Abstract
One-step apexification using mineral trioxide aggregate (MTA) has been reported as an alternative treatment modality with more benefits than the use of long-term calcium hydroxide for teeth with open apex. However, orthograde placement of MTA is a challenging procedure in terms of length control. This case series describes the sequence of events following apical extrusion of MTA into the periapical area during a one-step apexification procedure for maxillary central incisor with an infected immature apex. Detailed long-term observation revealed complete resolution of the periapical radiolucent lesion around the extruded MTA. These cases revealed that direct contact with MTA had no negative effects on healing of the periapical tissues. However, intentional MTA overfilling into the periapical lesion is not to be recommended.
Keywords: extrusion, immature apex, mineral trioxide aggregate, one-step apexification, open apex, periapical healing
Introduction
When an infected or traumatized immature tooth has vital pulps, the general consensus for clinical treatment is to preserve normal pulp tissue by using traditional apexogenesis or recent alternative revascularization to allow complete formation of the root.1,2,3 The success rate for the apexogenesis was reported as high as 92%.4 On the other hand, a completion of the root filling may be facilitated by inducing a hard tissue apical barrier for those teeth with non-vital pulps. There have been many attempts to achieve an apical barrier for the incompletely formed apex, a process also known as apexification. The longest advocated medicament for apexification has been Ca(OH)2 (calcium hydroxide).5,6,7 Although the clinical success rates for Ca(OH)2 apexification is high, complications like risk of re-infection and root fracture has gradually steered clinician toward the use of alternative material such as mineral trioxide aggregate (MTA) as an apical barrier.8,9,10
Successful outcome of MTA apexification has been reported.11 The essence of this treatment approach lies in the expedient cleaning and shaping of the root canal system, followed by replacement of an apical seal with a material that is amenable to tissue healing and regeneration.12,13 Patients benefitted from this treatment approach.14,15 MTA apexification may be performed as a one- or two-visit procedure and alleviate the need for extended period of dressing with Ca(OH)2. This technique also allows for immediate restoration and reduces the potential of catastrophic, vertical or oblique root fracture that often affects such teeth.16
Obtaining an optimal apical seal in teeth with immature apices is a challenge because the wide apical foramen requires a large volume of filling material that may extrude into the periradicular tissue initiating the foreign-body reactions.5 The lack of an apical step or seat also contributes to the apical extrusion of the material. Special placement techniques, using manual,17 ultrasonic18 or ultrasonic-assisted hand delivery for MTA19 have been suggested to minimize extrusion of the material. Despite this, difficulties in delivering and filling the canal with MTA material from an orthograde direction have been reported.17,20 Apical extrusion of the material sometimes occurs.
While favorable clinical outcomes from one-visit MTA apexification have been reported,21,22,23 in complete closure of the immature apex was observed in the case of overfilling.24 On the other hand, there is no long-term report of cases with MTA extruded into the periapical lesion. This report presented a series of cases with apical extrusion of MTA into the periradicular lesion during the one-step apexification procedure of maxillary central incisors with an immature or resorbed apex and their long-term clinical outcome.
Case 1
A 15-year-old male was referred to a dental teaching hospital for the management of a large periapical lesion on the right maxillary incisor (tooth #11) in 2006. Clinical examination revealed a labial swelling and tenderness to percussion associated with tooth #11. Periapical radiograph showed a wide open, incompletely formed apex surrounded by a radiolucent lesion of approximately 20 mm in diameter. The patient reported a history of trauma involving the central incisor 5 years before. The tooth was diagnosed as pulp necrosis with acute exacerbation of a chronic apical abscess. Non-surgical root canal therapy of tooth #11 was initiated.
