Abstract
Effective Plasmodium falciparum immunity requires a precisely timed and balanced response of inflammatory and anti-inflammatory immune regulators. These responses begin with innate immune effectors and are modulated over the course of an infection and between episodes to limit inflammation. To date, there are no effective immunomodulatory therapies for severe malaria. Some of the most potent immunomodulators are naturally occurring infections, including helminthic and chronic viral infections. This review examines malaria coinfection with these organisms, and their impact on malaria morbidity and immune responses. Overall, there is compelling evidence to suggest that chronic coinfections can modulate deleterious malaria-specific immune responses, suggesting that therapeutic agents may be effective if utilized early in infection. Examination of the mechanisms of these effects may serve as a platform to identify more targeted and effective malaria immunomodulatory therapeutics.
Keywords: coinfection, helminth, hepatitis, HIV, immunomodulation, malaria, Plasmodium falciparum
The pathophysiology of malaria has been difficult to characterize over the past century, in part because of the wide spectrum in the severity and presentation of disease. In some cases, it presents as a profoundly life-threatening disease, and in other cases as a benign infection without any symptoms. With increased focus on the immunology of Plasmodium infection in pursuit of a vaccine, it has become clear that this spectrum of disease can be explained in part by the complex relationship between the parasite and the host immune response. Like many other infections, the pathology experienced by the host is often a consequence of the immune system response to the parasite. There is now evidence that the host immune response is modulated over the course of infection and in subsequent infections to limit malaria morbidity. Because of this, there has been significant investigation into immunomodulators as adjunctive therapy for the treatment of severe malaria. However, despite promising results in mouse studies, little to no benefit has been demonstrated in human studies.
How can we determine which immune responses influence human malaria protection and pathology, so that we can utilize immunmoodulation as a therapeutic modality? We have relied heavily on mouse models, which can give us valuable clues through direct augmentation of the immune responses with cell line depletions and knockout studies. However, the conclusions that can be drawn from these studies are limited by the fundamental differences in immune responses between mice and humans, and the differences in murine and human Plasmodium species. The same immunomodulatory experiments cannot be carried out in humans, and therefore, we are often limited to observational studies. One way to examine the relative importance of these immune responses in humans is to observe the effect of naturally occurring immunomodulators on the vulnerability to malaria infection and disease. In this review, understanding about effective natural immune responses to malaria and the data on how common malaria coinfections affect this response, specifically helminthic infections and chronic viral infections, are examined. This area of research holds tremendous opportunity for the discovery of therapeutic modalities, as it increases understanding of the role that parasites and viral infections play in modulating immune responses. Indeed as a result of these kinds of studies, whole organisms such as Trichuris suis for allergic diseases are already being pursued as immunomodulatory therapies [1].
Since Plasmodium falciparum is the most common cause of severe disease by far, this review focuses almost exclusively on immunomodulation in P. falciparum malaria in humans and in animal models designed to mimic P. falciparum infection in humans. It should also be noted that in many areas of this review, severe malaria is described as a single clinical entity; but in fact, this description includes multiple syndromes with varying pathophysiology. For this review, the general category of ‘severe malaria’ is used because many of the clinical studies cited did not explicitly differentiate between these syndromes when published. The lack of clarity on the precise pathology being studied may explain some of the discrepant results described in malaria coinfection literature. As we move forward in our understanding of severe malaria pathophysiology, it will be important for future studies to differentiate between these syndromes, particularly when examining immunopathology and modulation.
Host immune responses in malaria infection & disease
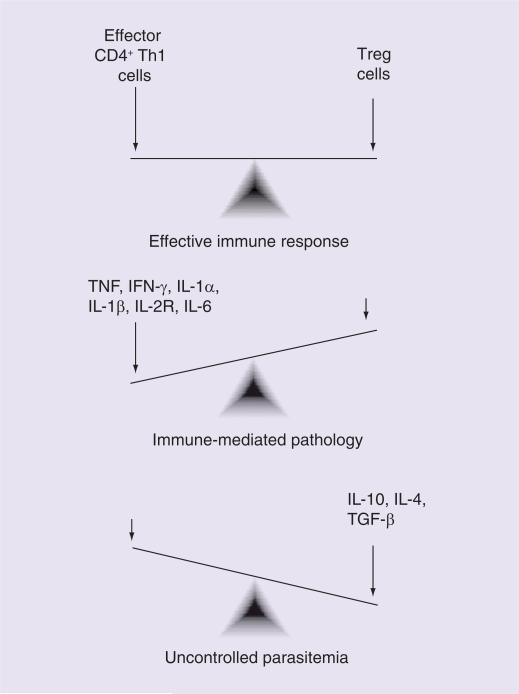
Evidence in both humans and mice suggests that effective natural immunity is characterized by a rapid initial inflammatory response, quickly followed by regulation of this response [2,3]. The critical role of this initial inflammatory response has been shown in a variety of ways, including multiple studies that have demonstrated that peripheral blood mononuclear cell IFN-γ production in response to ex vivo malaria antigen stimulation is associated with malaria infection protection [4]. However, a number of proinflammatory cytokines have also been implicated in the development of severe disease, including IFN-γ, TNF, IL-1α, IL-1β, IL-6 and IL-2 receptor [4,5]. Furthermore, relatively low levels of the anti-inflammatory cytokines, such as IL-10 and IL-6, have been found in individuals suffering from severe malaria [5–7]. Overall, this suggests that an unregulated inflammatory response is deleterious to the host. The importance of a balanced response was demonstrated in a Ghanian study where a higher ratio of proinflammatory (IFN-γ, TNF-α and IL-12) to anti-inflammatory (IL-10 and TGF-β) cytokines was associated with lower rates of infection, but a greater likelihood of having clinical disease when infected [8]. Overall, it seems that a lack of the initial inflammatory stage will lead to uncontrolled proliferation of the parasite. However, an uncontrolled inflammatory immune response will lead to immune-mediated pathology (Figure 1). Further investigations are focusing on what factors tip the balance in one direction or the other, ultimately leading to disease.
Figure 1. Balancing inflammatory and anti-inflammatory immune responses to malaria.
This figure represents cellular immune responses theorized to mediate disease outcomes in the setting of Plasmodium falciparum coinfection, notably intestinal helminth infections. The figure is a simplified representation of these responses, which highlights mechanisms that may be amenable to therapeutic targets, but does not fully represent the multiple and complex immune mechanisms that occur in humans in response to P. falciparum infection.
Early inflammatory response
The role of innate immunity in the initial inflammatory response to malaria is under investigation. The strongest evidence for the interaction between malaria and the innate immune system comes from mouse models, specifically Plasmodium berghei, Plasmodium yoelii and Plasmodium chabaudi. Toll-like receptor (TLR) and other pattern recognition receptor signaling play a critical role in the activation of early immune system responders including macrophages, dendritic cells and natural killer cells. In vitro studies have demonstrated that the activation of TLR2 and TLR9 by various parasite targets leads to proinflammatory cytokine responses [9–12]. However, as these responses are examined over the course of infection, the picture becomes more complex. Activation of dendritic cell (DC) TLRs taken from mice early in Plasmodium infection appears to cause inflammatory cytokine release from T cells through the production of IL-12 [13]. However, DCs isolated from later stages in infection, when activated through TLRs, produce and stimulate T cells to produce IL-10 [14]. The molecular signals that modulate this change have yet to be understood.
Whether these early innate immune responses contribute to disease pathology is under investigation. There is evidence that cerebral malaria pathophysiology is immune mediated, with a priming of αβ T cells on first exposure to the parasite leading to the exaggerated inflammatory response in subsequent infections [15]. This was supported by the observations that children afflicted with cerebral malaria already have high levels of antimalaria antibodies, suggesting earlier infection without severe disease. As mentioned, the inflammatory cytokine IFN-γ plays a critical role in controlling parasitemia and in disease protection, and also in cerebral malaria pathophysiology [16–20]. Mice that do not express IFN-γ receptor do not develop cerebral malaria [21]. Similarly, early innate responses seem necessary for the development of cerebral disease. In mouse models, cerebral malaria-type syndromes are seen in P. berghei ANKA-infected wild-type mice. Mice that are deficient in TLR2, TLR9 or MyD88 (the downstream mediator of most TLR activation pathways) do not experience CNS symptoms when infected with P. berghei ANKA, which normally causes murine cerebral malaria syndromes [22]. The suggestion that TLRs play a role in disease severity has spurred multiple studies on TLR polymorphisms. Polymorphisms in human TLR4, TLR9 and Toll-IL-1 receptor domain-containing adaptor proteins have all been associated with increased susceptibility to severe malaria disease [23–26]. These findings support the role of early innate immune responses in the development of malaria-related pathology and present potential targets for immunomodulating therapy to augment disease therapy or vaccine efficacy.
