 mutant larvae produced different fluorescence accumulation patterns in contrast to their controls (Table 2). Isolation rearing in fact significantly reduced fluorescence accumulation in Hk (gsts1 showed a similar trend, although not statistically significant) and after washout, the further increase in fluorescence was proportionally greater in Hk, contrary to the smaller increase in WT after washout (Table 2).
At present time, it is not possible to provide a simple interpretation for the observed differences in DHR fluorescence signals because the identity of the ROS involved is unknown. Moreover, the kinetics of accumulation of Rh-123 in mitochondria and the decline of the fluorescence accumulation after washout of external DHR may depend on the dynamics of ROS generation, consumption, and clearance (see Discussion).
Discussion
Effects of social isolation and a potential link with ROS
This paper presents the first documentation of isolation rearing effects on nerve and muscle excitability. Isolation rearing is known to increases aggressive behaviors in various vertebrate and invertebrate species, including rats (Hatch et al., 1965), crustaceans (Yeh et al., 1996), and insects (Alexander, 1961; Breed 1983; Pfennig & Reeve, 1989; Hoffmann 1990; Ueda & Kidokoro 2002). Our study demonstrated that Hk and gsts1 mutations altered adult female aggressive behaviors as well as larval neuromuscular excitability. Furthermore, these mutations inhibited the robust behavioral and neuromuscular modifications in response to isolated rearing detected in WT control animals. As described in Introduction, both Hk and gsts1 have been implicated in ROS metabolisms (Singh et al., 2001; Weng et al., 2006; Ueda & Wu, 2008). A potential role of ROS in mediating social isolation-induced phenomena has been inferred from increased ROS levels in mouse brain (Huong et al., 2005) and urine (Miyashita et al., 2006) after social isolation. Interestingly, recent independent studies have demonstrated the involvement of a cytochrome p450 gene, a potential player in ROS regulation, in Drosophila male-male aggression (Dierick & Greenspan 2006). Mutations of this gene cause excessive aggressive behaviors and block the enhancement effect of isolation rearing on aggression (Wang et al., 2008). Additional examples of the involvement of free radicals in aggression include nitric oxide synthase-knockout mice (Nelson et al., 1995) and certain coelenterates using ROS to injure conspecific neighbors (Bartosz et al., 2008). It will be interesting to investigate whether altered ROS regulation also modify membrane excitability in these preparations.
Effects of ROS on membrane excitability
It is worth pointing out that isolation rearing led to different consequences in motoneurons (Figure 3) and muscles (Figure 9) and the same is true for treatment with a redox agent DTT (see Figure 8 for motoneurons, and Figures 8 and 9 for muscles). This may reflect the pervasive actions of different ROS molecules on diverse targets, including certain types of ion channels. It has been shown that oxidation of the -SH group can lead to profound functional modifications of Ca2+ (Chiamvimonvat et al., 1995) channels and several K+ channels, including Drosophila Sh IA (Ruppersberg et al., 1991; Ciorba et al., 1997) and slowpoke BK (DiChiara & Reinhart 1997) channels. Thus, our results of DTT treatment can be interpreted in the light of ROS modification of these Drosophila K+ channels (Figures 8 and 9).
Different neurons and muscle cells in Drosophila display a variety of excitability properties and firing patterns (e.g. Elkins et al., 1986; Elkins & Ganetzky, 1988; Singh & Wu, 1990; Zhao & Wu, 1997; Yao & Wu, 1999; Peng & Wu, 2007), as a result of differential expression of ion channels. For example, action potentials in Drosophila nerve and muscles are controlled by Na+ and Ca2+ channels, respectively (Wu & Ganetzky, 1980; Salkoff & Wyman 1983; Singh & Wu, 1990), along with different subsets of K+ channels (Salkoff & Wyman 1981, Singh & Wu, 1989; Baker & Salkoff, 1990; Peng & Wu, 2007). Therefore, the consequences of altered ROS regulation may lead to non-uniform effects on membrane excitability in different excitable cells. It will be desirable to employ additional redox reagents attacking other chemicals bonds or functional groups to further examine the differential responses of different channel types. In addition, voltage-clamp experiments in specific cell types will be required to identify the specific channels and their redox modifications.