After rubber dam isolation, the central incisor cavity was accessed; hemorrhagic, purulent exudate was seen discharging from the root canal. Working length was determined using both electronic apical locator (e-Magic Finder; S-Denti, Cheonan, Korea) and intraoral digital radiograph (Figure 1a). The canal was prepared using hand and rotary instruments, with 5.25% sodium hypochlorite (NaClO) and ethylene diaminetetraacetic acid (EDTA). as irrigants. The dimension of master apical file could not be confirmed because the apical foramen measured greater than size 140. A water-based Ca(OH)2 paste (Calcipex II; J. Morita, Osaka, Japan) was placed as medicament for 2 weeks. In the next visit, the canal was thoroughly irrigated with 5.25% NaClO, dried and filled with MTA (ProRoot MTA; Dentsply Tulsa Dental Specialties, Johnson City, TN, USA) for an apical step. The material was mixed according to the manufacturer's directions and carried to root canal with an amalgam carrier. Small increments of MTA were delivered apically using a size 120 gutta-percha cone and plugged to 4 mm short of working length, followed by paper points to remove excess moisture. This procedure was repeated until a 3–4 mm thickness of the apical MTA plug was obtained, guided by tactile sensation and measurement on paper points. Digital radiographs (PDX1000; PointNix, Seoul, Korea) were taken to monitor the progress. In spite of these efforts to control the length of MTA placement, an extrusion of the MTA beyond the apex was detected (Figure 1b and 1c). The remaining canal space was obturated with MTA, patient and his parents were informed of the extrusion, and the patient scheduled for follow-up.
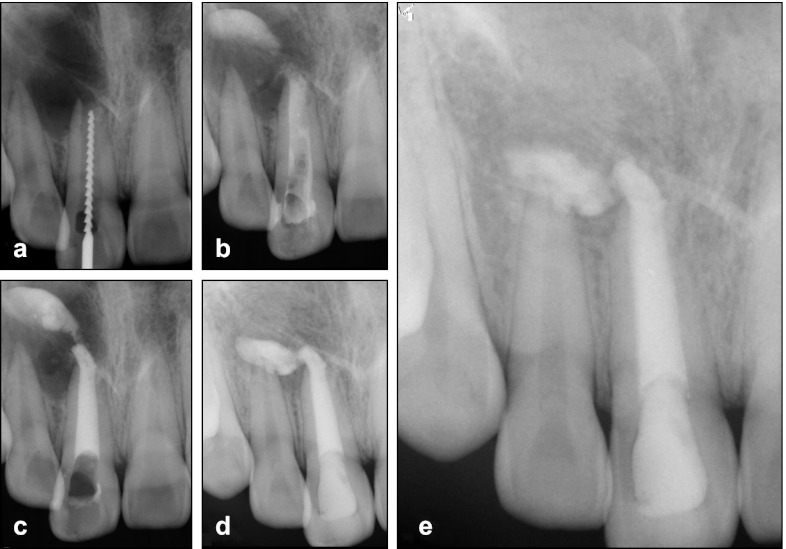
Figure 1.
Radiographs of case 1. (a) The first visit during working length measurement. (b) The MTA extrusion into the periapical lesion during the one-step apexification. (c) Completion of MTA placement. (d) 11-month follow-up presenting the displacement of MTA. (e) 36-month follow-up showing complete resolution of periapical radiolucency around the extruded MTA. MTA, mineral trioxide aggregate.
The patient was reviewed every week for 1 month, and then every 6 month. After about 1 year, the extruded MTA appeared to have shifted in its location (Figure 1d). At the final recall after 36 months, a significant reduction of periapical radiolucent lesion was noted and the overfilled MTA was completely surrounded by bony (or bone-like) tissues (Figure 1e).