Immune tolerance & Tregs
Early effective immune responses are characterized by inflammatory responses set in motion by the innate responders detailed earlier, but how are these responses later replaced by anti-inflammatory cytokine signaling? It may be reasonable to surmise that this change takes place as the erythrocytic parasite load is controlled. However, murine studies suggest that inflammatory cytokines peak early but decline prior to the decline of peripheral parasitemia [27–29]. Tregs, characterized as CD4+CD25+FoxP3+, are now being investigated as the mediator of this switch from a predominantly inflammatory to a predominately anti-inflammatory response. Data in murine and human studies suggest that these cells are associated with a muted inflammatory response to malaria and increased parasite burden [30]. In human observational studies, it appears that malaria induces FoxP3 expression and increased number of Treg cells [31,32]. Furthermore, longitudinal evaluation of CD4+FoxP3+ Treg cell levels in a human sporozoite challenge study documented that an increased number of these cells was associated with higher parasite loads and a decline in proinflammatory cytokines [31]. Recent studies have examined the development of Treg-mediated immune tolerance to malaria in utero. There is evidence that newborns of women with placental malaria are at an increased risk of malaria infection and disease, a vulnerability that persists for years [33–35]. Data suggest that this effect may be mediated by induction of tolerance to blood-stage malaria antigens in utero. These findings include an increased frequency of Treg (CD4+CD25hi FoxP3+) in the cord blood of newborns born in malaria endemic areas and in vitro data that Th1-responses to P. falciparum blood-stage antigens are suppressed in these study subjects [36,37].
In vitro culture experiments suggest that IL-2, IL-10 and TGF-β may drive the induction and expansion of these Tregs [38]. IL-10 plays an important role in balancing inflammatory responses to infections versus immune-related pathology [39,40]. This has been demonstrated both in viruses [41] and in parasites, where increased IL-10 is associated with a chronic infection, while low levels lead to acute infectious disease patterns. Increased IL-10 levels in asymptomatic malaria have been found in pregnant women [42] and children [6]. It has been demonstrated that despite the seemingly short life of inflammatory immune responses to malaria (as characterized by the presence of IFN-γ-producing T cells), IL-10-producing T cells persist for many years after infection in a low transmission setting [43]. This is consistent with the observation that those with historical immunity of malaria but a recent lack of exposure will still maintain protection from severe disease.
Nitric oxide/CD23 pathway
Modulation of signaling in the CD23/nitric oxide (NO) pathway has also been the proposed mechanism by which malaria immune responses are modulated, an area in which coinfection research has contributed considerably. NO production via inducible nitric oxide synthetase (iNOS) upregulation has been intensely investigated as a mediator, either as the cause or alleviator of human malaria disease. On the whole, NO production has been associated with protection from cerebral malaria in both murine and human studies [44–47]. Reactive nitrogen intermediates (RNIs), which are present when NO is produced, have been negatively correlated with the peripheral schizont to trophozoite ratio, which is used as a surrogate for the quantity of sequestered parasites [48]. It is postulated that NO decreases endothelial activation and parasite sequestration by downregulating the production of receptors such as ICAM-1 by way of the angiopoietin/TIE2 system and systemic anti-inflammatory effects [46]. NO can be produced as the downstream effect of an inflammatory cytokine response, and can also result from the activation of CD23 through low-affinity binding with IgE complexes. CD23 activation will induce iNOS transcription and also can lead to the release of cytokines that both potentiate an inflammatory reaction, such as TNF, as well as regulate it, such as IL-10, when activated chronically [48]. CD23 when not bound is cleaved and becomes a soluble signaling molecule, increasing IgE expression and activating multiple immune effector cells [49]. With chronic activation of this pathway, it is thought that less soluble CD23 is produced, leading to a more restrained NO and inflammatory cytokine reaction during an acute malaria infection [50].
Antibody-dependent cellular cytotoxicity
Finally, as mentioned earlier, control of parasitemia by antibody-dependent mechanisms may not, on its own, explain the transition from an inflammatory to anti-inflammatory profile. However, antibody-dependent cellular cytotoxicity has been a focus of investigation as a possible mechanism behind develop ment of immunity to symptomatic parasitemia. Certain P. falciparum antibodies are recognized as being associated with protection from malaria disease, and particular subclasses have been more strongly associated with these protective effects, specifically the cytophilic antibody subclasses IgG1 and IgG3 [51,52]. It has been theorized that early, ineffective antibody responses are later replaced by effective responses through subclass switching, and with this subclass switching comes a more effective immune response [53]. The growing data on the protection from disease provided by blood-stage antigen antibodies support the role of this mechanism as a part of the immunomodulatory response to malaria infection, and the differentiation of B cells to produce a particular type of IgG subclass is probably in part dictated by the presence and timing of specific cytokine signals, set in motion by the mechanisms described earlier.
Immunoregulation in the setting of coinfections
Microorganisms can have powerful effects on immune responses of their host. This is particularly the case in chronic infections that modulate immune responses in order to escape detection or in diseases that attack or exhaust the host immune response. A significant amount of research has been devoted to examining the impact of malaria coinfection with other microorganisms. The findings from this research provide insight into the effects of known immunomodulators on malaria morbidity.
Helminth infections
As with malaria infection, helminthic infection is often asymptomatic and when there is pathology, it is often derived from the host immune response. These organisms, which include both soil-transmitted helminths, schistosomes and filarial infections, have well-described potent immunomodulatory effects on their hosts, a trait that allows them to live in humans for long periods of time undetected. This has been attributed to their anti-inflammatory effect, which dampens IFN-γ responses and biases immune responses towards a Th2-type response through Tregs, Th2-inducing DCs and alternatively activated macrophages [54,55]. Early on, it was demonstrated that helminthic infection decreases host responses to various vaccines, including tetanus [56] and cholera [57]. Given the anti-inflammatory effects of helminths, it is expected that, based on what is known of malaria immunology and pathophysiology, a helminth infection may lead to increased rates of infection and parasitemia by dampening inflammatory signaling and decreased rates of immune-mediated disease. Overall, this hypothesis is born out in some studies but contradicted in others. This suggests that our understanding of malaria immunity and the homogeneity of helminthic immunomodulation may be overly simplistic [58]. Most important, however, is the overwhelming evidence that these infections can influence malaria-specific immune responses, and helminth infection must, therefore, be assessed in studies aimed at characterizing malaria immunity.
Soil-transmitted helminths
The impact of soil-transmitted helminths on malaria infection and severity is the best characterized among all the helmithic infections (Table 1). Several studies on Ascaris lumbricoides have been performed out of concern that intestinal worm treatment campaigns may have the unintended effect of increasing the rate and severity of malaria infection. In studies that screen purely for infection, both symptomatic and asymptomatic, some have found increased malaria infection rates among individuals with Ascaris infection [59], while others have shown decreased rates [60] or no effect [61]. Randomized controlled trials of the antihelminthic levamisole in the 1990s showed that after children were treated for Ascaris infection, they had increased parasitemia rates with malaria infections [62,63]. Studies that went further, examining the malaria morbidity, have found that Ascaris infection is associated with decreased drops in hemoglobin from malaria [64], and decreased odds of cerebral malaria and renal complications of severe malaria [65], all complications that may occur in part owing to immune-mediated mechanisms [66]. By contrast, a study suggested that Ascaris infection was associated with increased rates of severe malaria among children, although the majority of subjects in this study met criteria for severe malaria through vomiting, which can be symptoms of Ascaris infection itself [67]. On the whole, there are insufficient data on whether Ascaris infection increases or decreases vulnerability to infection; but the preponderance of data suggests that Ascaris infection may decrease the morbidity associated with malaria infection.
Table 1.
Key clinical publications on soil-transmitted helminth and malaria coinfection.
