On the basis of the reported accuracies of various imaging methods and taking into account cost, this multidisciplinary consensus panel has proposed algorithms for use in the imaging evaluation of the rotator cuff in patients with a native rotator cuff, those with a repaired rotator cuff, and those who have undergone shoulder replacement.
Abstract
The Society of Radiologists in Ultrasound convened a panel of specialists from a variety of medical disciplines to reach a consensus about the recommended imaging evaluation of painful shoulders with clinically suspected rotator cuff disease. The panel met in Chicago, Ill, on October 18 and 19, 2011, and created this consensus statement regarding the roles of radiography, ultrasonography (US), computed tomography (CT), CT arthrography, magnetic resonance (MR) imaging, and MR arthrography. The consensus panel consisted of two co-moderators, a facilitator, a statistician and health care economist, and 10 physicians who have specialty expertise in shoulder pain evaluation and/or treatment. Of the 13 physicians on the panel, nine were radiologists who were chosen to represent a broad range of skill sets in diagnostic imaging, different practice types (private and academic), and different geographical regions of the United States. Five of the radiologists routinely performed musculoskeletal US as part of their practice and four did not. There was also one representative from each of the following clinical specialties: rheumatology, physical medicine and rehabilitation, orthopedic surgery, and nonoperative sports medicine. The goal of this conference was to construct several algorithms with which to guide the imaging evaluation of suspected rotator cuff disease in patients with a native rotator cuff, patients with a repaired rotator cuff, and patients who have undergone shoulder replacement. The panel hopes that these recommendations will lead to greater uniformity in rotator cuff imaging and more cost-effective care for patients suspected of having rotator cuff abnormality.
© RSNA, 2013
Introduction
Shoulder pain has a self-reported prevalence in the general population of between 16% and 26% and is the third most common musculoskeletal symptom for which patients seek medical attention, trailing only low back pain and knee pain (1,2). The most common causes of shoulder pain include rotator cuff disease (defined as tendinosis and/or tear), frozen shoulder, instability, and osteoarthritis. Rotator cuff disease is the most prevalent cause of shoulder pain, occurring in approximately 65%–70% of patients (3). The prevalence of rotator cuff disease increases with age, and it is estimated that, by the age of 70 years, more than 50% of the population will have a full- or partial-thickness rotator cuff tear, although not always symptomatic (4). Early diagnosis of rotator cuff tears is important because untreated tears may enlarge, cause increased pain (5), and lead to irreversible fatty degeneration and atrophy of the shoulder musculature (6). Once these muscle changes occur, the risk of a recurrent tear after surgical repair is substantial and has been reported to be as high as 94% (7,8). In addition, larger and retracted tears can be technically difficult to repair, and intrinsic changes in tendon properties may preclude an anatomic repair to the footprint (9,10).
There is a wealth of literature evaluating imaging techniques that can be used to detect rotator cuff abnormality. These techniques include radiography (11), ultrasonography (US) (12), magnetic resonance (MR) imaging (12), MR arthrography (13), and computed tomographic (CT) arthrography (14). Nevertheless, the choice of imaging test is often based on individual physician preference rather than scientific evidence. The American College of Radiology has developed appropriateness criteria that rate the relative usefulness of various imaging modalities for the evaluation of shoulder pain in different clinical scenarios (15). Although these criteria are informative, they do not define an algorithmic approach to the imaging evaluation of suspected rotator cuff disease.
The Society of Radiologists in Ultrasound convened a panel of specialists from a variety of medical disciplines to reach a consensus about the recommended imaging evaluation of painful shoulders with clinically suspected rotator cuff disease. The panel met in Chicago, Ill, on October 18 and 19, 2011, and created this consensus statement. The goal of this conference was to construct several algorithms with which to guide the imaging evaluation of common clinical scenarios where rotator cuff disease is suspected. The panel believes that such algorithms would help guide practicing physicians, improve patient care, and potentially reduce unnecessary imaging costs.