Case 2
A 36-year-old male was referred to the same teaching hospital for endodontic re-treatment of the right maxillary incisor (tooth #11) in 2006. The chief complaint was discomfort of tooth #11 with a history of intermittent swelling on the labial aspect. He presented a root canal treatment on that tooth 9 years ago. Medical history was not contributory. Clinical examination showed that the tooth was tender to percussion, and a sinus tract was present on the labial vestibule that was traced to the apical area of 21. Radiographically, tooth #11 had a periapical rarefaction, measured approximately 11 mm (width)×15 mm (height), with a resorbed apex (Figure 2a). A diagnosis of chronic apical abscess was made and nonsurgical re-treatment of tooth #11 planned. After access cavity preparation, the gutta-percha was removed using gates-glidden drill and ProFile rotary instruments. Canal was instrumented with K-files to size #55, irrigated with 5.25% NaClO and dressed with Ca(OH)2. At the second visit, the sinus tract had resolved and the tooth asymptomatic. However, tissue fluid exudate was still present in the root canal. Thus, irrigation and dressing was repeated at this visit. Ten days later, only a trace amount of exudates could be blotted by paper point, and a one-step apexification with MTA as apical barrier was performed. MTA (ProRoot MTA; Dentsply Tulsa Dental Specialties, Johnson City, TN, USA) was delivered to the apical 3 mm of the canal using an amalgam carrier and then downpacked with measured endodontic pluggers under an operating dental microscope (Pico; Zeiss, Oberkochen, Germany). A large paper points were used to remove excess moisture. A couple of radiographs were taken to determine the positioning of the MTA, which revealed a mass of MTA present in the periapical radiolucency (Figure 2b and 2c). The tooth was restored with dentine-bonded composite and the patient was informed about the possibility of surgical intervention. Patient came back for 1-month recall, but reported no episodes of discomfort and/or swelling.
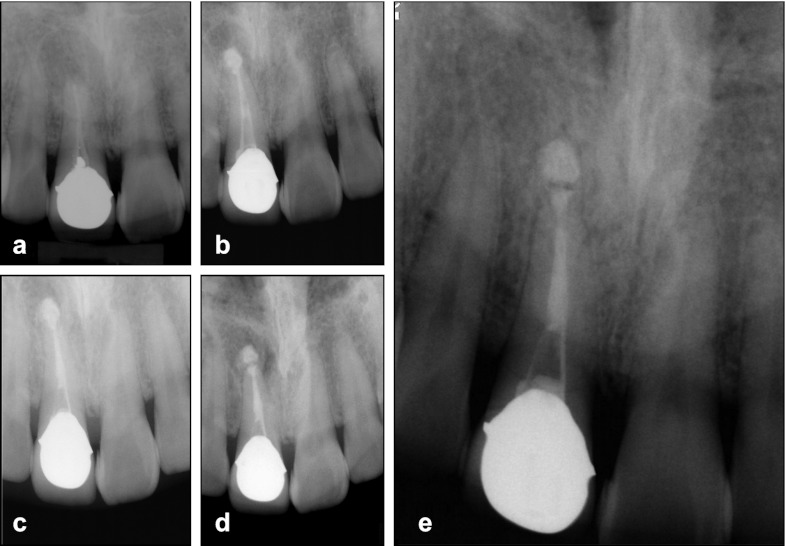
Figure 2.
Radiographs of case 2. (a) The first visit for diagnosis. (b) The MTA extrusion into the periapical lesion during the one-step apexification. (c) Completion of MTA placement. (d) 3-month recall showing the gradual healing of the apical lesion with radiolucent halo around the extruded MTA. (e) 54-month follow-up showing complete osseous repair with continuum of lamina dura-like structure along the extruded MTA. MTA, mineral trioxide aggregate.
Further periapical radiographs were taken at 3 (Figure 2d), 14 (not shown) and 36 months (not shown) to evaluate the periapical condition. Gradual healing of the periapical rarefaction was noted. At the 54-month follow-up, osseous repair and a continuous lamina dura-like structure could be observed along with the extruded MTA (Figure 2e). Clinical examination revealed the absence of clinical signs and symptoms with adequate function.