ROS localization, action and dynamics
We have previously described measurements of fluorescent signals from the ROS probe DHR at larval NMJs (Ueda & Wu, 2008). The signal was enhanced in Hk and gsts1 as well as in WT treated with hydrogen peroxide. In the present study, we found no significant differences between grouped and isolated WT larvae in fluorescence accumulation during DHR incubation (Table 2). However, this result does not preclude a role of altered ROS metabolism in isolation-induced hyperexcitability. The DHR signal does not reflect in real time the generation and action of ROS, which may have a brief turnover time in the local microenvironment, whereas accumulation of oxidized DHR trapped in mitochondria can follow a slower time scale of tens of minutes (Ueda & Wu, 2008). Another potential complication is that significant ROS increase may trigger cellular responses to mobilize reserved or redundant redox mechanisms, as demonstrated in other preparations (for review, Scandalios 2005). For instance, neuronal excitation imposed by sustained high K+ stimulus can increase the cellular capacity for ROS clearance (Yermolaieva et al., 2000). Such homeostatic feedback regulation may change the apparent ROS levels indicated by oxidized DHR fluorescence accumulation in mitochondria.
Importantly, in WT (CS) and ry control, isolation rearing lead to slower further accumulation of fluorescence upon removal of external DHR. This is consistent with the possibility of an increased clearance capability by isolation rearing such that cytosolic ROS is more effectively removed. After stopping DHR influx by washing, the local DHR supply would be less effective in competition with the enhanced clearance mechanisms for ROS reaction, i.e. more ROS is removed locally before reacting with DHR remaining in the cell. In the same vein, homeostatic ROS clearance mechanisms may also be evoked in Hk and gsts1 mutants, especially in the isolated larvae. However, the above assumptions await further experimental verification.
Pleiotropy of Hk and gsts1 mutations and isolation rearing-induced phenotypes
Our results are consistent with the roles of Hk and gsts1 in redox regulation and their mutations are expected to have potential pleiotropic effects on a variety of cellular processes. The various physiological and behaviorial phenotypes examined here may involve non-overlapping subsets of excitable cells or neural circuits, upon which Hk and gsts1 mutations or isolation rearing exert differential effects.
Similar to the effects of isolation rearing and DTT treatment, the effects of Hk and gsts1 mutations were not identical for individual behavioral components of female aggression and physiological parameters of NMJs. Specifically, different aggressive behaviors were more extreme in Hk1 and gsts106 mutants than isolation-reared WT and ry control (Table 1), whereas the reverse was true for the NMJ hyperexcitability phenotype (Figures 2 and 5). Furthermore, these mutant phenotypes are not strictly correlated in the order of severity among alleles of Hk and gsts1. For example, the sequences of severity in the mutant alleles and control lines (iso, isolated and grp, grouped) are compared bellow for three different phenotypes.
Head butt: gsts106 ≈ Hk1 > ryiso > gsts104 > Hk2 > WTiso ≈ rygrp ≫ WTgrp
Approach: gsts106 ≈ ryiso > gsts104 > Hk1 ≈ WTiso ≈ rygrp ≫ WTgrp ≈ Hk2
NMJ hyperexcitability: ryiso > WTiso ≈ Hk1 > gsts104 ≈ gsts106 > Hk2 ≈ rygrp > WTgrp
The mechanism why Hk1 is different from Hk2 is not known because mutation sites of these EMS-induced alleles have not been reported. However, milder aggression phenotype in gsts104227 compared to gsts106253 might be due to the difference in the expression levels of the GSTS1 protein. P-element in gsts104227 is inserted in an upstream region of the gene, while that in gsts106253 is in the untranslated region of the first exon (Tweedie et al., 2009). It is possible that both mutants are hypomorph.
It should be noted that in this study, our gsts1 stocks contained a ry marker derived from the parental line. Since ry encodes xanthine dehydrgenase (Glassman & Mitchell 1959), we can not exclude the possibility that it contributes to redox metabolism. Certain mild phenotypes were noticeable in ry larvae. First, enhancement of aggression by isolation rearing was less extreme in the ry control than in WT CS (Figure 1) and second, at a physiological Ca2+ level, isolation-induced nerve repetitive firing persisted in CS but was suppressed in ry (Figure 7). Therefore, it is possible that the gsts1 phenotypes described here are modified by the ry background effect. It will be important to investigate the separate roles of gsts1 and ry in isolation in future studies.