| Organism | Country | Ascaris spp. | Hookworm | Trichuris spp. | Strongyloides spp. | All soil-transmitted helminths | Findings | Ref. |
|---|---|---|---|---|---|---|---|---|
| Hookworm | Malawi | ↑ IR | Hookworm infection associated with malaria infection in a cross-sectional study | [74] | ||||
| Ascaris lumbricoides | Gabon | ↑ IR | A. lumbricoides associated with Plasmodium infection during pregnancy. Sensitivity of hookworm assay not considered sufficient for analysis. Study was a cross-sectional analysis within a clinical trial | [59] | ||||
| Soil-transmitted helminths | Thai–Burmese border | ↓ IR | ↑ IR ↑ DS | A. lumbricoides infection was associated with a decreased risk of malaria in a cross-sectional analysis. Hookworm infection was associated with an increased risk of malaria and anemia. Any spp. of malaria was evaluated | [60] | |||
| A. lumbricoides, hookworm, Trichuris spp. | Brazil | ↓ DS | ↓ DS | ↓ DS | Decreased drop in hemoglobin during acute Plasmodium vivax infection for helminth-infected children | [64] | ||
| A. lumbricoides, hookworm, Trichuris spp. | Uganda | ↑ DS | Hookworm infection associated with lower hemoglobin level during malaria infection in school-aged children. Spatial and household clustering of coinfection noted | [69] | ||||
| A. lumbricoides, hookworm, Trichuris spp. | Ethiopia | ↑ DS | ↓ DS | Higher malaria parasite density among those with hookworm infection. Soil-transmitted helminth infection negatively correlated with the severe malaria symptoms | [68] | |||
| A. lumbricoides, hookworm, Trichuris spp. | Ghana | Whole blood samples from helminth-infected children were exposed to malaria infected RBCs and expressed increased levels of IL-10, SOCS-3, FoxP3 and PD-1 | [78] | |||||
| A. lumbricoides, hookworm, Trichuris spp., Strongyloides spp. | Ghana | ↑ IR | ↑ IR | No Assoc. | No Assoc. | Pregnant women with hookworm and Ascaris infection had increased rates of malaria infection. No association was seen with Strongyloides or Trichura spp. | [73] | |
| All gastrointestinal helminth, Ascaris | Kenya | No Assoc. | Gastrointestinal helminth infection and eosinophilia were statistically not associated with malaria susceptiblity | [76] | ||||
| Hookworm, Trichuris spp., Strongyloides spp. | Uganda | ↑ IR | No Assoc. | No Assoc. | Cross-sectional study in which hookworm infection is associated with malaria infection. No association noted with Trichuris or Strongyloides spp. infection | [71] | ||
| Hookworm, Trichuris, Ascaris | Zimbabwe | ↑ IR | Hookworm infection is associated in a cross-sectional analysis with P. falciparum malaria infection | [70] | ||||
| A. lumbricoides | Madagascar | ↓ DS | Ascaris treatment with levamisole associated with increase in P. falciparum densities among 4–15-year-olds | [62,63] | ||||
| A. lumbricoides, hookworm, Trichuris spp. | Uganda | No Assoc. | No association observed between intestinal helminth infection and malaria risk in a cross-sectional household-based sample | [61] | ||||
| A. lumbricoides | Senegal | ↑ DS | Higher prevalence in a prospective case–control study of Ascaris infection in individuals with severe malaria | [67] | ||||
| Soil-transmitted helminths | Thailand | ↑ DS | Helminth-infected subjects were more likely to develop falciparum clinically symptomatic malaria in a prospective trial | [75] | ||||
| A. lumbricoides, hookworm | Thailand | ↓ DS | ↓ DS | A. lumbricoides and Necator americanus (hookworm) was associated with protection from cerebral malaria in a case–control trial. Helminth infection was associated with lower rates of renal failure, jaundice and peripheral mature schizonts. Helminth-infected individuals had higher reactive nitrogen intermediates in blood samples | [65,75,79] | |||
DS measures include parasite density, severity of anemia and prevalence/incidence of severe malaria (or one of the severe malaria criteria including cerebral malaria). IR refers to any measure of infection, including incidence or prevalence.
Assoc.: Association; DS: Disease severity; IR: Infection rate; PD: Programmed death; RBC: Red blood cell.
There are less data on other soil-transmitted helminths including hookworm and Trichuris trichiura, and many studies do not differentiate by the type of soil-transmitted helminth. The studies that have examined the effect of hookworm alone found more consistently that malaria infection is greater among hookworm-infected individuals, including increased infection rates and increased Plasmodium parasitemia [60,68–74]. Clinical studies examining any type of soil-transmitted helminth have yielded mixed results [60,61,68,75–77]; however, an interesting study demonstrated that stool soil-transmitted helminth infection was associated with protection from cerebral malaria in Thai adults, and that individuals with helminth infections had increased concentrations of RNIs than those without helminth infection [50]. This was attributed to the chronic activation of the CD23 receptor and decreased circulating levels of soluble CD23. This group has postulated that acute CD23 activation, in the setting of high levels of sCD23, produces pathologic NO and TNF production. In the setting of low circulated soluble CD23, CD23 activation will lead to a restrained NO release, limiting parasite sequestration.
With the inconsistent data, research addressing the immunologic mechanisms at play is useful. Cytokine profiling in Plasmodium and helminth coinfected individuals reveals increased expression of IL-10 when compared with those with Plasmodium infection alone [78]. Another study has also demonstrated increased expression of the Treg marker FoxP3 among those with helminthic infection [78]. There were similar findings in an Asian retrospective case–control study examining the influence of worm infection on malaria vulnerability [79]. In Ascaris, in particular, it seems that soil-transmitted helminths may be able to decrease the severity of infection by biasing a Th2-mediated response. However, the effect of helminthic infection of controlling parasitemia is less clear. If the initial inflammatory response is critical for controlling parasitemia, then one might expect higher parasitemia in the setting of helminthic infection. This has been seen in hookworm infections, but the opposite has been demonstrated with Ascaris. Overall, there is evidence that soil-transmitted helminths immunomodulate responses to malaria infection, but there are inconsistencies between helminth type and other factors including host age.
Schistosomes
The same issue of conflicting findings exists in the research on malaria-schistosome coinfection (Table 2). Limited studies in humans suggest that Schistosoma mansoni is associated with increased risk of malaria infection [80]. In murine models, S. mansoni coinfection results in higher parasitemia and lethal disease from P. yoelii, an infection that is normally not fatal in mouse models [81]. In an examination of possible immune-mediated pathology, a P. berghei murine model found that coexisting S. mansoni infection appears to decrease rates of cerebral malaria [82]. Even more confusingly, researchers examining P. falciparum and Schistosoma haematobium have found decreased rates of uncomplicated malaria among individuals with S. haematobium [83,84]. In this population, S. haematobium is associated with elevated IL-6, IL-4 and IFN-γ levels. When infected with malaria, IL-6 and IL-10 levels rose in all children, but were blunted in Schistsoma-infected individuals [85]. Further studies showed that S. haematobium infection was associated with decreased Tregs during malaria infection, decreased Plasmodium parasitemia and increased IFN-γ and IL-2 [74,86]. The parasitemia and cytokine profile is similar to what would be expected based on the results of the sporozoite challenge studies examining Treg cells, but why does S. haematobium infection, which in isolation causes a potent Th2 response, lead to an increased inflammatory response to malaria infection? It is likely that we are oversimplifying the immunomodulatory of S. haematobium and other helminths. Interestingly, one factor addressed by the schistosomiasis coin-fection literature is the role of the intensity of helminth infection in malaria outcomes [83]. In the same study cited above, where S. mansoni infection was associated with increased malaria risk, a subanalysis demonstrated that those with only moderate intensity infection, malaria attack rates were lower, although this analysis did not reach statistical significance [80].
Table 2.
Key clinical publications on schistosomiasis and malaria coinfection.
| Organisms | Country | Findings | Ref. |
|---|---|---|---|
| Schistosoma haematobium | Malawi | S. haematobium associated with lower malaria parasite densities | [74] |
| S. haematobium | Gabon | S. hematobium was not found to be associated with malaria infection | [59] |
| S. haematobium | Kenya | Schistosomiasis was not associated with malaria susceptiblity | [76] |
| Schistosoma mansoni | Uganda | No association between S. mansoni and malaria infection found | [71] |
| S. mansoni, S. haematobium | Zimbabwe | S. mansoni infection associated with Plasmodium falciparum malaria infection | [70] |
| S. haematobium | Mali | Levels of Tregs were lower in malaria–S. haematobium coinfected children versus children with malaria alone. Increased Tregs were associated with decreased serum Th1 cytokine levels and elevated parasitemia | [86] |
| S. haematobium | Mali | IL-6 and IL-10 elevations in acute malaria were blunted in schistosomiasis-infected children from 4 to 8 years old | [85] |
| S. haematobium | Senegal | Subjects with low-density S. haematobium had lower P. falciparum parasitemia | [83] |
| S. haematobium | Mali | Prospective study demonstrating delay in time to clinical malaria infection, decreased number of malaria episodes and decreased Plasmodium parasitemia among schistosomiasis-infected children | [86] |
| S. mansoni | Senegal | S. mansoni-infected individuals had increased incidence of clinical malaria. Effect most apparent in those with high schistosomiasis organism burden. Nonsignificant observation that malaria attack rates were lower in ‘medium-grade’ S. mansoni infections | [80] |
Filaria
Chronic filarial infections (Wuchereria bancrofti, Mansonella perstans, Brugia malayi and Brugia timori), like other helminth infections have been associated with an anti-inflammatory immune response (decreased IL-2 and IFN-γ), thought to be mediated through increased circulating IL-10-producing CD4+ lymphocytes [87–89]. In fact, the filarial glycoprotein ES-62 is an excellent example of a potential immunomodulatory therapy to arise from coinfection research. This protein has been shown to inhibit mast cell activity in hypersentivity reactions and may hold therapeutic promise in allergy-based pathophysiology [90]. In malaria coinfection research, filarial infection has been demonstrated to modulate malaria-specific IL-12p70, IP-10 (CXCL-10), IFN-γ, TNF-α and IL-17A levels [91,92]. This is thought to take place through interferon regulatory factor-1 signaling by CD4 cells, suppressing IL-12 secretion from antigen presenting cells [93]. In the murine cerebral malaria model using P. berghei ANKA, filiarial infection with Litomosoide sigmodontis was protective against the development of cerebral malaria, an effect that was lost when the same experiment was carried out in IL-10-knockout mice [94]. Although robust research has been carried out on the immunologic mechanisms of filarial and Plasmodium coinfection, there are limited clinical data on these coinfections. Therefore, it is not known whether the bias towards a regulatory immune environment actually leads to more infection and less immune-mediated disease.