Case 3
A 16-year-old female student was referred to the Samsung Medical Center for treatment of a large periapical pathosis associated with the left maxillary central incisor (tooth #21) in December 2006. She presented a history of trauma to this #21 tooth about 10 years ago. She complained of tenderness on palpation at the vestibular area. On examination, a sinus tract was noted between teeth #21 and #22 near the mucogingival junction. Periapical radiograph revealed an incompletely formed, open apex of tooth #21 with a large periapical lesion of 15 mm in diameter. The sinus tract was traced but could not differentiate the origin (Figure 3a). Electric pulp testing revealed that the #22 tooth was vital. A diagnosis of chronic apical abscess of tooth #21 was made and non-surgical re-treatment with MTA apexification was planned.
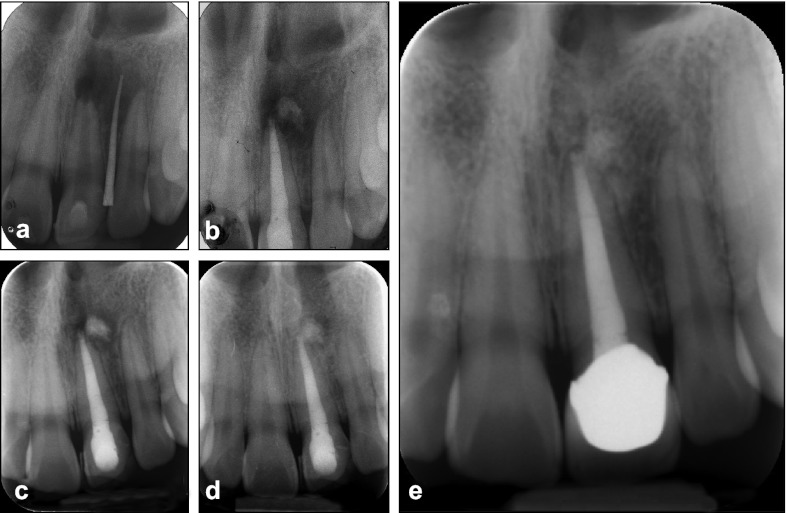
Figure 3.
Radiographs of case 3. (a) The preoperative diagnostic procedure with tracing of sinus tract. (b) The MTA extrusion into the periapical lesion during the one-step apexification. (c) 8-month recall check with gradual healing of periapical lesion. (d) 24-month recall check presenting irregular margin with radiolucent halo around extruded MTA during the osseous healing. (e) 48-month follow-up showing complete healing of periapical lesion and intermingled MTA with regenerated bone. MTA, mineral trioxide aggregate.
After isolating the tooth #21, access to its root canal was gained, and then the working length determined with the use of digital radiograph and electronic apex locator (Root-ZX; Morita, Tokyo, Japan). The canal was cleaned chemomechanically using hand and rotary instruments, irrigated with 5.25% NaClO and EDTA. The dimension of the apical foramen was larger than size 140. A water-based calcium hydroxide paste (Calcipex II; J. Morita, Osaka, Japan) was placed using lentulospirals to the working length as medicament.
One week later, the canal of this #21 tooth was copiously irrigated with 5% NaClO, dried with sterilized paper points and obturated with MTA (ProRoot MTA; Dentsply Tulsa Dental Specialities, Johnson City, TN, USA) as a one-step apexification, with a procedure described for in Case 1. Despite considerable care during MTA plugging, there was some extrusion of MTA into the periapical lesion (Figure 3b). The rest of the root canal was obturated with thermoplasticized injectable gutta-percha (Obtura II; Obtura-Spartan, Fenton, MO, USA) and the access cavity was restored with a composite resin. The patient was scheduled for follow-up. After 8 months (August 2007), the patient presented with no symptoms. Periapical radiograph showed decrease in size of the periapical radiolucency (Figure 3c). Thereafter, at 12-, 24- and 36-month recall, the patient was free from any signs and symptoms. There was a radiolucent halo with irregular margin around the extruded MTA on the 24-month recall radiograph (Figure 3d). The 48-month radiograph showed complete healing of the periapical lesion with the extruded MTA intermingled with regenerated bone with a periodontal ligament-like space between them (Figure 3e).