Imaging of the Shoulder
Radiography is routinely used in the evaluation of the rotator cuff because imaging findings may indirectly suggest rotator cuff disease or provide additional information important to clinical management. For example, cortical irregularity of the greater tuberosity at the attachment site of the supraspinatus indirectly indicates the presence of a rotator cuff tear with a sensitivity of 90% and a negative predictive value of 96% (16). Other information gained from radiography includes the presence of a subacromial enthesophyte or acromioclavicular osteophyte, which can cause cuff impingement. Calcification of the rotator cuff and, possibly, the adjacent bursa can also be visualized. Superior migration of the humeral head with narrowing of the acromiohumeral distance is an important radiographic finding that is indicative of the presence of a large or massive rotator cuff tear that has disrupted the force couples of the glenohumeral joint. Goutallier et al (17) reported that an acromiohumeral distance of less than 6 mm was almost always associated with a full-thickness chronic infraspinatus tear and, therefore, surgical repair is not always amenable owing to poor quality of the cuff and advanced fatty degeneration. Conversely, an acromiohumeral distance of 6 mm or more was of no diagnostic relevance (17). Other studies have also reported that the presence of cephalad migration of the humeral head is indicative of a chronic rotator cuff tear that cannot be surgically repaired (18). Other potential radiographic findings include os acromiale, fracture, osteoarthrosis of the acromioclavicular or glenohumeral joint, and, less commonly, other bone abnormality such as malignancy.
CT has a limited role in the setting of suspected rotator cuff disease but can be used to evaluate muscle atrophy and fatty degeneration; however, grading of such findings can be unreliable (19,20). CT after the intraarticular injection of iodinated contrast material (CT arthrography) may be used to evaluate the intraarticular structures (eg, labrum) and rotator cuff tears that communicate with the articular surface (21). Because MR arthrography can provide more information than CT arthrography about interstitial and bursal-sided tears and does not use ionizing radiation, the use of CT arthrography is not common in the evaluation of the rotator cuff. CT arthrography may have a more important role after surgery because metal anchors and their associated artifacts may compromise the diagnostic value of MR images.
MR imaging is an effective imaging method for evaluating the rotator cuff. With MR imaging, a full-thickness rotator cuff tear can be diagnosed with 92.1% sensitivity and 92.9% specificity, whereas a partial-thickness tear can be diagnosed with 63.6% sensitivity and 91.7% specificity (12). Other important information about the rotator cuff obtained with MR imaging is the presence of muscle fatty degeneration and atrophy, which is associated with poor outcome after rotator cuff repair, although grading of fatty degeneration can be unreliable (20,22). Advantages of the use of MR imaging include a global assessment of all shoulder structures, including cartilage and bone marrow, whereas disadvantages relate to patient issues (claustrophobia and contraindications owing to certain metallic implants and electronic devices), cost, and accessibility. MR imaging after intraarticular administration of contrast material (MR arthrography) can also be used to assess the rotator cuff, with 95.4% sensitivity and 98.9% specificity for the diagnosis of full-thickness tear and 85.9% sensitivity and 96% specificity for the diagnosis of partial-thickness tear (12). The use of intraarticular contrast material with MR imaging is ideal for the evaluation of an intraarticular abnormality related to labrum and cartilage (23).
US evaluation of the rotator cuff was introduced in 1977 (24) and has become more widespread with advances in technology, increased portability, and decreased costs (25).With proper training, high accuracies in the diagnosis of rotator cuff disease can be achieved. A full-thickness rotator cuff tear can be diagnosed with 92.3% sensitivity and 94.4% specificity by using US, whereas a partial-thickness tear can be diagnosed with 66.7% sensitivity and 93.5% specificity (12). US can also help diagnose rotator cuff muscle fatty degeneration and atrophy (6,26,27). Advantages of US include portability, low cost, and lack of contraindications, whereas disadvantages relate to limited assessment of the capsule, labrum, and cartilage as well as the inability to evaluate purely intraosseous abnormalities. The spatial resolution of images obtained with US is higher than that of images obtained with routine MR imaging (28), and patients prefer US examination to MR imaging (29).
With US, both image acquisition and image interpretation tend to be dependent on the skill of the interpreting physician. With the other imaging modalities, however, the greater operator dependence occurs during image interpretation. With regard to US evaluation of the rotator cuff, low interobserver variability has been demonstrated, although variability is higher in the diagnosis of partial-thickness tears (30,31). Operator dependence also exists with MR imaging, where interobserver variability has been reported as moderate; poor agreement is reported for partial-thickness tears (32,33). Defined imaging techniques and protocols are important to minimize such operator dependence, especially with US. Although a review of shoulder imaging protocols is beyond the scope of this article, it is essential that a comprehensive imaging protocol be followed for both US (34) and MR imaging (35). Regardless of whether images of the rotator cuff are obtained with US or MR imaging, the goals are the same—namely to obtain high-spatial-resolution images of the rotator cuff in the short and long axis in such a way as to avoid artifacts that would limit interpretation.