The variability in responses, both clinical and immunologic, reinforces that helminthic infection should not be considered a uniform immunologic entity. Investigation into the precise mechanisms by which helminthic infection augments the immune response is currently underway. Multiple TLR targets have been identified so perhaps distinct immunologic signaling with the innate immune system is one explanation for the observed variability. Lipids derived from Schistosoma and Ascaris activate TLR2 [95], schistosomal egg dsRNA activates TLR3 [96] and a schistosomal mansoni egg polylactosamine sugar ( lacto-N-fucopentaose III) and filarial glycoprotein ES-62 activate TLR4. Each of these is thought to do so through unknown coreceptors that push DC response in an anti-inflammatory direction [89], and also possibly interfere with TLR signaling of other microorganisms.
HIV infection
There is great interest in understanding the interaction of HIV and malaria, given the magnitude and geographic distribution of the two epidemics. However, there are major obstacles in studying these two infections. Diagnostically, the high prevalence of multiple opportunistic infections in persons with HIV infection and immunodeficiency makes accurate assessments of malaria burden difficult. Furthermore, the widespread use of cotrimoxazole prophylaxis, which has antimalarial activity, may obscure the immunomodulatory effects of HIV on malaria infection. Finally, from a mechanistic standpoint, immunomodulation in HIV is difficult to characterize because it has such profound effects on multiple aspects of the immune system, causing dysfunction in innate, cell-mediated and humoral aspects of immunity. Despite this, there is considerable clinical evidence that HIV increases susceptibility to malaria (Table 3) [97–101], and this increased vulnerability has been reported both in infection rates and disease severity [102]. Interestingly, observations that HIV-positive persons are at increased risk for severe disease have come primarily from studies carried out in areas of unstable transmission and in pregnant women. This suggests that the impact of HIV infection seems to be greatest in those who have a greater likelihood of being without pre-existing immunity to malaria at the time of HIV infection. Perhaps, the immunomodulatory effects of HIV most profoundly impact one's ability to effectively acquire immunity to malaria and to a lesser degree impairs already acquired immune responses.
Table 3.
Key clinical publications on chronic viral and malaria coinfection.
| Infection | Country | Findings | Ref. |
|---|---|---|---|
| HIV | Malawi | HIV infection was associated with malaria infection in a cross-sectional analysis within a clinical trial | [74] |
| Uganda | HIV-1 infection is associated with cerebral malaria in children in a cross-sectional analysis | [102] | |
| Cameroon | HIV-1 infection was not associated with placental and peripheral Plasmodium falciparum infection. HIV was associated with increased placental P. falciparum parasitemia | [105] | |
| Zambia | Case–control trial in which HIV-1 infection was a risk factor for adults with severe malaria compared with controls with uncomplicated malaria asymptomatic controls. Disease severity was associated with low CD4 | [99] | |
| Malawi | Prospective study evaluating malaria treatment failure with SP in the setting of HIV infection and considerable levels of SP resistance. Treatment failure was not associated with CD4 cell count | [100] | |
| Malawi | A longitudinal observational trial demonstrating an increased incidence of clinical malaria episodes in subjects with CD4 cell counts <200 cells/mm3 | [101] | |
| Malawi | Prospective trial showing association of HIV-1 infection with parasitemia. Treatment failure was not associated with HIV infection | [98] | |
| Uganda | Prospective observational trial in which HIV infection, low CD4 cell count and advanced HIV clinical stage was associated with increased incidence of clinical malaria. Among HIV-infected subjects, low CD4-cell counts were associated with increased parasitemia | [97] | |
| Hepatitis B | Brazil | An observational study with active and passive Plasmodium case finding. Subjects with active HBV were more likely to be asymptomatic when infected with malaria and have decreased parasitemia. HBV infection was also associated with a decreased inflammatory cytokine profile | [106] |
| Brazil | Among subjects with acute P. falciparum and Plasmodium vivax malaria, clinical characteristics were similar between those with and without serologic markers for HBV infection | [107] | |
| Vietnam | Among patients hospitalized for severe P. falciparum malaria, the prevalence of hepatitis B surface antigen positivity was higher than that in the reported population | [108] | |
| Gambia | In a case–control trial of severe malaria and match controls, severe cases were more likely to carry hepatitis B | [109] | |
HBV: Hepatitis B virus; SP: Sulfadoxine–pyrimethamine.
In terms of the mechanisms behind this immunomodulation, it was found early in the HIV epidemic that progression of the disease is characterized by the gradual transition to lower levels of inflammatory cytokines and higher levels of anti-inflammatory cytokines [103]. In viremic HIV-infected persons, IL-10 mRNA is found to be unregulated in several peripheral blood mononuclear cell lines [104]. Cytokine profiles in response to another chronic parasitic infection, leishmaniasis, have found that there are decreased IFN-γ:IL-10 ratios in individuals with HIV and leishmaniasis as compared with HIV-negative individuals with the infection. There is very little direct research examining the immunologic mechanisms by which HIV affects malaria vulnerability. A study on pregnant women found that HIV infection significantly alters the cytokine milieu of the placenta. IL-10 mRNA levels in the placenta decrease while TNF-α mRNA levels increase. However, the presence of peripheral or placental P. falciparum parasitemia did not differ according to HIV infection status, although HIV infection was associated with higher level of placental parasitemia [105]. Further research is required in the area of HIV and malaria coinfection.
Hepatitis B infection
Viral hepatitis coinfection in malaria has been largely overlooked, with only a handful of clinical studies available in the literature (Table 3). These infections, like the other chronic infections discussed in this article, have been described as having anti-inflammatory effects on their hosts. A study conducted in the Amazon included immunologic outcomes and found that individuals with active hepatitis B infection were more likely to be asymptomatic in response to Plasmodium infection, tended to present with lower levels of parasitemia and had decreased proinflammatory cytokine levels [106]. However, another study in the same region found that there were no clinical differences in those individuals with coinfection versus malaria infection alone [107]. Parasitemia rates were lower and antibody levels were higher in hepatitis B-infected subjects; but these findings did not reach statistical significance. In Thailand, one group found that the prevalence of hepatitis B among severe malaria patients was much higher than in the general population (24 vs 10%) [108]. This phenomenon has also been documented in Africa among children with uncomplicated malaria infection [109]. Few conclusions can be drawn, given that there are only a handful of studies addressing this coinfection, and it would also be valuable to understand the impact of hepatitis C coinfection.
Other viral infections
Other immunomodulatory viruses include Epstein–Barr virus (EBV) and cytomegalovirus (CMV). While numerous articles have been written about the effects of malaria on EBV infection [110–112], little is known about how EBV infection affects the risk of malaria or immune response to P. falciparum. Very little is known about CMV infection in malaria endemic areas, and the few studies published on CMV and malaria have noted the effect of malaria on CMV infection (e.g., placental malaria is associated with increased congenital CMV infection) [113], but not the effect of CMV infection on risk of malaria or on immune response to malaria. Further research in this area is also strongly indicated.
Conclusion
Implications of immunomodulatory therapeutics
In this review, we have examined the effect on malaria infection of common infections with known immunomodulatory effects, but can we apply what we learn from these ‘experiments in nature’ on malaria coinfections to help develop immunomodulatory therapeutics? Based on the data on helminthic or viral coinfections with malaria reviewed here, immunomodulatory therapies in malaria will need to ride the fine balance between control of parasitemia and control of the immune response to P. falciparum. Excessive attenuation of the proinflammatory response could lead to an increased level of parasitemia, while therapies that do not adequately control the proinflammatory response may be ineffective at decreasing severe disease. Therapies that have been investigated include broadly anti-inflammatory agents such as glucocorticoids and TNF-α-targeting agents whether through antibodies, pentoxifylline or thalidomide [114,115]. Smaller studies have also examined targeting other cytokines, and there are also agents that target downstream immune effects including drugs targeting the expression of adherence molecules and drugs aimed at limiting oxidative damage.