Discussion
Conservative approaches of apexogenesis or revascularization have been advocate for the treatment of vital immature teeth with apical periodontitis. Meanwhile, in the case of non-vital teeth with incompletely formed apex, one- or two-step MTA apical barrier technique can be introduced as an alternative to calcium hydroxide apexification. From a clinical perspective, MTA has a unique advantage for the treatment of immature teeth because one of the problems often found in those cases is the presence of tissue fluid or exudation into the canals.25 MTA is able to set in the presence of moisture. When it was used as an apical barrier, it can stimulate the cells to differentiate into hard tissue-forming cells to produce a hard tissue matrix. Masuda et al.26 reported that MTA does not produce any adverse effect on microcirculation of the connective tissues. Torabinejad et al.27 also demonstrated that MTA implanted into the animal bone resulted in minimal inflammatory reactions with favorable bone healing with direct bone apposition. However, these results from these animal studies, although favorable, cannot justify the any deliberate placement of MTA into the periapical tissue obturation material preferably should be confines within the root canal system, because extrusion can reduce decreased the chance of success of endodontic treatment.28 In an effort to prevent extrusion, practicing the placement of this material in artificial canals would be very helpful in developing clinical skills.
For the present case series, unfortunately, a considerable amount of MTA was extruded into the apical lesion during placement. It may be speculated that the MTA might be dislodged through the wide apical foramen or be pushed actively beyond apical foramen. The healing outcomes of these three cases are very similar. The extruded MTA seems to have been compressed and/or resorbed slightly and moved down (to gravitational direction) from original location. Regardless of the final location of the detached MTA, favorable healing around this material was observed. These healing responses were remarkably favorable when considering that the prognosis of overfilling with common root canal filling materials is generally poor.24,28 According to Holland et al.,28 there would be cell adhesion and differentiation with consequent deposition of hard tissue by the periapical tissue opposing the MTA, with biological closure of the apical foramen as well as absence of inflammation in the periapical tissues. The outcomes of these present cases mirrored the results from animal studies well.
A resorbable matix has been suggested for the easy length control and for prevention of overfilling.29 The use of calcium sulfate has been used as such an internal matrix.30 Collaplug (Zimmer Dental, Warsaw, IN, USA) was also used as an apical matrix; however, it did not appear to prevent extrusion or improve the sealing ability of MTA.31 The short-term application of Ca(OH)2 might bring about an additional benefit;9,11,21 however, its use prior to MTA apexification has been associated with extrusion of the MTA material.32 In this case series, we had used Ca(OH)2 as an intracanal medicament because the debridement of infected immature root can be difficult to achieve as a one-visit procedure and dressing with Ca(OH)2 would allow sufficient time to disinfect the root canal without significantly weakening of the root strength.33 Therefore, the apical extrusion of the MTA material might be related to the use of Ca(OH)2. Others have reported that the use of MTA in a one-visit apexification procedure (without Ca(OH)2 medication) was clinically effective.18 Nonetheless, the follow-up results of these patients indicated the clinical effectiveness of MTA as an apical plug via an orthograde approach, regardless of any internal matrix or medicament used.
For the present clinical observations, MTA favors the apexification and periapical healing even when a considerable amount of this material had inadvertently been extruded. While it is recognized that extrusion of MTA via an open apex is not a common mishap during the apexification procedure, the extruded material does not negatively affect the healing of the periapical tissues, as supported by follow-up observations of 36–54 months. If a complete closure of the root apex is considered the ideal goal to be achieved for the apexification procedure, this goal has been achieved in the present case series. However, deliberate overfilling of MTA into the periapical lesion is not advocated in any clinical circumstance. Revascularization procedure, as a further advanced procedure, is highly suggested for this kind of cases to get a biological healing including continuing of root formation without the risk of material extrusion.
Acknowledgments
This study was supported by the National Research Foundation (NRF) of Korea, funded by the Ministry of Education, Science and Technology (MEST) (No. 2011-0014231, Dr. Seok-Wood Chang), Korea.
