Abstract
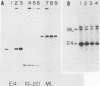
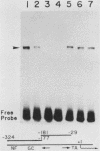
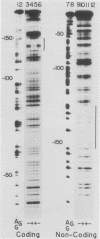
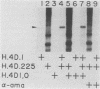
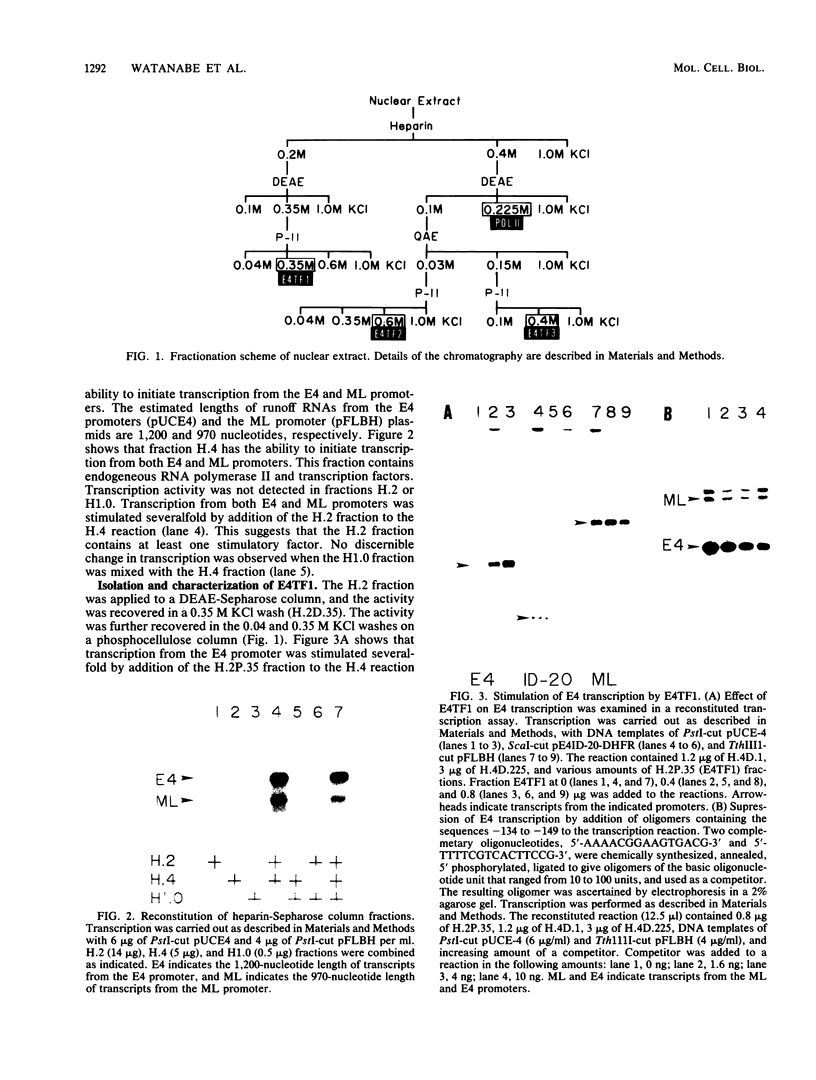
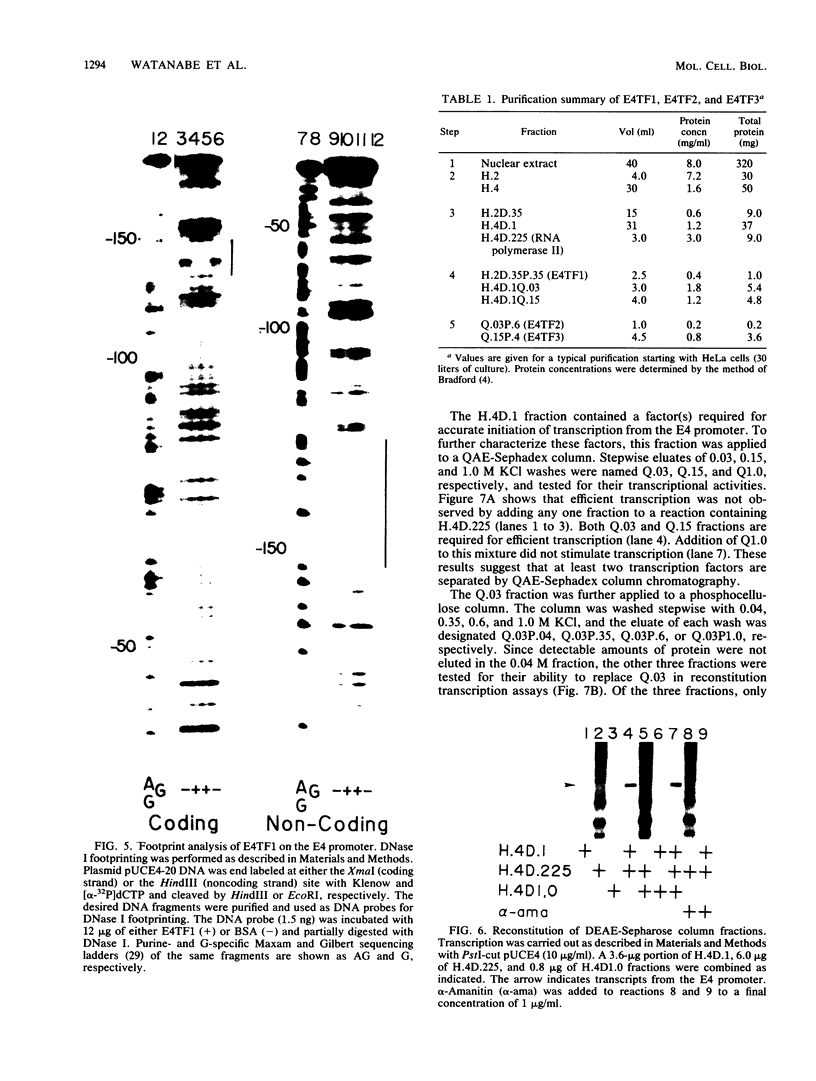
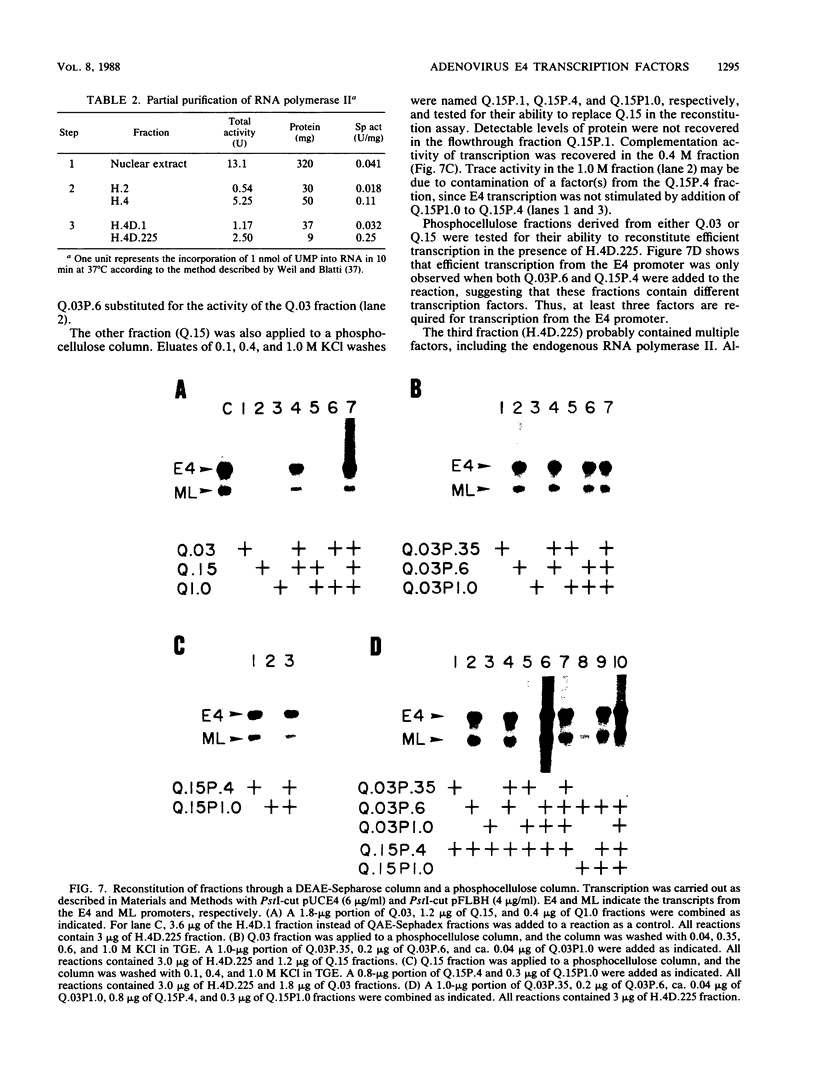
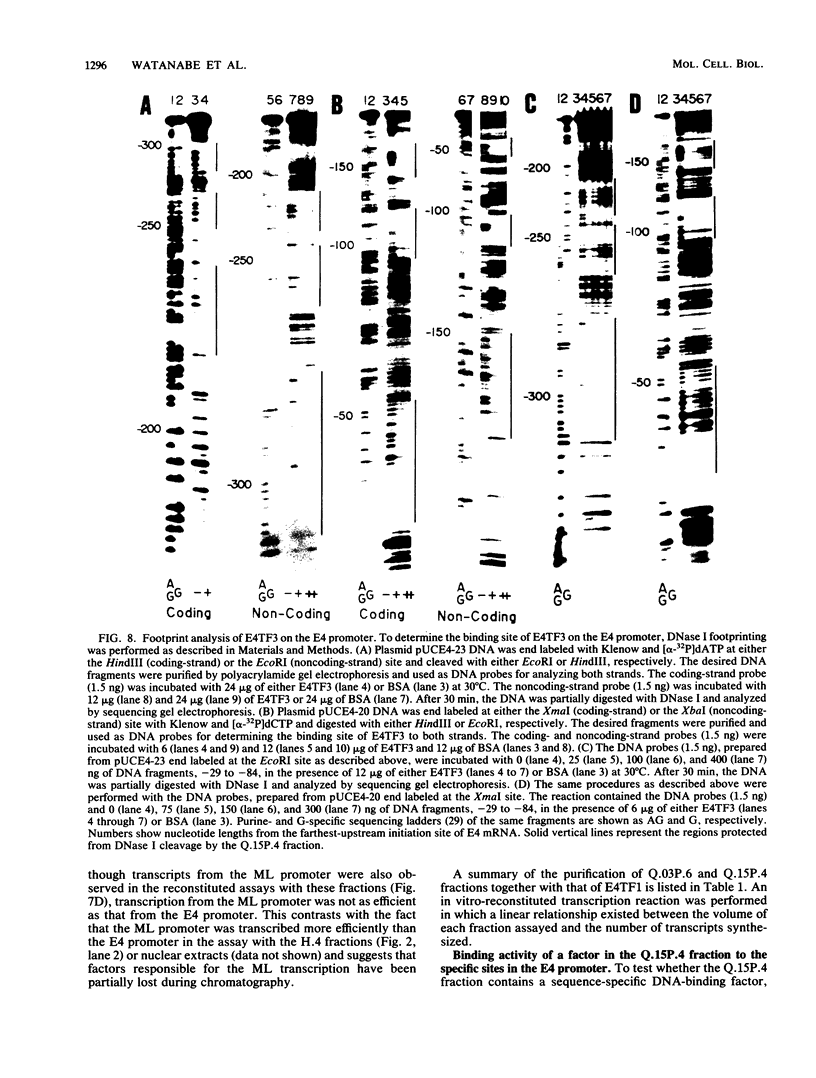
Two kinds of trans-acting factors that regulate transcription from the promoter of the adenovirus early-region 4 (E4) have been identified by reconstituting nuclear extracts of HeLa cells. They were designated E4TF1 and E4TF3 for E4 transcription factors. These factors were responsible for efficient and accurate transcription in vitro from the E4 promoter, as were another transcription factor, designated E4TF2, and a crude fraction containing endogenous RNA polymerase II. E4TF1 stimulated transcription from the E4 promoter but not from the major late promoter or the E4 mutant promoter lacking the E4TF1-binding site. Footprint analysis of E4TF1 revealed that it binds to a specific region, residing between 132 and 152 base pairs upstream from the initiation site of the E4 mRNA. E4TF3 also regulated transcription from the E4 promoter. E4TF3 protected four ca. 20-base-pair regions in a DNase I footprinting assay. They were located around 40, 160, 230, and 260 base pairs upstream from the initiation site of E4 mRNA. Specific inhibition of E4 transcription was observed by addition of DNA fragments covering one of the E4TF1- and E4TF3-binding sites to in vitro transcription assays. These results suggest that both E4TF1 and E4TF3 regulate E4 transcription by binding to the specific upstream elements in the E4 promoter. These factors may be involved in the E1A transactivation of E4 transcription.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Blanton R. A., Carter T. H. Autoregulation of adenovirus type 5 early gene expression. III. Transcription studies in isolated nuclei. J Virol. 