At present, none of the major immunomodulatory therapies have been shown to improve clinical outcomes in humans, although attenuation of the inflammatory response might have an effect on long-term effects such as cognitive impairment in survivors of severe malaria [116]. In terms of improving short-term outcomes including mortality, the timing of immunomodulatory therapy remains an issue. It has been postulated that these agents have not had great success because they are administered too late in the pathogenesis of the disease; at the time a child presents with severe disease, an already strong proinflammatory response is underway. The demonstrated ability of coinfections, which are present at the onset of infection, to modulate disease outcomes supports this hypothesis. It is possible that concomitant highly effective antiparasitic therapy such as artemisinin combination therapy with immunomodulatory agents may decrease the risk of uncontrolled parasitemia with these agents; but there are no well-designed trials to provide a strong basis for such a prediction. Murine models may provide some insight into timing of immunomodulatory therapy, but the differences in Plasmodium species between human infection and murine models and in the human and murine immune response may limit the extrapolation of these data [117]. Two recent reviews highlighted some promising new therapies, including arginine and inhaled NO to increase NO concentrations, and PPAR-γ agonists such as rosiglitazone, which can modify CD36 transcription and TLR2-dependent innate inflammatory immune responses, as potential agents to favorably modulate human immune response to malaria [114,115]. These studies reflect insights learned from murine models and also from the studies of helminth coinfection with malaria, ‘experiments in nature’ that provided support for helminth-induced increase in RNIs as a factor in protection against cerebral malaria [50], and helminth-associated alterations in TLR responses [118,119].
In the case of limiting or preventing severe disease, the only way to administer pharmacologic immunomodulators sooner may be to treat all infected individuals with risk factors of severe malaria, but before they develop severe disease in order to prevent rather than treat severe malaria [89]. There are investigations underway to identify biomarkers for severe disease [120]; but in reality we are still a long way from useful clinical tools to identify high-risk individuals. With this in mind, when considering prevention strategies for those with severe disease, we should consider what has been seen with HIV infection. It is possible that immunomodulation may prevent the acquisition of immunity. Any immunomodulatory therapy that is studied should be evaluated longitudinally to assure that it does not impair the acquisition of effective natural immunity in a way that leads to greater risk later in life.
Finally, the diversity of immunomodulatory responses seen among the helminth infections suggests that certain anti-inflammatory pathways decrease malaria severity while others do not. This may point towards choosing specific immunomodulatory targets rather than using broadly anti-inflammatory agents. This variability also brings up the possibility that confounding factors may be at play here, and that more data are needed to understand the true impact of coinfection on malaria morbidity and mortality. Epidemiological studies may not be able to completely control the increased risk of both infections among certain sub populations. Age and socioeconomic factors have been linked with the risk of coinfection [73]. Studies examining only clinical outcomes do not allow us to understand the mechanism for increased or decreased malaria vulnerability. It is possible that in the setting of a pre-existing condition such as HIV or helminthic infection, an individual has less physiologic reserve to deal with malaria infection as opposed to a truly immunomodulatory mechanism. Because of these issues with clinical and epidemiologic studies, it is valuable to have studies that examine immunologic mechanisms behind interactions including cytokine profiling and T-cell phenotyping.
In conclusion, we have evaluated some of the critical naturally acquired immune responses to malaria infection and examined how some of these responses are altered by naturally occurring immunomodulators. Overall, this line of research may lend insight into effective ways to alter immune response to decrease malaria morbidity, but sometimes the contradictory evidence from different studies points to the complexity of the interplay of pathogens and host immune response. The relative paucity of data on coinfections, notably but not exclusively for viral coinfections, points to a need for continued research in these areas including both clinical assessments and studies on immunologic mechanisms of disease or protection.
Expert commentary
At present, there are still major research gaps in the field of immunomodulation in malaria. Limited data on coinfection with known immunomodulatory infections suggest that immunomodulation can ameliorate severe disease outcomes. However, the precise targets that can simultaneously control deleterious immune responses without leading to uncontrolled parasitemia remain elusive. More research is required to provide insights that could lead to new immunomodulatory therapies in malaria. The research presented here on experiments in nature suggests that coinfection research can lend insights into the development of these therapies. Data from animal models cannot be the sole basis for human immunomodulatory targets, because the immunology of severe malaria in animal models differs in important ways from that of human malaria. Data from studies of coinfections in humans may inform us about important factors in the human immune response that modulate parasitemia and severity of disease.
Five-year view
The field of malaria immunology is rapidly expanding. Over the next several years, the ‘optimal’ immune response to P. falciparum may be understood more fully through high-throughput assays and systems biology approaches. Diagnostic tools that allow for more accurate field-based diagnostics may also be developed, including tools that identify children who are at greatest risk for severe disease early in their course. These studies and tools could allow for the development of efficacious immunomodulatory therapies by providing insights on how to more precisely modulate the host immune response, and by allowing introduction of this therapy in a timely manner. The development of these therapeutics would be greatly facilitated by continued research on coinfections in malaria.
Key issues.
Effective Plasmodium falciparum immunity requires a precisely timed and balanced response of inflammatory and anti-inflammatory immune regulators.
Limited data from parasitic and viral coinfections in individuals with P. falciparum infection suggest that immunomodulatory therapies that alter host Toll-like receptor and Treg responses to P. falciparum or augment host nitric oxide levels are most likely to result in reduction in disease severity.
Data on helminthic coinfection in malaria suggest that specific anti-inflammatory profiles are associated with protection, while others are not.
HIV infection increases the incidence and severity of malaria infection. This phenomenon is particularly notable in areas of low transmission.
There is a paucity of data on coinfection in malaria, particularly viral coinfection, and further studies in this area are needed.
To date, no immunomodulatory therapies have reproducibly demonstrated improvement in clinical outcomes in human studies, but some promising adjunctive therapies are currently in clinical trials.
Acknowledgments
This work was supported in part by a grant awarded to CC John from the National Institute of Neurological Disorders and Stroke and the Fogarty International Center (R01 NS055349).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
•• of considerable interest
- 1.Croft AM, Bager P, Kumar S. Helminth therapy (worms) for allergic rhinitis. Cochrane Database Syst. Rev. 2012;4:CD009238. doi: 10.1002/14651858.CD009238.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riley EM, Wahl S, Perkins DJ, Schofield L. Regulating immunity to malaria. Parasite Immunol. 2006;28(1–2):35–49. doi: 10.1111/j.1365-3024.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- 3.Artavanis-Tsakonas K, Tongren JE, Riley EM. The war between the malaria parasite and the immune system: immunity, immunoregulation and immunopathology. Clin. Exp. Immunol. 2003;133(2):145–152. doi: 10.1046/j.1365-2249.2003.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCall MB, Sauerwein RW. Interferon-γ – central mediator of protective immune responses against the pre-erythrocytic and blood stage of malaria. J. Leukoc. Biol. 2010;88(6):1131–1143. doi: 10.1189/jlb.0310137. [DOI] [PubMed] [Google Scholar]
- 5••.Clark IA, Budd AC, Alleva LM, Cowden WB. Human malarial disease: a consequence of inflammatory cytokine release. Malar. J. 2006;5:85. doi: 10.1186/1475-2875-5-85. [Excellent historical perspective on the evolving understanding of the role of inflammatory immune cytokines in malaria pathophysiology.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurtzhals JA, Adabayeri V, Goka BQ, et al. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet. 1998;351(9118):1768–1772. doi: 10.1016/S0140-6736(97)09439-7. [DOI] [PubMed] [Google Scholar]