Methods and Conference Preparations
The Society of Radiologists in Ultrasound consensus panel consisted of two co-moderators (J.A.J., L.N.N.), a facilitator (C.B.B.), a statistician and health care economist (L.P.), and 10 physicians who have specialty expertise in shoulder pain evaluation and/or treatment. Of the 13 physicians on the panel, nine were radiologists who were chosen to represent a broad range of skill sets in diagnostic imaging, different practice types (private and academic), and different geographical regions of the United States. Five of the radiologists routinely performed musculoskeletal US as part of their practice (J.A.J., L.N.N., C.B.B., T.T.M., and S.A.T.), and four were musculoskeletal radiologists who did not routinely perform US (L.W.B., L.S.S., M.J.T., and J.N.W.). There was also one representative from each of the following clinical specialties: rheumatology (R.G.T.), physical medicine and rehabilitation (J.S.), orthopedic surgery (K.Y.), and nonoperative sports medicine (J.M.M.). Several months before the conference the moderators assigned speaking topics to nine of the panelists, and these speakers provided outlines and references for their presentations to the other panelists ahead of time. A second orthopedic surgeon (A.B.) was recruited for additional input during manuscript preparation.
Role of the Moderators and Facilitator
The moderators designed the schedule for the consensus conference and invited the speakers. During the conference, the moderators introduced the formal presentations, led the panel discussions, and created the algorithms from a laptop projected onto a video screen while receiving the panelists’ input. The facilitator took comprehensive notes during the presentations for later reference during algorithm creation and helped focus the panel discussions.
Formal Presentations
Formal presentations were given in front of the entire panel. The length of the presentations ranged from 20 to 45 minutes. The nine formal presentations and their authors were as follows: “Shoulder Pain: What the Surgeon Wants to Know from Imaging” (K.Y.), “Shoulder Pain: What the Nonoperative Sports Physician Wants to Know” (J.S.), “Shoulder Pain: What the Rheumatologist Wants to Know” (R.G.T.), “Routine Shoulder Radiography and Conventional Arthrography” (M.J.T.), “Shoulder CT” (L.W.B.), “Shoulder MR Imaging/MR Arthrography” (L.S.S.), “Shoulder US” (S.A.T.), “Economic Considerations” (L.P.), and “American College of Radiology Appropriateness Criteria for Shoulder Imaging” (J.N.W.).
Panel Discussions
Panel discussions were scheduled throughout the day to mirror the preceding presentations. These discussions were an open exchange of ideas among the panelists. The specific topics of discussion were as follows: imaging algorithm for shoulder pain from the clinicians’ perspective; relative roles of shoulder MR imaging, CT, and US for shoulder pain; and diagnostic algorithm in context of American College of Radiology Appropriateness Criteria
Imaging Algorithms
The algorithms were developed gradually throughout the panel discussions and were finalized during a 4-hour discussion session on the 2nd day of the conference. No algorithm was finalized unless all of the panelists agreed with every branch of the algorithm. If one of the panelists objected to a portion of the algorithm, it was revised until all panelists were in agreement. Minor changes were also incorporated by consensus during the drafting of the manuscript until all panelists signed off on the final version of the manuscript.
When developing the imaging algorithms, the panelists agreed on the following statements: (a) The need for imaging should be determined by a history and physical examination performed by a competent clinician. (b) It is assumed that image acquisition and interpretation are completed only by qualified individuals with the appropriate expertise. The panel recognizes that not all practitioners will have expertise in all of the modalities. For example, in the United States there are prominent musculoskeletal radiologists who do not read shoulder radiographs, do not read MR images, do not perform MR or CT arthrography, and/or do not perform shoulder US. The algorithms are therefore based on a “best case” scenario. (c) When imaging tests are judged equally valid in diagnostic accuracy, the algorithm should include the test of lower cost and/or exposure to ionizing radiation.
The panelists agreed that three separate imaging algorithms were needed to address different patient populations: one for the native shoulder (Fig 1), one for a shoulder that has undergone rotator cuff repair (Fig 2), and one for a shoulder that has undergone glenohumeral joint replacement (Fig 3). Each algorithm would begin with a patient suspected of having a rotator abnormality and would end with a positive diagnosis, related either to the rotator cuff or to another abnormality.