1979 Feb;29(2):458–465. doi: 10.1128/jvi.29.2.458-465.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Chen I. S., Cann A. J., Shah N. P., Gaynor R. B. Functional relation between HTLV-II x and adenovirus E1A proteins in transcriptional activation. Science. 1985 Nov 1;230(4725):570–573. doi: 10.1126/science.2996140. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Martin P. L., Shastry B. S., Roeder R. G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Isolation of transcription factors that discriminate between different promoters recognized by RNA polymerase II. Cell. 1983 Mar;32(3):669–680. doi: 10.1016/0092-8674(83)90053-3. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Egly J. M., Miyamoto N. G., Moncollin V., Chambon P. Is actin a transcription initiation factor for RNA polymerase B? EMBO J. 1984 Oct;3(10):2363–2371. doi: 10.1002/j.1460-2075.1984.tb02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A., Baker C. C., Manley J. L., Ziff E. B., Sharp P. A. In vitro transcription of adenovirus. J Virol. 1981 Dec;40(3):703–719. doi: 10.1128/jvi.40.3.703-719.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi P., Perricaudet M. The E4 promoter of adenovirus type 2 contains an E1A dependent cis-acting element. Nucleic Acids Res. 1986 Nov 25;14(22):9035–9049. doi: 10.1093/nar/14.22.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi P., Perricaudet M. The E4 transcriptional unit of Ad2: far upstream sequences are required for its transactivation by E1A. Nucleic Acids Res. 1984 Oct 25;12(20):7877–7888. doi: 10.1093/nar/12.20.7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaka S., Nishigaki T., Sharp P. A., Handa H. Regulation of in vitro and in vivo transcription of early-region IV of adenovirus type 5 by multiple cis-acting elements. Mol Cell Biol. 1987 Jul;7(7):2578–2587. doi: 10.1128/mcb.7.7.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H., Kaufman R. J., Manley J., Gefter M., Sharp P. A. Transcription of Simian virus 40 DNA in a HeLa whole cell extract. J Biol Chem. 1981 Jan 10;256(1):478–482. [PubMed] [Google Scholar]