- 7.Day NP, Hien TT, Schollaardt T, et al. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J. Infect. Dis. 1999;180(4):1288–1297. doi: 10.1086/315016. [DOI] [PubMed] [Google Scholar]
- 8••.Dodoo D, Omer FM, Todd J, Akanmori BD, Koram KA, Riley EM. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J. Infect. Dis. 2002;185(7):971–979. doi: 10.1086/339408. [Interesting study looking at the combined effect of inflammatory and anti-inflammatory cytokine changes on malaria susceptibility.] [DOI] [PubMed] [Google Scholar]
- 9.Erdman LK, Kain KC. Taking the sting out of malaria. Immunity. 2011;35(2):149–151. doi: 10.1016/j.immuni.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Coban C, Ishii KJ, Kawai T, et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J. Exp. Med. 2005;201(1):19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parroche P, Lauw FN, Goutagny N, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc. Natl Acad. Sci. USA. 2007;104(6):1919–1924. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Gowda NM, Kumar S, Gowda DC. Protein-DNA complex is the exclusive malaria parasite component that activates dendritic cells and triggers innate immune responses. J. Immunol. 2010;184(8):4338–4348. doi: 10.4049/jimmunol.0903824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry JA, Rush A, Wilson RJ, Olver CS, Avery AC. Dendritic cells from malaria-infected mice are fully functional APC. J. Immunol. 2004;172(1):475–482. doi: 10.4049/jimmunol.172.1.475. [DOI] [PubMed] [Google Scholar]
- 14••.Perry JA, Olver CS, Burnett RC, Avery AC. Cutting edge: the acquisition of TLR tolerance during malaria infection impacts T cell activation. J. Immunol. 2005;174(10):5921–5925. doi: 10.4049/jimmunol.174.10.5921. [An elegant set of experiments in the Plasmodium yoelii mice model demonstrating the progression of proinflammatory signaling to anti-inflammatory signaling from T cells through dendritic cell activation over the course of infection.] [DOI] [PubMed] [Google Scholar]
- 15.Riley EM. Is T-cell priming required for initiation of pathology in malaria infections? Immunol. Today. 1999;20(5):228–233. doi: 10.1016/s0167-5699(99)01456-5. [DOI] [PubMed] [Google Scholar]
- 16.Su Z, Stevenson MM. Central role of endogenous γ interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect. Immun. 2000;68(8):4399–4406. doi: 10.1128/iai.68.8.4399-4406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler S, Willheim M, Baier K, et al. Frequency of cytokine-producing T cells in patients of different age groups with Plasmodium falciparum malaria. J. Infect. Dis. 1999;179(1):209–216. doi: 10.1086/314571. [DOI] [PubMed] [Google Scholar]
- 18.Torre D, Speranza F, Giola M, Matteelli A, Tambini R, Biondi G. Role of Th1 and Th2 cytokines in immune response to uncomplicated Plasmodium falciparum malaria. Clin. Diagn. Lab. Immunol. 2002;9(2):348–351. doi: 10.1128/CDLI.9.2.348-351.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torre D, Giola M, Speranza F, Matteelli A, Basilico C, Biondi G. Serum levels of interleukin-18 in patients with uncomplicated Plasmodium falciparum malaria. Eur. Cytokine Netw. 2001;12(2):361–364. [PubMed] [Google Scholar]
- 20.Luty AJ, Lell B, Schmidt-Ott R, et al. Interferon-γ responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J. Infect. Dis. 1999;179(4):980–988. doi: 10.1086/314689. [DOI] [PubMed] [Google Scholar]
- 21.Amani V, Vigário AM, Belnoue E, et al. Involvement of IFN-γ receptor-medicated signaling in pathology and anti-malarial immunity induced by Plasmodium berghei infection. Eur. J. Immunol. 2000;30(6):1646–1655. doi: 10.1002/1521-4141(200006)30:6<1646::AID-IMMU1646>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Coban C, Ishii KJ, Uematsu S, et al. Pathological role of Toll-like receptor signaling in cerebral malaria. Int. Immunol. 2007;19(1):67–79. doi: 10.1093/intimm/dxl123. [DOI] [PubMed] [Google Scholar]
- 23.Khor CC, Chapman SJ, Vannberg FO, et al. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat. Genet. 2007;39(4):523–528. doi: 10.1038/ng1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mockenhaupt FP, Hamann L, von Gaertner C, et al. Common polymorphisms of Toll-like receptors 4 and 9 are associated with the clinical manifestation of malaria during pregnancy. J. Infect. Dis. 2006;194(2):184–188. doi: 10.1086/505152. [DOI] [PubMed] [Google Scholar]
- 25.Mockenhaupt FP, Cramer JP, Hamann L, et al. Toll-like receptor (TLR) polymorphisms in African children: common TLR-4 variants predispose to severe malaria. Proc. Natl Acad. Sci. USA. 2006;103(1):177–182. doi: 10.1073/pnas.0506803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sam-Agudu NA, Greene JA, Opoka RO, et al. TLR9 polymorphisms are associated with altered IFN-γ levels in children with cerebral malaria. Am. J. Trop. Med. Hyg. 2010;82(4):548–555. doi: 10.4269/ajtmh.2010.09-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omer FM, Riley EM. Transforming growth factor β production is inversely correlated with severity of murine malaria infection. J. Exp. Med. 1998;188(1):39–48. doi: 10.1084/jem.188.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect. Immun. 1999;67(9):4435–4442. doi: 10.1128/iai.67.9.4435-4442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsutsui N, Kamiyama T. Transforming growth factor β-induced failure of resistance to infection with blood-stage Plasmodium chabaudi in mice. Infect. Immun. 1999;67(5):2306–2311. doi: 10.1128/iai.67.5.2306-2311.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berretta F, St-Pierre J, Piccirillo CA, Stevenson MM. IL-2 contributes to maintaining a balance between CD4+FoxP3+ regulatory T cells and effector CD4+ T cells required for immune control of blood-stage malaria infection. J. Immunol. 2011;186(8):4862–4871. doi: 10.4049/jimmunol.1003777. [DOI] [PubMed] [Google Scholar]
- 31.Walther M, Tongren JE, Andrews L, et al. Upregulation of TGF-β, FoxP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity. 2005;23(3):287–296. doi: 10.1016/j.immuni.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Finney OC, Nwakanma D, Conway DJ, Walther M, Riley EM. Homeostatic regulation of T effector to Treg ratios in an area of seasonal malaria transmission. Eur. J. Immunol. 2009;39(5):1288–1300. doi: 10.1002/eji.200839112. [DOI] [PubMed] [Google Scholar]
- 33.Le Hesran JY, Cot M, Personne P, et al. Maternal placental infection with Plasmodium falciparum and malaria morbidity during the first 2 years of life. Am. J. Epidemiol. 1997;146(10):826–831. doi: 10.1093/oxfordjournals.aje.a009200. [DOI] [PubMed] [Google Scholar]
- 34.Schwarz NG, Adegnika AA, Breitling LP, et al. Placental malaria increases malaria risk in the first 30 months of life. Clin. Infect. Dis. 2008;47(8):1017–1025. doi: 10.1086/591968. [DOI] [PubMed] [Google Scholar]
- 35.Mutabingwa TK, Bolla MC, Li JL, et al. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med. 2005;2(12):e407. doi: 10.1371/journal.pmed.0020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brustoski K, Moller U, Kramer M, et al. Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum infection. J. Infect. Dis. 2006;193(1):146–154. doi: 10.1086/498578. [DOI] [PubMed] [Google Scholar]
- 37.Mackroth MS, Malhotra I, Mungai P, Koech D, Muchiri E, King CL. Human cord blood CD4+CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J. Immunol. 2011;186(5):2780–2791. doi: 10.4049/jimmunol.1001188. [DOI] [PubMed] [Google Scholar]
- 38.Scholzen A, Mittag D, Rogerson SJ, Cooke BM, Plebanski M. Plasmodium falciparum-mediated induction of human CD25FoxP3 CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFβ. PLoS Pathog. 2009;5(8):e1000543. doi: 10.1371/journal.ppat.1000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J. Immunol. 2008;180(9):5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 40.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 41.Blackburn SD, Wherry EJ. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15(4):143–146. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Wilson NO, Bythwood T, Solomon W, et al. Elevated levels of IL-10 and G-CSF associated with asymptomatic malaria in pregnant women. Infect. Dis. Obstet. Gynecol. 2010;2010:317430. doi: 10.1155/2010/317430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wipasa J, Okell L, Sakkhachornphop S, et al. Short-lived IFN-γ effector responses, but long-lived IL-10 memory responses, to malaria in an area of low malaria endemicity. PLoS Pathog. 2011;7(2):e1001281. doi: 10.1371/journal.ppat.1001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabrales P, Zanini GM, Meays D, Frangos JA, Carvalho LJ. Nitric oxide protection against murine cerebral malaria is associated with improved cerebral microcirculatory physiology. J. Infect. Dis. 2011;203(10):1454–1463. doi: 10.1093/infdis/jir058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zanini GM, Cabrales P, Barkho W, Frangos JA, Carvalho LJ. Exogenous nitric oxide decreases brain vascular inflammation, leakage and venular resistance during Plasmodium berghei ANKA infection in mice. J. Neuroinflammation. 2011;8:66. doi: 10.1186/1742-2094-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serghides L, Kim H, Lu Z, et al. Inhaled nitric oxide reduces endothelial activation and parasite accumulation in the brain, and enhances survival in experimental cerebral malaria. PLoS ONE. 2011;6(11):e27714. doi: 10.1371/journal.pone.0027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeo TW, Lampah DA, Gitawati R, et al. Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc. Natl Acad. Sci. USA. 2008;105(44):17097–17102. doi: 10.1073/pnas.0805782105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nacher M. Interactions between worm infections and malaria. Clin. Rev. Allergy Immunol. 2004;26(2):85–92. doi: 10.1007/s12016-004-0003-3. [DOI] [PubMed] [Google Scholar]