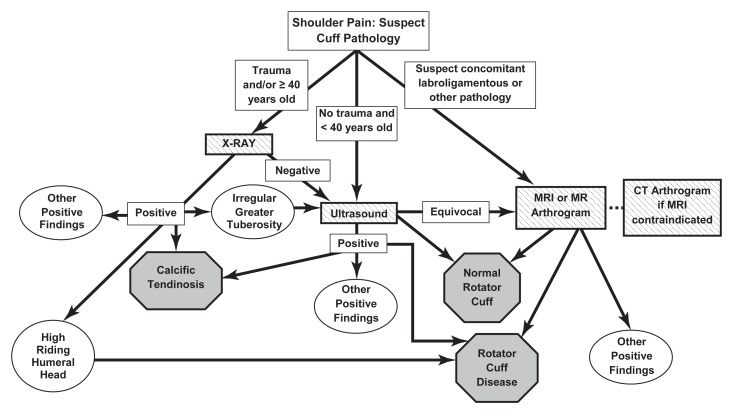
Figure 1:
Diagnostic algorithm for painful native shoulders suspected of having rotator cuff abnormality. Endpoints for algorithm include normal rotator cuff, rotator cuff abnormality, or other positive findings, which include, but are not limited to, osteoarthritis, fractures, bone tumors, or labroligamentous abnormality.
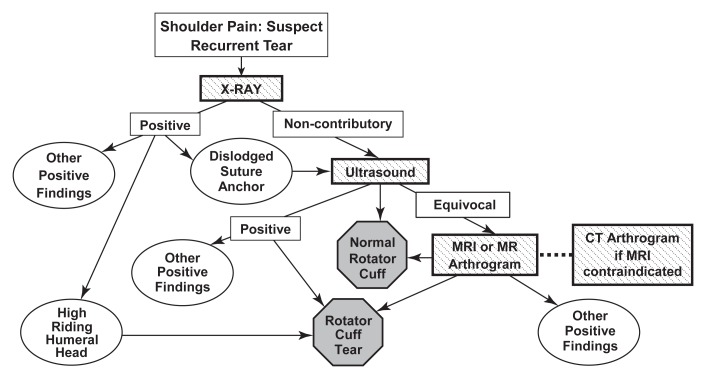
Figure 2:
Diagnostic algorithm for painful shoulders with history of rotator cuff repair suspected of having recurrent rotator cuff abnormality.
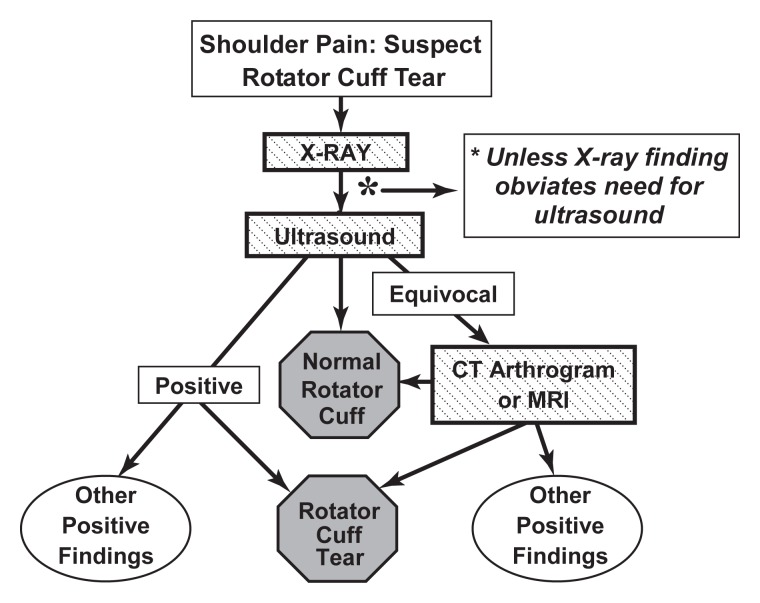
Figure 3:
Diagnostic algorithm for painful shoulders suspected of having rotator cuff abnormality after arthroplasty.
Native Shoulder
The algorithm for native shoulders is shown in Figure 1. Panelists first addressed when radiography was indicated in patients suspected of having a rotator cuff abnormality. Although it was agreed among the entire panel that a radiograph could potentially be helpful in all age groups in the evaluation of suspected rotator cuff abnormality, the panel believed that such a study was of low yield in patients younger than 40 years if there was no history of trauma. Therefore, the consensus was to perform radiography as the first test only in patients with a history of acute trauma and/or those who are at least 40 years of age. If the radiograph demonstrates calcific tendinosis and if the clinical physician believes that the calcification is causing the symptoms, then treatments such as US-guided needle lavage and aspiration and subacromial-subdeltoid bursa corticosteroid injection can be considered (36). If superior migration of the humeral head is present with an acromiohumeral distance of less than 6 mm, then the findings are diagnostic of rotator cuff tear (37). If the radiograph demonstrates cortical irregularity of the greater tuberosity, then further evaluation is warranted given its association with rotator cuff tears (11). If the radiograph shows a fracture, dislocation, or other non–rotator cuff abnormality, then the patient can be treated appropriately.