Larval NMJs and isolation-induce hyperexcitability
The Drosophila larval NMJs have been shown to be remarkably plastic in response to environmental influences. NMJ growth can be enhanced by exposure to high temperature (Zhong & Wu, 2004) or by growing larvae at low densities (Stewart & McLean. 2004). Larvae that maintained prolonged crawling activity on agar plates are associated with increased synaptic strength at NMJs (Sigrist et al., 2003; Steinert et al., 2006). The cAMP pathway has been implicated in these studies in the regulation of the observed developmental plasticity (Zhong & Wu, 2004; Steinert et al., 2006). However, additional signaling mechanisms, including those directly or indirectly influenced by ROS, may also be involved in similar developmental plasticity. It is known that dysfunction of several signaling pathways can also cause motorneuron hyperexcitability leading to giant ejps, e.g. mutations of a Ca2+-sensitive regulator of guanylyl cyclase, frq (Mallart et al., 1991, Pongs et al., 1993) and forced expression of mutated tyrosine protein kinase, Fak (Ueda et al., 2008). Potential cross-talks between redox reactions and these signaling pathways have been implicated (Gozin et al., 1998; Mancuso et al., 2008). Thus, further exploration using the larval neuromuscular preparation may provide insights into the potential links between ROS levels and the various signaling pathways in isolation rearing-induced hyperexcitability.
Identification of neural circuits underlying altered behavioral components
Considering the pervasive effects of Hk and gsts1 mutations in different cell types, the role of these genes in aggressive behaviors requires further investigation with refined temporal and spatial control. It is likely that expression of aggressive behaviors requires functional modulation of specific types of neurons or neural circuits in the CNS. Our characterization of the robust modifications in motor neurons and muscles by social isolation provides a foundation for studying functional modifications of relevant neural circuits in the CNS. It is known that aggressive behaviors are affected by the biogenic amine systems (Baier et al., 2002; Certel et al., 2007; Dierick & Greenspan 2007; Hoyer et al., 2008), which are vulnerable to ROS stress (Zhou & Freed 2005; Whitworth et al., 2005). It is also known that forced expression of Gsts1 proteins can rescue the loss of dopamine neurons in the Drosophila Parkinson disease model (parkin mutations, Whitworth et al., 2005). The Gal4-UAS binary expression system (Brand & Perrimon 1993) used in the above study will enable targeted expression of Hk and Gsts1 proteins in specific neural circuits. It will be of great interest to determine the types of central neurons and neural circuits responsible for aggressive behaviors and how Hk and gsts1 mutations separately or jointly modify different functional aspects of these neurons.
ACKNOWLEDGMENTS
We thank Ms. Jennifer Heacock, Ning Lu, and Atulya Iyengar for assistance in preparing the manuscript. We also thank Mr. James Brown and Ms. Sun Lee for collecting behavioral data. This work was supported by NIH grants NS26528 and HD18577.