References
- Chueh LH, Huang GT. Immature teeth with periradicular periodontitis or abscess undergoing apexogenesis: a paradigm shift. J Endod. 2006;32 12:1205–1213. doi: 10.1016/j.joen.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Jung IY, Lee SJ, Hargreaves KM. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. J Endod. 2008;34 7:876–887. doi: 10.1016/j.joen.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Ding RY, Cheung GS, Chen J, et al. Pulp revascularization of immature teeth with apical periodontitis: a clinical study. J Endod. 2009;35 5:745–749. doi: 10.1016/j.joen.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Witherspoon DE, Small JC, Harris GZ. Mineral trioxide aggregate pulpotomies: a case series outcomes assessment. J Am Dent Assoc. 2006;137 5:610–618. doi: 10.14219/jada.archive.2006.0256. [DOI] [PubMed] [Google Scholar]
- Rafter M. Apexification: a review. Dent Traumatol. 2005;21 1:1–8. doi: 10.1111/j.1600-9657.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- Citrome GP, Kaminski EJ, Heuer MA. A comparative study of tooth apexification in the dog. J Endod. 1979;5 10:290–297. doi: 10.1016/S0099-2399(79)80077-1. [DOI] [PubMed] [Google Scholar]
- Weisenseel JA, Jr, Hicks ML, Pelleu GB., Jr Calcium hydroxide as an apical barrier. J Endod. 1987;13 1:1–5. doi: 10.1016/S0099-2399(87)80084-5. [DOI] [PubMed] [Google Scholar]
- Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18 3:134–137. doi: 10.1034/j.1600-9657.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- Pace R, Giuliani V, Pini Prato L, et al. Apical plug technique using mineral trioxide aggregate: results from a case series. Int Endod J. 2007;40 6:478–484. doi: 10.1111/j.1365-2591.2007.01240.x. [DOI] [PubMed] [Google Scholar]
- Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25 3:197–205. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- Holden DT, Schwartz SA, Kirkpatrick TC, et al. Clinical outcomes of artificial root-end barriers with mineral trioxide aggregate in teeth with immature apices. J Endod. 2008;34 7:812–817. doi: 10.1016/j.joen.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Steinig TH, Regan JD, Gutmann JL. The use and predictable placement of mineral trioxide aggregate in one-visit apexification cases. Aust Endod J. 2003;29 1:34–42. doi: 10.1111/j.1747-4477.2003.tb00496.x. [DOI] [PubMed] [Google Scholar]
- Matt GD, Thorpe JR, Strother JM, et al. Comparative study of white and gray mineral trioxide aggregate (MTA) simulating a one- or two-step apical barrier technique. J Endod. 2004;30 12:876–879. doi: 10.1097/01.don.0000136213.93171.45. [DOI] [PubMed] [Google Scholar]
- Wen PH, Liou JU, Duh BR. Apexification of nonvital immature mandibular premolars using two different techniques. J Dent Sci. 2009;4 2:96–101. [Google Scholar]
- Hayashi M, Shimizu A, Ebisu S. MTA for obturation of mandibular central incisors with open apices: case report. J Endod. 2004;30 2:120–122. doi: 10.1097/00004770-200402000-00015. [DOI] [PubMed] [Google Scholar]
- Hatibovic-Kofman S, Raimundo L, Chong L, et al. Mineral trioxide aggregate in endodontic treatment for immature teeth. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2094–2097. doi: 10.1109/IEMBS.2006.259851. [DOI] [PubMed] [Google Scholar]
- Bogen G, Kuttler S. Mineral trioxide aggregate obturation: a review and case series. J Endod. 2009;35 6:777–790. doi: 10.1016/j.joen.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Witherspoon DE, Ham K. One-visit apexification: technique for inducing root-end barrier formation in apical closures. Pract Proced Aesthet Dent. 2001;13 6:455–460. [PubMed] [Google Scholar]