- 49.Acharya M, Borland G, Edkins AL, et al. CD23/FceRII: molecular multi-tasking. Clin. Exp. Immunol. 2010;162(1):12–23. doi: 10.1111/j.1365-2249.2010.04210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nacher M, Singhasivanon P, Traore B, et al. Helminth infections are associated with protection from cerebral malaria and increased nitrogen derivatives concentrations in Thailand. Am. J. Trop. Med. Hyg. 2002;66(3):304–309. doi: 10.4269/ajtmh.2002.66.304. [DOI] [PubMed] [Google Scholar]
- 51.Taylor RR, Allen SJ, Greenwood BM, Riley EM. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am. J. Trop. Med. Hyg. 1998;58(4):406–413. doi: 10.4269/ajtmh.1998.58.406. [DOI] [PubMed] [Google Scholar]
- 52.Roussilhon C, Oeuvray C, Müller-Graf C, et al. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 2007;4(11):e320. doi: 10.1371/journal.pmed.0040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olesen CH, Brahimi K, Vandahl B, et al. Distinct patterns of blood-stage parasite antigens detected by plasma IgG subclasses from individuals with different level of exposure to Plasmodium falciparum infections. Malar. J. 2010;9:296. doi: 10.1186/1475-2875-9-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54••.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites–masters of regulation. Immunol. Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [In-depth review of the immunologic investigations underway to understand helminthic immunomodulation.] [DOI] [PubMed] [Google Scholar]
- 55.Nacher M. Malaria vaccine trials in a wormy world. Trends Parasitol. 2001;17(12):563–565. doi: 10.1016/s1471-4922(01)02117-1. [DOI] [PubMed] [Google Scholar]
- 56.Sabin EA, Araujo MI, Carvalho EM, Pearce EJ. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J. Infect. Dis. 1996;173(1):269–272. doi: 10.1093/infdis/173.1.269. [DOI] [PubMed] [Google Scholar]
- 57.Cooper PJ, Chico M, Sandoval C, et al. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin β subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect. Immun. 2001;69(3):1574–1580. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58••.Nacher M. Interactions between worms and malaria: good worms or bad worms? Malar. J. 2011;10:259. doi: 10.1186/1475-2875-10-259. [Concise review of clinical evidence available on the influence of helminth on malaria infection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adegnika AA, Ramharter M, Agnandji ST, et al. Epidemiology of parasitic co-infections during pregnancy in Lambaréné, Gabon. Trop. Med. Int. Health. 2010;15(10):1204–1209. doi: 10.1111/j.1365-3156.2010.02598.x. [DOI] [PubMed] [Google Scholar]
- 60.Boel M, Carrara VI, Rijken M, et al. Complex Interactions between soil-transmitted helminths and malaria in pregnant women on the Thai–Burmese border. PLoS Negl. Trop. Dis. 2010;4(11):e887. doi: 10.1371/journal.pntd.0000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shapiro AE, Tukahebwa EM, Kasten J, et al. Epidemiology of helminth infections and their relationship to clinical malaria in southwest Uganda. Trans. R. Soc. Trop. Med. Hyg. 2005;99(1):18–24. doi: 10.1016/j.trstmh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 62.Brutus L, Watier L, Hanitrasoamampionona V, Razanatsoarilala H, Cot M. Confirmation of the protective effect of Ascaris lumbricoides on Plasmodium falciparum infection: results of a randomized trial in Madagascar. Am. J. Trop. Med. Hyg. 2007;77(6):1091–1095. [PubMed] [Google Scholar]
- 63.Brutus L, Watier L, Briand V, Hanitrasoamampionona V, Razanatsoarilala H, Cot M. Parasitic co-infections: does Ascaris lumbricoides protect against Plasmodium falciparum infection? Am. J. Trop. Med. Hyg. 2006;75(2):194–198. [PubMed] [Google Scholar]
- 64.Melo GC, Reyes-Lecca RC, Vitor-Silva S, et al. Concurrent helminthic infection protects schoolchildren with Plasmodium vivax from anemia. PLoS ONE. 2010;5(6):e11206. doi: 10.1371/journal.pone.0011206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nacher M, Singhasivanon P, Silachamroon U, et al. Helminth infections are associated with protection from malaria-related acute renal failure and jaundice in Thailand. Am. J. Trop. Med. Hyg. 2001;65(6):834–836. doi: 10.4269/ajtmh.2001.65.834. [DOI] [PubMed] [Google Scholar]
- 66.Das BS. Renal failure in malaria. J. Vector Borne Dis. 2008;45(2):83–97. [PubMed] [Google Scholar]
- 67.Le Hesran JY, Akiana J, Ndiaye el HM, Dia M, Senghor P, Konate L. Severe malaria attack is associated with high prevalence of Ascaris lumbricoides infection among children in rural Senegal. Trans. R. Soc. Trop. Med. Hyg. 2004;98(7):397–399. doi: 10.1016/j.trstmh.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 68.Degarege A, Animut A, Legesse M, Erko B. Malaria severity status in patients with soil-transmitted helminth infections. Acta Trop. 2009;112(1):8–11. doi: 10.1016/j.actatropica.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 69.Pullan RL, Kabatereine NB, Bukirwa H, Staedke SG, Brooker S. Heterogeneities and consequences of Plasmodium species and hookworm coinfection: a population based study in Uganda. J. Infect. Dis. 2011;203(3):406–417. doi: 10.1093/infdis/jiq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Midzi N, Sangweme D, Zinyowera S, et al. The burden of polyparasitism among primary schoolchildren in rural and farming areas in Zimbabwe. Trans. R. Soc. Trop. Med. Hyg. 2008;102(10):1039–1045. doi: 10.1016/j.trstmh.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 71.Hillier SD, Booth M, Muhangi L, et al. Plasmodium falciparum and helminth coinfection in a semi urban population of pregnant women in Uganda. J. Infect. Dis. 2008;198(6):920–927. doi: 10.1086/591183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nacher M. Worms and malaria: blind men feeling the elephant? Parasitology. 2008;135(7):861–868. doi: 10.1017/S0031182008000358. [DOI] [PubMed] [Google Scholar]
- 73.Yatich NJ, Yi J, Agbenyega T, et al. Malaria and intestinal helminth co-infection among pregnant women in Ghana: prevalence and risk factors. Am. J. Trop. Med. Hyg. 2009;80(6):896–901. [PubMed] [Google Scholar]
- 74.Thigpen MC, Filler SJ, Kazembe PN, et al. Associations between peripheral Plasmodium falciparum malaria parasitemia, human immunodeficiency virus, and concurrent helminthic infection among pregnant women in Malawi. Am. J. Trop. Med. Hyg. 2011;84(3):379–385. doi: 10.4269/ajtmh.2011.10-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nacher M, Singhasivanon P, Yimsamran S, et al. Intestinal helminth infections are associated with increased incidence of Plasmodium falciparum malaria in Thailand. J. Parasitol. 2002;88(1):55–58. doi: 10.1645/0022-3395(2002)088[0055:IHIAAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 76.Bejon P, Mwangi TW, Lowe B, Peshu N, Hill AV, Marsh K. Helminth infection and eosinophilia and the risk of Plasmodium falciparum malaria in 1- to 6-year-old children in a malaria endemic area. PLoS Negl. Trop. Dis. 2008;2(1):e164. doi: 10.1371/journal.pntd.0000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Druilhe P, Tall A, Sokhna C. Worms can worsen malaria: towards a new means to roll back malaria? Trends Parasitol. 2005;21(8):359–362. doi: 10.1016/j.pt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 78.Hartgers FC, Obeng BB, Kruize YC, et al. Responses to malarial antigens are altered in helminth-infected children. J. Infect. Dis. 2009;199(10):1528–1535. doi: 10.1086/598687. [DOI] [PubMed] [Google Scholar]
- 79.Nacher M, Gay F, Singhasivanon P, et al. Ascaris lumbricoides infection is associated with protection from cerebral malaria. Parasite Immunol. 2000;22(3):107–113. doi: 10.1046/j.1365-3024.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 80.Sokhna C, Le Hesran JY, Mbaye PA, et al. Increase of malaria attacks among children presenting concomitant infection by Schistosoma mansoni in Senegal. Malar. J. 2004;3:43. doi: 10.1186/1475-2875-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sangweme D, Shiff C, Kumar N. Plasmodium yoelii: adverse outcome of non-lethal P. yoelii malaria during co-infection with Schistosoma mansoni in BALB/c mouse model. Exp. Parasitol. 2009;122(3):254–259. doi: 10.1016/j.exppara.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Waknine-Grinberg JH, Gold D, Ohayon A, et al. Schistosoma mansoni infection reduces the incidence of murine cerebral malaria. Malar. J. 2010;9:5. doi: 10.1186/1475-2875-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Briand V, Watier L, LE Hesran JY, Garcia A, Cot M. Coinfection with Plasmodium falciparum and Schistosoma haematobium: protective effect of schistosomiasis on malaria in senegalese children? Am. J. Trop. Med. Hyg. 2005;72(6):702–707. [PubMed] [Google Scholar]
- 84.Lyke KE, Dicko A, Dabo A, et al. Association of Schistosoma haematobium infection with protection against acute Plasmodium falciparum malaria in Malian children. Am. J. Trop. Med. Hyg. 2005;73(6):1124–1130. [PMC free article] [PubMed] [Google Scholar]