On the basis of the literature, most notably the meta-analysis by de Jesus et al (12), the panelists agreed that US should be the next examination in the evaluation of suspected rotator cuff abnormality. This decision was based on the nearly equivalent accuracy of US and MR imaging for full- and partial-thickness tears (12) combined with the fact that US has a lower cost (38), greater patient acceptance (29), and no contraindications. The panel also agreed that US can provide information concerning fatty degeneration and atrophy of the rotator cuff musculature (6,26,27). A discussion arose over whether the greater field of view afforded by MR imaging gives a better “road map” to the shoulder surgeon, especially in the setting of a torn rotator cuff with retraction beneath the acromion. The panel agreed that, in most cases, US provides accurate information about tear size and location such that the patient will get the appropriate surgical treatment and that the need to obtain additional MR images would be at the discretion of the treating physician (39).
If the US findings are diagnostic for a normal rotator cuff, rotator cuff disease, or other positive findings such as impingement, long head of the biceps brachii abnormality, or subacromial-subdeltoid bursal abnormality that explain the patient’s pain, then the patient work-up is complete. If, however, the US findings do not explain the cause of pain, then MR imaging or MR arthrography should be performed next. CT arthrography should be performed if the patient has a contraindication to MR imaging or a history of surgery that could cause substantial metallic artifact at MR imaging. The panelists noted that although MR arthrography has been shown in a meta-analysis to be more sensitive and specific than either US or MR imaging for rotator cuff tears (12), the procedure is invasive because it requires intraarticular injection of a gadolinium chelate. Because the panel agreed that neither routine MR imaging nor MR arthrography has a clear advantage over the other test in all situations, the panel agreed that one or the other could be ordered on the basis of referring physician and/or radiologist preference. MR arthrography would be preferred in a patient with shoulder instability, where detailed assessment of the labrum and labroligamentous structures is required. MR imaging or MR arthrography findings could include normal rotator cuff, rotator cuff abnormality, or other abnormality such as labral tear, fracture, or bone tumor—any of which complete the diagnostic imaging evaluation.
In patients younger than 40 years who have no history of trauma, the consensus was to begin the imaging evaluation with US if there is clinical suspicion of isolated rotator cuff disease, although it is acknowledged that it may be difficult to differentiate between isolated rotator cuff injuries and rotator injuries associated with other shoulder injuries at clinical examination. MR imaging or MR arthrography (or CT arthrography if there is a contraindication to MR imaging) should be used if there is suspicion of rotator cuff abnormality and concomitant labroligamentous or other glenohumeral abnormality (ie, cartilage injury, intraarticular bodies). The rationale for going directly to MR imaging or MR arthrography in the latter situation is that MR imaging and MR arthrography have been shown to be highly sensitive for labroligamentous abnormality, including tears (40,41). Because there are insufficient data regarding the sensitivity of US for diagnosing labroligamentous abnormality (42), the consensus of the panel is that US examination should not be used for suspected capsular or labral injuries.
Shoulder with Previous Rotator Cuff Repair
The algorithm for shoulders that have previously undergone rotator cuff repair and in which there is clinical suspicion of either a recurrent or new rotator cuff tear is shown in Figure 2. Panelists agreed that radiography would be the initial imaging method of choice. Superior migration of the humeral head may be suggestive of a large recurrent rotator cuff tear, whereas a dislodged suture anchor should be followed by US evaluation of the rotator cuff. If the initial radiograph is noncontributory, US would be the next test because it has been shown to be highly accurate in the evaluation of the postoperative shoulder (43). An equivocal US scan of the rotator cuff would prompt MR imaging or MR arthrography (44)—with CT arthrography performed if there is a contraindication to MR imaging.
Shoulder after Arthroplasty
The algorithm for a patient suspected of having rotator cuff abnormality after shoulder replacement surgery is shown in Figure 3. Panelists agreed that patients with shoulder pain after arthroplasty should undergo both radiography and US in all circumstances unless an obvious radiographic finding such as a fracture or prosthetic loosening obviates the need for further imaging (45). A review of serial radiographs postoperatively is also crucial to the differentiation of stable radiolucent lines from findings of loosening, implant subsidence, or osteolysis. US has been shown to be very accurate in the evaluation of the rotator cuff in patients who have undergone arthroplasty (45,46). Only if the US scan is equivocal would another test be necessary. In such cases, the panel favored either CT arthrography or MR imaging performed with techniques to suppress the artifacts caused by the metallic prosthesis.