- Handa H., Kingston R. E., Sharp P. A. Inhibition of adenovirus early region IV transcription in vitro by a purified viral DNA binding protein. Nature. 1983 Apr 7;302(5908):545–547. doi: 10.1038/302545a0. [DOI] [PubMed] [Google Scholar]
- Handa H., Sharp P. A. Requirement for distal upstream sequences for maximal transcription in vitro of early region IV of adenovirus. Mol Cell Biol. 1984 Apr;4(4):791–798. doi: 10.1128/mcb.4.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. E1A transcription induction: enhanced binding of a factor to upstream promoter sequences. Science. 1986 Feb 14;231(4739):719–722. doi: 10.1126/science.2935935. [DOI] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986 Apr 25;45(2):219–228. doi: 10.1016/0092-8674(86)90386-7. [DOI] [PubMed] [Google Scholar]
- Lee D. C., Roeder R. G. Transcription of adenovirus type 2 genes in a cell-free system: apparent heterogeneity of initiation at some promoters. Mol Cell Biol. 1981 Jul;1(7):635–651. doi: 10.1128/mcb.1.7.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A., Green M. R. A cellular transcription factor E4F1 interacts with an E1a-inducible enhancer and mediates constitutive enhancer function in vitro. EMBO J. 1987 May;6(5):1345–1353. doi: 10.1002/j.1460-2075.1987.tb02374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Specific binding of a cellular DNA replication protein to the origin of replication of adenovirus DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6177–6181. doi: 10.1073/pnas.80.20.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Winkler J. J. Regulation of early adenovirus transcription: a protein product of early region 2 specifically represses region 4 transcription. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1893–1897. doi: 10.1073/pnas.77.4.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishigaki T., Hanaka S., Kingston R. E., Handa H. A specific domain of the adenovirus EIV promoter is necessary to maintain susceptibility of the integrated promoter to EIA transactivation. Mol Cell Biol. 1988 Jan;8(1):353–360. doi: 10.1128/mcb.8.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn G. J., van Driel W., van der Vliet P. C. Nuclear factor III, a novel sequence-specific DNA-binding protein from HeLa cells stimulating adenovirus DNA replication. Nature. 1986 Aug 14;322(6080):656–659. doi: 10.1038/322656a0. [DOI] [PubMed] [Google Scholar]
- Samuels M., Fire A., Sharp P. A. Separation and characterization of factors mediating accurate transcription by RNA polymerase II. J Biol Chem. 1982 Dec 10;257(23):14419–14427. [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Factors involved in specific transcription by human RNA polymerase II: analysis by a rapid and quantitative in vitro assay. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4394–4398. doi: 10.1073/pnas.82.13.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SivaRaman L., Subramanian S., Thimmappaya B. Identification of a factor in HeLa cells specific for an upstream transcriptional control sequence of an EIA-inducible adenovirus promoter and its relative abundance in infected and uninfected cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5914–5918. doi: 10.1073/pnas.83.16.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil P. A., Blatti S. P. Partial purification and properties of calf thymus deoxyribonucleic acid dependent RNA polymerase III. Biochemistry. 1975 Apr 22;14(8):1636–1642. doi: 10.1021/bi00679a015. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]