REFERENCES
- Alexander RD. Social behaviour in crickets. Behaviour. 1961;17:130–223. [Google Scholar]
- Anstey M, Rogers SM, Ott SR, Burrows M, Simpson SJ. Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts. Science. 2009;323:627–630. doi: 10.1126/science.1165939. [DOI] [PubMed] [Google Scholar]
- Baier A, Wittek B, Brembs B. Drosophila as a new model organism for the neurobiology of aggression? J. Exp. Biol. 2002;205:1233–1240. doi: 10.1242/jeb.205.9.1233. [DOI] [PubMed] [Google Scholar]
- Baker K, Salkoff L. The Drosophila Shaker gene codes for a distinctive K+ currents in a subset of neurons. 1990 doi: 10.1016/0896-6273(90)90449-p. [DOI] [PubMed] [Google Scholar]
- Balling A, Technau GM, Heisenberg M. Are the structural changes in adult Drosophila mushroom bodies memory traces? Studies on biochemical learning mutants. J. Neurogenet. 1989;4:65–73. [PubMed] [Google Scholar]
- Barrot M, Wallace DL, Bolaños CA, Graham DL, Perrotti LI, Neve RL, Chambliss H, Yin JC, Nestler EJ. Regulation of anxiety and initiation of sexual behavior by CREB in the nucleus accumbens. Proc. Natl. Acad. Sci. U S A. 2005;102:8357–8362. doi: 10.1073/pnas.0500587102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosz G, Finkelshtein A, Przygodzki T, Bsor T, Nesher N, Sher D, Zlotkin E. A pharmacological solution for a conspecific conflict: ROS-mediated territorial aggression in sea anemones. Toxicon. 2008;51:1038–1050. doi: 10.1016/j.toxicon.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Beall C, Fyrberg C, Song S, Fyrberg E. Isolation of a Drosophila gene encoding glutathione S-transferase. Biochem. Genet. 1992;30:515–527. doi: 10.1007/BF01037590. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Breed MD. Correlations between aggressiveness and corpora allata. Iinsects Sociaux. 1983;30:482–495. [Google Scholar]
- Certel SJ, Savella MG, Schlegel DC-F, Kravitz EA. Modulation of Drosophila male behavioral choice. Proc. Natl. Acad. Sci. USA. 2007;104:4706–4711. doi: 10.1073/pnas.0700328104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamvimonvat N, O'Rourke B, Kamp TJ, Kallen RG, Hofmann F, Flockerzi V, Marban E. Functional consequences of sulfhydryl modification in the pore-forming subunits of cardiovascular Ca2+ and Na+ channels. Circ. Res. 1995;76:325–334. doi: 10.1161/01.res.76.3.325. [DOI] [PubMed] [Google Scholar]
- Chouinard SW, Wilson GF, Schlimgen AK, Ganetzky B. A potassium channel beta subunit related to the aldo-keto reductase superfamily is encoded by the Drosophila Hyperkinetic locus. Proc. Natl. Acad. Sci. USA. 1995;92:6763–6767. doi: 10.1073/pnas.92.15.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciorba MA, Heinemann SH, Weissbach H, Brot N, Hoshi T. Modulation of potassium channel function by methionine oxidation and reduction. Proc. Natl. Acad. Sci. USA. 1997;94:9932–9937. doi: 10.1073/pnas.94.18.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo J, Escobar I, Gijon E. Effects of the electrophoretic application of sulfhydryl reagents to the end-plate receptors. Int. J. Neurosci. 1971;1:199–209. doi: 10.3109/00207457109146971. [DOI] [PubMed] [Google Scholar]
- DiChiara TJ, Reinhart PH. Redox Modulation of hslo Ca2+-Activated K+ Channels. J. Neurosc. 1997;17:4942–4955. doi: 10.1523/JNEUROSCI.17-13-04942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierick HA, Greenspan RJ. Molecular analysis of flies selected for aggressive behavior. Nat. Genet. 2006;38:1023–1031. doi: 10.1038/ng1864. [DOI] [PubMed] [Google Scholar]
- Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat. Genet. 2007;39:678–682. doi: 10.1038/ng2029. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Kravitz EA. Serotonin, social status and aggression. Curr. Biol. 1997;7:812–819. doi: 10.1016/s0959-4388(97)80140-7. [DOI] [PubMed] [Google Scholar]