- 85.Lyke KE, Dabo A, Sangare L, et al. Effects of concomitant Schistosoma haematobium infection on the serum cytokine levels elicited by acute Plasmodium falciparum malaria infection in Malian children. Infect. Immun. 2006;74(10):5718–5724. doi: 10.1128/IAI.01822-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lyke KE, Dabo A, Arama C, et al. Reduced T regulatory cell response during acute Plasmodium falciparum infection in malian children co-infected with Schistosoma haematobium. PLoS ONE. 2012;7(2):e31647. doi: 10.1371/journal.pone.0031647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.King CL, Kumaraswami V, Poindexter RW, et al. Immunologic tolerance in lymphatic filariasis. Diminished parasite-specific T and B lymphocyte precursor frequency in the microfilaremic state. J. Clin. Invest. 1992;89(5):1403–1410. doi: 10.1172/JCI115729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mahanty S, Ravichandran M, Raman U, Jayaraman K, Kumaraswami V, Nutman TB. Regulation of parasite antigen-driven immune responses by interleukin-10 (IL-10) and IL-12 in lymphatic filariasis. Infect. Immun. 1997;65(5):1742–1747. doi: 10.1128/iai.65.5.1742-1747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89••.van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology. 2007;212(6):475–490. doi: 10.1016/j.imbio.2007.03.009. [Clear and comprehensive review of the literature on the immunomodulatory effects of helminths, including an examination of the molecular mechanisms.] [DOI] [PubMed] [Google Scholar]
- 90.Melendez AJ, Harnett MM, Pushparaj PN, et al. Inhibition of Fc epsilon RI-mediated mast cell responses by ES-62, a product of parasitic filarial nematodes. Nat. Med. 2007;13(11):1375–1381. doi: 10.1038/nm1654. [DOI] [PubMed] [Google Scholar]
- 91.Metenou S, Dembélé B, Konate S, et al. Patent filarial infection modulates malaria-specific type 1 cytokine responses in an IL-10-dependent manner in a filaria/malaria-coinfected population. J. Immunol. 2009;183(2):916–924. doi: 10.4049/jimmunol.0900257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Metenou S, Dembele B, Konate S, et al. Filarial infection suppresses malaria-specific multifunctional Th1 and Th17 responses in malaria and filarial coinfections. J. Immunol. 2011;186(8):4725–4733. doi: 10.4049/jimmunol.1003778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Specht S, Ruiz DF, Dubben B, Deininger S, Hoerauf A. Filaria-induced IL-10 suppresses murine cerebral malaria. Microbes Infect. 2010;12(8–9):635–642. doi: 10.1016/j.micinf.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 94.Metenou S, Kovacs M, Dembele B, Coulibaly YI, Klion AD, Nutman TB. Interferon regulatory factor modulation underlies the bystander suppression of malaria antigen-driven IL-12 and IFN-γ in filaria-malaria co-infection. Eur. J. Immunol. 2012;42(3):641–650. doi: 10.1002/eji.201141991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van der Kleij D, Latz E, Brouwers JF, et al. A novel host–parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates Toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 2002;277(50):48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 96.Aksoy E, Zouain CS, Vanhoutte F, et al. Double-stranded RNAs from the helminth parasite Schistosoma activate TLR3 in dendritic cells. J. Biol. Chem. 2005;280(1):277–283. doi: 10.1074/jbc.M411223200. [DOI] [PubMed] [Google Scholar]
- 97.Whitworth J, Morgan D, Quigley M, et al. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet. 2000;356(9235):1051–1056. doi: 10.1016/S0140-6736(00)02727-6. [DOI] [PubMed] [Google Scholar]
- 98.Patnaik P, Jere CS, Miller WC, et al. Effects of HIV-1 serostatus, HIV-1 RNA concentration, and CD4 cell count on the incidence of malaria infection in a cohort of adults in rural Malawi. J. Infect. Dis. 2005;192(6):984–991. doi: 10.1086/432730. [DOI] [PubMed] [Google Scholar]
- 99.Chalwe V, Van geertruyden JP, Mukwamataba D, et al. Increased risk for severe malaria in HIV-1-infected adults, Zambia. Emerging Infect. Dis. 2009;15(5):749. doi: 10.3201/eid1505.081009. quiz 858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Laufer MK, van Oosterhout JJ, Thesing PC, et al. Malaria treatment efficacy among people living with HIV: the role of host and parasite factors. Am. J. Trop. Med. Hyg. 2007;77(4):627–632. [PubMed] [Google Scholar]
- 101••.Laufer MK, van Oosterhout JJ, Thesing PC, et al. Impact of HIV-associated immunosuppression on malaria infection and disease in Malawi. J. Infect. Dis. 2006;193(6):872–878. doi: 10.1086/500245. [Well-designed study that shows both the effect of HIV infection and CD4 count on clinical malaria susceptibility.] [DOI] [PubMed] [Google Scholar]
- 102.Imani PD, Musoke P, Byarugaba J, Tumwine JK. Human immunodeficiency virus infection and cerebral malaria in children in Uganda: a case–control study. BMC Pediatr. 2011;11:5. doi: 10.1186/1471-2431-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Klein SA, Dobmeyer JM, Dobmeyer TS, et al. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. AIDS. 1997;11(9):1111–1118. doi: 10.1097/00002030-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 104.Brockman MA, Kwon DS, Tighe DP, et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114(2):346–356. doi: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kfutwah A, Mary JY, Lemen B, et al. ANRS 1267 study team. Plasmodium falciparum infection significantly impairs placental cytokine profile in HIV infected Cameroonian women. PLoS ONE. 2009;4(12):e8114. doi: 10.1371/journal.pone.0008114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Andrade BB, Santos CJ, Camargo LM, et al. Hepatitis B infection is associated with asymptomatic malaria in the Brazilian Amazon. PLoS ONE. 2011;6(5):e19841. doi: 10.1371/journal.pone.0019841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Braga WS, Souza RA, Silva EB, Fonseca JC, Tosta CE. [Coinfection between hepatitis B virus and malaria: clinical, serologic and immunologic aspects]. Rev. Soc. Bras. Med. Trop. 2006;39(1):27–31. doi: 10.1590/s0037-86822006000100005. [DOI] [PubMed] [Google Scholar]
- 108.Barcus MJ, Hien TT, White NJ, et al. Short report: hepatitis B infection and severe Plasmodium falciparum malaria in Vietnamese adults. Am. J. Trop. Med. Hyg. 2002;66(2):140–142. doi: 10.4269/ajtmh.2002.66.140. [DOI] [PubMed] [Google Scholar]
- 109.Thursz MR, Kwiatkowski D, Torok ME, et al. Association of hepatitis B surface antigen carriage with severe malaria in Gambian children. Nat. Med. 1995;1(4):374–375. doi: 10.1038/nm0495-374. [DOI] [PubMed] [Google Scholar]
- 110.Njie R, Bell AI, Jia H, et al. The effects of acute malaria on Epstein–Barr virus (EBV) load and EBV-specific T cell immunity in Gambian children. J. Infect. Dis. 2009;199(1):31–38. doi: 10.1086/594373. [DOI] [PubMed] [Google Scholar]
- 111.Piriou E, Kimmel R, Chelimo K, et al. Serological evidence for long-term Epstein–Barr virus reactivation in children living in a holoendemic malaria region of Kenya. J. Med. Virol. 2009;81(6):1088–1093. doi: 10.1002/jmv.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Snider CJ, Cole SR, Chelimo K, et al. Recurrent Plasmodium falciparum malaria infections in Kenyan children diminish T-cell immunity to Epstein–Barr virus lytic but not latent antigens. PLoS ONE. 2012;7(3):e31753. doi: 10.1371/journal.pone.0031753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van der Sande MA, Kaye S, Miles DJ, et al. Risk factors for and clinical outcome of congenital cytomegalovirus infection in a peri-urban West-African birth cohort. PLoS ONE. 2007;2(6):e492. doi: 10.1371/journal.pone.0000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Higgins SJ, Kain KC, Liles WC. Immunopathogenesis of falciparum malaria: implications for adjunctive therapy in the management of severe and cerebral malaria. Expert Rev. Anti. Infect. Ther. 2011;9(9):803–819. doi: 10.1586/eri.11.96. [DOI] [PubMed] [Google Scholar]
- 115.John CC, Kutamba E, Mugarura K, Opoka RO. Adjunctive therapy for cerebral malaria and other severe forms of Plasmodium falciparum malaria. Expert Rev. Anti. Infect. Ther. 2010;8(9):997–1008. doi: 10.1586/eri.10.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.John CC, Bangirana P, Byarugaba J, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122(1):e92–e99. doi: 10.1542/peds.2007-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.White NJ, Turner GD, Medana IM, Dondorp AM, Day NP. The murine cerebral malaria phenomenon. Trends Parasitol. 2010;26(1):11–15. doi: 10.1016/j.pt.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kane CM, Cervi L, Sun J, et al. Helminth antigens modulate TLR-initiated dendritic cell activation. J. Immunol. 2004;173(12):7454–7461. doi: 10.4049/jimmunol.173.12.7454. [DOI] [PubMed] [Google Scholar]
- 119.Venugopal PG, Nutman TB, Semnani RT. Activation and regulation of Toll-like receptors (TLRs) by helminth parasites. Immunol. Res. 2009;43(1–3):252–263. doi: 10.1007/s12026-008-8079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Conroy AL, Phiri H, Hawkes M, et al. Endothelium-based biomarkers are associated with cerebral malaria in Malawian children: a retrospective case–control study. PLoS ONE. 2010;5(12):e15291. doi: 10.1371/journal.pone.0015291. [DOI] [PMC free article] [PubMed] [Google Scholar]