- Yeung P, Liewehr FR, Moon PC. A quantitative comparison of the fill density of MTA produced by two placement techniques. J Endod. 2006;32 5:456–459. doi: 10.1016/j.joen.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Hachmeister DR, Schindler WG, Walker WA, III, et al. The sealing ability and retention characteristics of mineral trioxide aggregate in a model of apexification. J Endod. 2002;28 5:386–390. doi: 10.1097/00004770-200205000-00010. [DOI] [PubMed] [Google Scholar]
- Giuliani V, Baccetti T, Pace R, et al. The use of MTA in teeth with necrotic pulps and open apices. Dent Traumatol. 2002;18 4:217–221. doi: 10.1034/j.1600-9657.2002.02107.x. [DOI] [PubMed] [Google Scholar]
- Maroto M, Barberia E, Planells P, et al. Treatment of a non-vital immature incisior with mineral trioxide aggregate (MTA) Dent Traumatol. 2003;19 3:165–169. doi: 10.1034/j.1600-9657.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo C, D'Amario M. Use of MTA for orthograde obturation of nonvital teeth with open apices: report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104 4:e98–e101. doi: 10.1016/j.tripleo.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Shabahang S, Torabinejad M, Boyne PP, et al. A comparative study of root-end induction using osteogenic protein-1, calcium hydroxide, and mineral trioxide aggregate in dogs. J Endod. 1999;25 1:1–5. doi: 10.1016/S0099-2399(99)80388-4. [DOI] [PubMed] [Google Scholar]
- Funteas UR, Wallace JA, Fochtman EW. A comparative analysis of mineral trioxide aggregate and Portland cement. Aust Endod J. 2003;29 1:43–44. doi: 10.1111/j.1747-4477.2003.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Masuda YM, Wang X, Hossain M, et al. Evaluation of biocompatibility of mineral trioxide aggregate with an improved rabbit ear chamber. J Oral Rehabil. 2005;32 2:145–150. doi: 10.1111/j.1365-2842.2004.01397.x. [DOI] [PubMed] [Google Scholar]
- Torabinejad M, Ford TR, Abedi HR, et al. Tissue reaction to implanted root-end filling materials in the tibia and mandible of guinea pigs. J Endod. 1998;24 7:468–471. doi: 10.1016/s0099-2399(98)80048-4. [DOI] [PubMed] [Google Scholar]
- Holland R, Mazuqueli L, de Souza V, et al. Influence of the type of vehicle and limit of obturation on apical and periapical tissue response in dogs' teeth after root canal filling with mineral trioxide aggregate. J Endod. 2007;33 6:693–697. doi: 10.1016/j.joen.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Lemon RR. Nonsurgical repair of perforation defects. Internal matrix concept. Dent Clin North Am. 1992;36 2:439–457. [PubMed] [Google Scholar]
- Al-Daafas A, Al-Nazhan S. Histological evaluation of contaminated furcal perforation in dogs' teeth repaired by MTA with or without internal matrix. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103 3:e92–e99. doi: 10.1016/j.tripleo.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Zou L, Liu J, Yin S, et al. In vitro evaluation of the sealing ability of MTA used for the repair of furcation perforations with and without the use of an internal matrix. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105 6:e61–e65. doi: 10.1016/j.tripleo.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Felippe WT, Felippe MC, Rocha MJ. The effect of mineral trioxide aggregate on the apexification and periapical healing of teeth with incomplete root formation. Int Endod J. 2006;39 1:2–9. doi: 10.1111/j.1365-2591.2005.01037.x. [DOI] [PubMed] [Google Scholar]
- Simon S, Rilliard F, Berdal A, et al. The use of mineral trioxide aggregate in one-visit apexification treatment: a prospective study. Int Endod J. 2007;40 3:186–197. doi: 10.1111/j.1365-2591.2007.01214.x. [DOI] [PubMed] [Google Scholar]