- Elkins T, Ganetzky B, Wu C-F. A Drosophila mutation that eliminates calcium-dependent potassium current. Proc. Natl. Acad. Sci. U S A. 1986;83:8415–8419. doi: 10.1073/pnas.83.21.8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins T, Ganetzky B. The role of potassium currents in Drosophila flight muscles. J. Neurosci. 1988;8:428–434. doi: 10.1523/JNEUROSCI.08-02-00428.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis PE. Social aggregation and gregarious behaviour in hoppers of locusta-migratoria-migratorioides (R-+-F) Behaviour. 1953;5:189–260. [Google Scholar]
- Emaus RK, Grunwald R, Lemasters JJ. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim. Biophys. Acta. 1986;850:436–448. doi: 10.1016/0005-2728(86)90112-x. [DOI] [PubMed] [Google Scholar]
- Emmendorffer A, Hecht M, Lohmannmatthes ML, Roesler J. A fast and easy method to determine the production of reactive oxygen intermediates by human and murine phagocytes using dihydrorhodamine 123. J. Immunol. Methods. 1990;131:269–275. doi: 10.1016/0022-1759(90)90198-5. [DOI] [PubMed] [Google Scholar]
- Feng Y, Ueda A, Wu C-F. A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. J. Neurogenet. 2004;18:377–402. doi: 10.1080/01677060490894522. [DOI] [PubMed] [Google Scholar]
- Ganetzky B, Wu C-F. Drosophila mutants with opposing effects on nerve excitability: genetic and spatial interactions in repetitive firing. J. Neurophys. 1982;47:501–514. doi: 10.1152/jn.1982.47.3.501. [DOI] [PubMed] [Google Scholar]
- Ganetzky B, Wu C-F. Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J. Neurogenet. 1983;1:17–28. doi: 10.3109/01677068309107069. [DOI] [PubMed] [Google Scholar]
- Gentsch C, Lichtsteiner M, Feer H. Locomotor activity, defecation score and corticosterone levels during an openfield exposure: a comparison among individually and group-housed rats, and genetically selected rat lines. Physiol. Behav. 1981;27:183–186. doi: 10.1016/0031-9384(81)90320-6. [DOI] [PubMed] [Google Scholar]
- Glassman E, Mitchell HK. Mutants of Drosophila melanogaster deficient in xanthine dehydrogenase. Genetics. 1959;44:153–162. doi: 10.1093/genetics/44.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozin A, Franzini E, Andrieu V, Costa LD, Rollet-Labelle E, Pasquier C. Reactive oxygen species activate focal adhesion kinase, paxillin and p130cas tyrosine phosphorylation in endothelial cells. Free Radic. Biol. Med. 1998;25:1021–1032. doi: 10.1016/s0891-5849(98)00134-8. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Harlow M. Social deprivation in monkeys. Sci. Am. 1962;207:136–146. doi: 10.1038/scientificamerican1162-136. [DOI] [PubMed] [Google Scholar]
- Hatch AM, Wiberg GS, Zawidzka Z, Cann M, Airth JM, Grice HC. Isolation syndrome in the rat. Toxicol. Appl. Pharmacol. 1965;7:737–745. doi: 10.1016/0041-008x(65)90132-8. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland: Sinauer; 2001. [Google Scholar]
- Hoffmann AA. The influence of age and experience with conspecifics on territorial behavior in Drosophila melanogaster . J. Insect Behav. 1990;3:1–12. [Google Scholar]
- Hoyer SC, Eckart A, Herrel A, Zars T, Fischer SA, Hardie SL, Heisenberg M. Octopamine in male aggression of Drosophila . Cur. Biol. 2008;18:159–167. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- Huong NT, Murakami Y, Tohda M, Watanabe H, Matsumoto K. Social isolation stress-induced oxidative damage in mouse brain and its modulation by majonoside-R2, a Vietnamese ginseng saponin. Biol. Pharm. Bull. 2005;28:1389–1393. doi: 10.1248/bpb.28.1389. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Properties of the larval neuromuscular junction of Drosophila melanogaster . J. Physiol. 1976;262:215–236. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Penning TM. Aldo-keto reductases and bioactivation/detoxication. Annu. Rev. Pharmacol. Toxicol. 2007;47:263–292. doi: 10.1146/annurev.pharmtox.47.120505.105337. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Walsh ML, Chen LB. Localization of mitochondria in living cells with rhodamine 123. Proc. Natl. Acad. Sci. U S A. 1980;77:990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan WD, Trout WE., III The behavior of four neurological mutants of Drosophila . Genetics. 1969;61:339–409. doi: 10.1093/genetics/61.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe P, Hoffman JH, Austin-LaFrance RJ, Bronzino JD. Neonatal isolation enhances hippocampal dentate response to tetanization in freely moving juvenile male rats. Exp. Neurol. 1995;136:89–97. doi: 10.1006/exnr.1995.1086. [DOI] [PubMed] [Google Scholar]