Recommendations for Future Research
The panel discussion emphasized the need for future research studies, including the need to (a) implement the proposed algorithms prospectively to determine their utility and practicality (it would be important to assess both patient outcomes and economic impact); (b) develop an economic model comparing US and either MR imaging or MR arthrography in patients suspected of having rotator cuff tear, taking into account patients who might require both imaging studies as part of their diagnostic imaging evaluation; (c) study the effectiveness of various US techniques to image rotator cuff tendons and muscles (eg, three-dimensional and extended field of view imaging, contrast media, color Doppler, and elastography); and (d) investigate how artifact suppression techniques for MR imaging may improve the performance of MR imaging in the evaluation of the postoperative rotator cuff.
In conclusion, on the basis of reported accuracies of various imaging methods and taking into account cost, this multidisciplinary consensus panel has proposed algorithms for use in the imaging evaluation of the rotator cuff in patients with a native rotator cuff, those with a repaired rotator cuff, and those who have undergone shoulder replacement. The panel hopes that these recommendations will lead to greater uniformity in rotator cuff imaging and more cost-effective care for patients suspected of having rotator cuff abnormality.
Disclosures of Conflicts of Interest: L.N.N. No relevant conflicts of interest to disclose. J.J.J. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a paid consultant for Bioclinica; receives royalties from Elsevier. Other relationships: none to disclose. C.B.B. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: received payment for expert testimony from various law firms and malpractice insurance companies; receives royalties from Thieme and Lippincott, Williams & Wilkins. Other relationships: none to disclose. L.W.B. No relevant conflicts of interest to disclose. A.B. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a paid consultant for Smith & Nephew. Other relationships: none to disclose. J.M.M. No relevant conflicts of interest to disclose. T.T.M. No relevant conflicts of interest to disclose. L.P. No relevant conflicts of interest to disclose. J.S. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a paid consultant for Tenex Health; institution receives money for consultancy from Tenex Health; receives payment for patents from Tenex Health; institution receives payment for patents from Tenex Health; receives royalties from Tenex Health; institution receives royalties from Tenex Health; has stock/stock options in Tenex Health; institution has stock/stock options in Tenex Health. Other relationships: none to disclose. L.S.S. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: receives payment for expert testimony from various law firms; receives royalties from Lippincott Williams & Wilkins. Other relationships: none to disclose. S.A.T. No relevant conflicts of interest to disclose. R.G.T. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: receives payment for lectures including service on speakers bureaus from Amgen and Abbott. Other relationships: received equipment support from SonoSite. M.J.T. No relevant conflicts of interest to disclose. J.N.W. No relevant conflicts of interest to disclose. K.Y. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: receives royalties from Tornier and Zimmer. Other relationships: none to disclose.
Received August 29, 2012; revision requested October 16; revision received November 1; final version accepted December 12.
Funding: This research was supported by the National Institutes of Health (grant RO1AR051026).