- Kinsey BM, Kassis AI, Fayad F, Layne WW, Adelstein SJ. Synthesis and biological studies of iodinated (127/125I) derivatives of rhodamine 123. J. Med. Chem. 1987;30:1757–1761. doi: 10.1021/jm00393a013. [DOI] [PubMed] [Google Scholar]
- Lee J, UEDA A, WU C-F. Pre- and post-synaptic mechanisms of synaptic strength homeostasis revealed by slowpoke and Shaker K+ channel mutations in Drosophila . Neuroscience. 2008;154:1283–1296. doi: 10.1016/j.neuroscience.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D. Effects of population density on larvae of Lepidoptera. Transactions of the Royal Entomological Society of London. 1953;104:543–585. [Google Scholar]
- Mallart A, Angautpetit D, Bourretpoulain C, Ferrus A. Nerve-terminal excitability and neuromuscular-transmission in T(X,Y)V7 and Shaker mutants of Drosophila melanogaster . J. Neurogenet. 1991;7:75–84. doi: 10.3109/01677069109066212. [DOI] [PubMed] [Google Scholar]
- Mancuso C, Capone C, Ranieri SC, Fusco S, Calabrese V, Eboli ML, Preziosi P, Galeotti T, Pani G. Bilirubin as an endogenous modulator of neurotrophin redox signaling. J. Neurosci. Res. 2008;86:2235–2249. doi: 10.1002/jnr.21665. [DOI] [PubMed] [Google Scholar]
- McCormack T, McCormack K. Shaker K+ channel β subunits belong to an NAD(P)H-dependent oxidoreductase superfamily. Cell. 1994;79:1133–1135. doi: 10.1016/0092-8674(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Yamaguchi T, Motoyama K, Unno K, Nakano Y, Shimoi K. Social stress increases biopyrrins, oxidative metabolites of bilirubin, in mouse urine. Biochem. Biophys. Res. Commun. 2006;349:775–780. doi: 10.1016/j.bbrc.2006.08.098. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378:383–386. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- Nilsen SP, Chan YB, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster . Proc Natl Acad Sci U S A. 2004;101:12342–12347. doi: 10.1073/pnas.0404693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Weng J, Cao Y, Bhosle RC, Zhou M. Functional coupling between the Kv1.1 channel and aldoketoreductase Kvβ1. J. Biol. Chem. 2008;283:8634–8642. doi: 10.1074/jbc.M709304200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pener MP, Yerushalmi Y. The physiology of locust phase polymorphism: an update. J Insect Physiol. 1998;44:365–377. doi: 10.1016/s0022-1910(97)00169-8. [DOI] [PubMed] [Google Scholar]
- Peng I-F, Wu C-F. Differential contributions of Shaker and Shab K+ currents to neuronal firing patterns in Drosophila . J. Neurophysiol. 2007;97:780–794. doi: 10.1152/jn.01012.2006. [DOI] [PubMed] [Google Scholar]
- Pfennig DW, Reeve HK. Neighbor recognition and contect-dendent aggression in a solitary wasp, Sphecius speciosus (Hymenoptera: Sphecidae) Ethology. 1989;80:1–18. [Google Scholar]
- Pongs O, Lindemeiera J, Zhua XR, Theila T, Engelkampa D, Krah-Jentgensa I, Lambrechtb H-G, Kochb KW, Schwemerc J, Rivosecchid R, Mallartd A, Galcerane J, Canale I, Barbase JA, Ferrúse A. Frequenin-A novel calcium-binding protein that modulates synaptic efficacy in the Drosophila nervous system. Neuron. 1993;11:15–28. doi: 10.1016/0896-6273(93)90267-u. [DOI] [PubMed] [Google Scholar]
- Rothe G, Oser A, Valet G. Dihydrorhodamine 123: a new flow cytometric indicator for respiratory burst activity in neutrophil granulocytes. Naturwissenschaften. 1988;75:354–355. doi: 10.1007/BF00368326. [DOI] [PubMed] [Google Scholar]
- Ruppersberg JP, Stocker M, Pongs O, Heinemann SH, Frank R, Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991;352:711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Robbins TW, Morgan MJ, Iversen SD. The effects of psychomotor stimulants on stereotypy and locomotor activity in socially-deprived and control rats. Brain. Res. 1975;84:195–205. doi: 10.1016/0006-8993(75)90975-0. [DOI] [PubMed] [Google Scholar]
- Salkoff L, Wyman R. Outward currents in developing Drosophila flight muscle. Science. 1981;212:461–463. doi: 10.1126/science.6259736. [DOI] [PubMed] [Google Scholar]
- Salkoff L, Wyman R. Ion currents in Drosophila flight muscles. J. Physiol. 1983;337:687–709. doi: 10.1113/jphysiol.1983.sp014649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 2005;38:995–1014. doi: 10.1590/s0100-879x2005000700003. [DOI] [PubMed] [Google Scholar]
- Schiller L, Donix M, Jähkel M, Oehler J. Serotonin 1A and 2A receptor densities, neurochemical and behavioural characteristics in two closely related mice strains after long-term isolation. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:492–503. doi: 10.1016/j.pnpbp.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Serra M, Sanna E, Mostallino MC, Biggio G. Social isolation stress and neuroactive steroids. Eur. Neuropsychopharmacol. 2007;17:1–11. doi: 10.1016/j.euroneuro.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Sigrist SJ, Reiff DF, Thiel PR, Steinert JR, Schuster CM. Experience-dependent strengthening of Drosophila neuromuscular junctions. J Neurosci. 2003;23:6546–6556. doi: 10.1523/JNEUROSCI.23-16-06546.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Wu C-F. Complete separation of four potassium currents in Drosophila. Neuron. 1989;2:1325–1329. doi: 10.1016/0896-6273(89)90070-6. [DOI] [PubMed] [Google Scholar]
- Singh S, Wu C-F. Properties of potassium currents and their role in membrane excitability in Drosophila larval muscle fibers. J. exp. Biol. 1990;152:59–76. doi: 10.1242/jeb.152.1.59. [DOI] [PubMed] [Google Scholar]
- Singh SP, Coronella JA, Benes H, Cochrane BJ, Zimniak P. Catalytic function of Drosophila melanogaster glutathione S-transferase DmGSTS1-1 (GST-2) in conjugation of lipid peroxidation end products. Eur. J. Biochem. 2001;268:2912–2923. doi: 10.1046/j.1432-1327.2001.02179.x. [DOI] [PubMed] [Google Scholar]
- Spitz RA. Hospitalism: An inquiry into the genesis of psychiatric conditions in early childhood. Psychoanal Study Child. 1945;1:58–74. [PubMed] [Google Scholar]
- Spradling AC, Stern D, Beaton A, Rhem EJ, Laverty T, Mozden N, Misra S, Rubin GM. The Berkeley Drosophila genome project gene disruption project: Single P-element insertions mutating 25% of vital Drosophila genes. Genetics. 1999;153:135–177. doi: 10.1093/genetics/153.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert JR, Kuromi H, Hellwig A, Knirr M, Wyatt AW, Kidokoro Y, Schuster CM. Experience-dependent formation and recruitment of large vesicles from reserve pool. Neuron. 2006;50:723–733. doi: 10.1016/j.neuron.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Stern M, Ganetzky B. Altered synaptic transmission in Drosophila hyperkinetic mutants. J. Neurogenet. 1989;5:215–228. doi: 10.3109/01677068909066209. [DOI] [PubMed] [Google Scholar]
- Stewart BA, Atwood HL, Renger JJ, Wang J, Wu C-F. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J. Comp. Physiol. A. 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- Stewart BA, McLean JR. Population density regulates Drosophila synaptic morphology in a Fasciclin-II-dependent manner. J. Neurobiol. 2004;61:392–399. doi: 10.1002/neu.20096. [DOI] [PubMed] [Google Scholar]
- Technau GM. Fiber number in the mushroom bodies of adult Drosophila melanogaster depends on age, sex and experience. J. Neurogenet. 1984;1:113–126. doi: 10.3109/01677068409107077. [DOI] [PubMed] [Google Scholar]
- Tweedie S, Ashburner M, Falls K, Leyland P, McQuilton P, Marygold S, Millburn G, Osumi-Sutherland D, Schroeder A, Seal R, Zhang H, The FlyBase Consortium FlyBase: enhancing Drosophila Gene Ontology annotations. Nucl. Acids Res. 2009;37:D555–D559. doi: 10.1093/nar/gkn788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Kidokoro Y. Aggressive behaviours of female Drosophila melanogaster are influenced by their social experience and food resources. Physiological Entomology. 2002;27:21–28. [Google Scholar]