References
- 1.Mitchell C, Adebajo A, Hay E, Carr A. Shoulder pain: diagnosis and management in primary care. BMJ 2005;331(7525):1124–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urwin M, Symmons D, Allison T, et al. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann Rheum Dis 1998;57(11):649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanahan EM, Sladek R. Shoulder pain at the workplace. Best Pract Res Clin Rheumatol 2011;25(1):59–68 [DOI] [PubMed] [Google Scholar]
- 4.Milgrom C, Schaffler M, Gilbert S, van Holsbeeck M. Rotator-cuff changes in asymptomatic adults: the effect of age, hand dominance and gender. J Bone Joint Surg Br 1995;77(2):296–298 [PubMed] [Google Scholar]
- 5.Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am 2010;92(16):2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HM, Dahiya N, Teefey SA, Keener JD, Galatz LM, Yamaguchi K. Relationship of tear size and location to fatty degeneration of the rotator cuff. J Bone Joint Surg Am 2010;92(4):829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med 2007;35(5):719–728 [DOI] [PubMed] [Google Scholar]
- 8.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am 2004;86-A(2):219–224 [DOI] [PubMed] [Google Scholar]
- 9.Jensen KL, Williams GR, Jr, Russell IJ, Rockwood CA., Jr Rotator cuff tear arthropathy. J Bone Joint Surg Am 1999;81(9):1312–1324 [DOI] [PubMed] [Google Scholar]
- 10.Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am 2004;86-A(Suppl 2):35–40 [PubMed] [Google Scholar]
- 11.Umans HR, Pavlov H, Berkowitz M, Warren RF. Correlation of radiographic and arthroscopic findings with rotator cuff tears and degenerative joint disease. J Shoulder Elbow Surg 2001;10(5):428–433 [DOI] [PubMed] [Google Scholar]
- 12.de Jesus JO, Parker L, Frangos AJ, Nazarian LN. Accuracy of MRI, MR arthrography, and ultrasound in the diagnosis of rotator cuff tears: a meta-analysis. AJR Am J Roentgenol 2009;192(6):1701–1707 [DOI] [PubMed] [Google Scholar]
- 13.Waldt S, Bruegel M, Mueller D, et al. Rotator cuff tears: assessment with MR arthrography in 275 patients with arthroscopic correlation. Eur Radiol 2007;17(2):491–498 [DOI] [PubMed] [Google Scholar]
- 14.Charousset C, Bellaïche L, Duranthon LD, Grimberg J. Accuracy of CT arthrography in the assessment of tears of the rotator cuff. J Bone Joint Surg Br 2005;87(6):824–828 [DOI] [PubMed] [Google Scholar]
- 15.Wise JN, Daffner RH, Weissman BN, et al. ACR appropriateness criteria on acute shoulder pain. J Am Coll Radiol 2011;8(9):602–609 [DOI] [PubMed] [Google Scholar]
- 16.Wohlwend JR, van Holsbeeck M, Craig J, et al. The association between irregular greater tuberosities and rotator cuff tears: a sonographic study. AJR Am J Roentgenol 1998;171(1):229–233 [DOI] [PubMed] [Google Scholar]
- 17.Goutallier D, Le Guilloux P, Postel JM, Radier C, Bernageau J, Zilber S. Acromio humeral distance less than six millimeters: its meaning in full-thickness rotator cuff tear. Orthop Traumatol Surg Res 2011;97(3):246–251 [DOI] [PubMed] [Google Scholar]
- 18.Gerber C, Wirth SH, Farshad M. Treatment options for massive rotator cuff tears. J Shoulder Elbow Surg 2011;20(2, Suppl):S20–S29 [DOI] [PubMed] [Google Scholar]
- 19.Williams MD, Lädermann A, Melis B, Barthelemy R, Walch G. Fatty infiltration of the supraspinatus: a reliability study. J Shoulder Elbow Surg 2009;18(4):581–587 [DOI] [PubMed] [Google Scholar]
- 20.Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res 2010;468(6):1558–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecouvet FE, Simoni P, Koutaïssoff S, Vande Berg BC, Malghem J, Dubuc JE. Multidetector spiral CT arthrography of the shoulder: clinical applications and limits, with MR arthrography and arthroscopic correlations. Eur J Radiol 2008;68(1):120–136 [DOI] [PubMed] [Google Scholar]
- 22.Lippe J, Spang JT, Leger RR, Arciero RA, Mazzocca AD, Shea KP. Inter-rater agreement of the Goutallier, Patte, and Warner classification scores using preoperative magnetic resonance imaging in patients with rotator cuff tears. Arthroscopy 2012;28(2):154–159 [DOI] [PubMed] [Google Scholar]
- 23.Sanders TG, Zlatkin M, Montgomery J. Imaging of glenohumeral instability. Semin Roentgenol 2010;45(3):160–179 [DOI] [PubMed] [Google Scholar]