- Ueda A, Wu CF. Distinct frequency-dependent regulation of nerve terminal excitability and synaptic transmission by IA and IK potassium channels revealed by Drosophila Shaker and Shab Mutations. 2006 doi: 10.1523/JNEUROSCI.0862-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Wu CF. Effects of Hyperkinetic, a β subunit of Shaker voltage-dependent K+ channels, on the oxidation state of presynaptic nerve terminals. J. Neurogenet. 2008;22:1–13. doi: 10.1080/01677060701807954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Grabbe C, Lee J, Lee J, Palmer RH, Wu C-F. Mutation of Drosophila focal adhesion kinase induces bang-sensitive behavior and disrupts glial function, axonal conduction and synaptic transmission. Eur. J. Neurosci. 2008;27:2860–2870. doi: 10.1111/j.1460-9568.2008.06252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Wu C-F. In vivo functional role of the Drosophila Hyperkinetic β subunit in gating and inactivation of Shaker K+ Channels. Biophys. J. 1996;71:3167–3176. doi: 10.1016/S0006-3495(96)79510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Humphreys JM, Phillips JP, Hilliker AJ, Wu C-F. A novel leg-shaking Drosophila mutant defective in a voltage-gated K+ current and hypersensitive to reactive oxygen species. J. Neurosci. 2000;20:5958–5964. doi: 10.1523/JNEUROSCI.20-16-05958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dankert H, Perona P, Anderson DJ. A common genetic target for environmental and heritable influences on aggressiveness in Drosophila . Proc. Natl. Acad. Sci. USA. 2008;105:5657–5663. doi: 10.1073/pnas.0801327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J, Cao Y, Moss N, Zhou M. Modulation of voltage-dependent Shaker family potassium channels by an aldo-keto reductase. J. Biol. Chem. 2006;281:15194–15200. doi: 10.1074/jbc.M513809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth AJ, Theodore DA, Greene JC, Benes H, Wes PD, Pallanck LJ. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson's disease. Proc. Natl. Acad. Sci. USA. 2005;102:8024–8029. doi: 10.1073/pnas.0501078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-F, Ganetzky B. Genetic alterations of nerve membrane excitability in temperature-sensitive paralytic mutant of Drosophila melanogaster . Nature. 1980;286:814–816. doi: 10.1038/286814a0. [DOI] [PubMed] [Google Scholar]
- Wu C-F, Ganetzky B, Jan LY, Jan Y-N, Benzer S. A Drosophila mutant with a temperature-sensitive block in nerve conduction. Proc. Natl. Acad. Sci. USA. 1978;75:4047–4051. doi: 10.1073/pnas.75.8.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W-D, Wu CF. Auxiliary Hyperkinetic β subunit of K+ channels: regulation of firing properties and K+ currents in Drosophila neurons. J. Neurophysiol. 1999;81:2472–2484. doi: 10.1152/jn.1999.81.5.2472. [DOI] [PubMed] [Google Scholar]
- Yeh S-R, Fricke RA, Edwards DH. The effect of social experience on serotonergic modulation of the escape circuit of crayfish. Science. 1996;271:366–369. doi: 10.1126/science.271.5247.366. [DOI] [PubMed] [Google Scholar]
- Yermolaieva O, Brot N, Weissbach H, Heinemann SH, Hoshi T. Reactive oxygen species and nitric oxide mediate plasticity of neuronal calcium signaling. Proc. Natl. Acad. Sci. USA. 2000;97:448–453. doi: 10.1073/pnas.97.1.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M-L, Wu C-F. Alterations in frequency coding and activity dependence of excitability in cultured neurons of Drosophila memory mutants. J. Neurosci. 1997;17:2187–2199. doi: 10.1523/JNEUROSCI.17-06-02187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Wu C-F. Neuronal activity and adenylyl cyclase in environment-dependent plasticity of axonal outgrowth in Drosophila . J. Neurosci. 2004;24:1439–1445. doi: 10.1523/JNEUROSCI.0740-02.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T α-synuclein toxicity. J. Biol. Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]