- 24.Mayer V. Ultrasonography of the rotator cuff. J Ultrasound Med 1985;4(11):608, 607. [DOI] [PubMed] [Google Scholar]
- 25.Beggs I. Shoulder ultrasound. Semin Ultrasound CT MR 2011;32(2):101–113 [DOI] [PubMed] [Google Scholar]
- 26.Kavanagh EC, Koulouris G, Parker L, et al. Does extended-field-of-view sonography improve interrater reliability for the detection of rotator cuff muscle atrophy? AJR Am J Roentgenol 2008;190(1):27–31 [DOI] [PubMed] [Google Scholar]
- 27.Khoury V, Cardinal E, Brassard P. Atrophy and fatty infiltration of the supraspinatus muscle: sonography versus MRI. AJR Am J Roentgenol 2008;190(4):1105–1111 [DOI] [PubMed] [Google Scholar]
- 28.Erickson SJ. High-resolution imaging of the musculoskeletal system. Radiology 1997;205(3):593–618 [DOI] [PubMed] [Google Scholar]
- 29.Middleton WD, Payne WT, Teefey SA, Hildebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol 2004;183(5):1449–1452 [DOI] [PubMed] [Google Scholar]
- 30.Le Corroller T, Cohen M, Aswad R, Pauly V, Champsaur P. Sonography of the painful shoulder: role of the operator’s experience. Skeletal Radiol 2008;37(11):979–986 [DOI] [PubMed] [Google Scholar]
- 31.Middleton WD, Teefey SA, Yamaguchi K. Sonography of the rotator cuff: analysis of interobserver variability. AJR Am J Roentgenol 2004;183(5):1465–1468 [DOI] [PubMed] [Google Scholar]
- 32.Theodoropoulos JS, Andreisek G, Harvey EJ, Wolin P. Magnetic resonance imaging and magnetic resonance arthrography of the shoulder: dependence on the level of training of the performing radiologist for diagnostic accuracy. Skeletal Radiol 2010;39(7):661–667 [DOI] [PubMed] [Google Scholar]
- 33.Robertson PL, Schweitzer ME, Mitchell DG, et al. Rotator cuff disorders: interobserver and intraobserver variation in diagnosis with MR imaging. Radiology 1995;194(3):831–835 [DOI] [PubMed] [Google Scholar]
- 34.Jacobson JA. Shoulder US: anatomy, technique, and scanning pitfalls. Radiology 2011;260(1):6–16 [DOI] [PubMed] [Google Scholar]
- 35.Opsha O, Malik A, Baltazar R, et al. MRI of the rotator cuff and internal derangement. Eur J Radiol 2008;68(1):36–56 [DOI] [PubMed] [Google Scholar]
- 36.Lee KS, Rosas HG. Musculoskeletal ultrasound: how to treat calcific tendinitis of the rotator cuff by ultrasound-guided single-needle lavage technique. AJR Am J Roentgenol 2010;195(3):638. [DOI] [PubMed] [Google Scholar]
- 37.Moosikasuwan JB, Miller TT, Burke BJ. Rotator cuff tears: clinical, radiographic, and US findings. RadioGraphics 2005;25(6):1591–1607 [DOI] [PubMed] [Google Scholar]
- 38.Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal imaging: medicare use, costs, and potential for cost substitution. J Am Coll Radiol 2008;5(3):182–188 [DOI] [PubMed] [Google Scholar]
- 39.Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears: comparison of ultrasonographic, magnetic resonance imaging, and arthroscopic findings in seventy-one consecutive cases. J Bone Joint Surg Am 2004;86-A(4):708–716 [PubMed] [Google Scholar]
- 40.Magee TH, Williams D. Sensitivity and specificity in detection of labral tears with 3.0-T MRI of the shoulder. AJR Am J Roentgenol 2006;187(6):1448–1452 [DOI] [PubMed] [Google Scholar]
- 41.Major NM, Browne J, Domzalski T, Cothran RL, Helms CA. Evaluation of the glenoid labrum with 3-T MRI: is intraarticular contrast necessary? AJR Am J Roentgenol 2011;196(5):1139–1144 [DOI] [PubMed] [Google Scholar]
- 42.Hammar MV, Wintzell GB, Aström KG, Larsson S, Elvin A. Role of US in the preoperative evaluation of patients with anterior shoulder instability. Radiology 2001;219(1):29–34 [DOI] [PubMed] [Google Scholar]
- 43.Prickett WD, Teefey SA, Galatz LM, Calfee RP, Middleton WD, Yamaguchi K. Accuracy of ultrasound imaging of the rotator cuff in shoulders that are painful postoperatively. J Bone Joint Surg Am 2003;85-A(6):1084–1089 [DOI] [PubMed] [Google Scholar]
- 44.Mohana-Borges AV, Chung CB, Resnick D. MR imaging and MR arthrography of the postoperative shoulder: spectrum of normal and abnormal findings. RadioGraphics 2004;24(1):69–85 [DOI] [PubMed] [Google Scholar]
- 45.Buck FM, Jost B, Hodler J. Shoulder arthroplasty. Eur Radiol 2008;18(12):2937–2948 [DOI] [PubMed] [Google Scholar]
- 46.Sofka CM, Adler RS. Sonographic evaluation of shoulder arthroplasty. AJR Am J Roentgenol 2003;180(4):1117–1120 [DOI] [PubMed] [Google Scholar]