Abstract
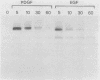
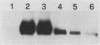
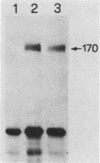
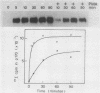
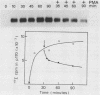
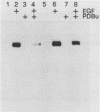
We have previously reported that antibodies to phosphotyrosine recognize the phosphorylated forms of platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) receptors (Zippel et al., Biochim. Biophys. Acta 881:54-61, 1986, and Sturani et al., Biochem. Biophys. Res. Commun. 137:343-350, 1986). In this report, the time course of receptor phosphorylation is investigated. In normal human fibroblasts, ligand-induced phosphorylation of PDGF and EGF receptors is followed by rapid dephosphorylation. However, in A431 cells the tyrosine-phosphorylated form of EGF receptor persists for many hours after EGF stimulation, allowing a detailed analysis of the conditions affecting receptor phosphorylation and dephosphorylation. In A431 cells, the number of receptor molecules phosphorylated on tyrosine was quantitated and found to be about 10% of total EGF receptors. The phosphorylated receptor molecules are localized on the cell surface, and they are rapidly dephosphorylated upon removal of EGF from binding sites by a short acid wash of intact cells and upon a mild treatment with trypsin. ATP depletion also results in rapid dephosphorylation, indicating that continuous phosphorylation-dephosphorylation reactions occur in the ligand-receptor complex at steady state. Phorbol 12-myristate 13-acetate added shortly before EGF reduces the rate and the final extent of receptor phosphorylation. Moreover, it also reduces the amount of phosphorylated receptors if it is added after EGF. Down-regulation of protein kinase C by chronic treatment with phorbol dibutyrate increases the receptor phosphorylation induced by EGF, suggesting a homologous feedback regulation of EGF receptor functions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N. Human platelet-derived growth factor (PDGF): purification of PDGF-I and PDGF-II and separation of their reduced subunits. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7314–7317. doi: 10.1073/pnas.78.12.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu M., Biswas R., Das M. 42,000-molecular weight EGF receptor has protein kinase activity. Nature. 1984 Oct 4;311(5985):477–480. doi: 10.1038/311477a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bertics P. J., Gill G. N. Self-phosphorylation enhances the protein-tyrosine kinase activity of the epidermal growth factor receptor. J Biol Chem. 1985 Nov 25;260(27):14642–14647. [PubMed] [Google Scholar]
- Brown K. D., Blay J., Irvine R. F., Heslop J. P., Berridge M. J. Reduction of epidermal growth factor receptor affinity by heterologous ligands: evidence for a mechanism involving the breakdown of phosphoinositides and the activation of protein kinase C. Biochem Biophys Res Commun. 1984 Aug 30;123(1):377–384. doi: 10.1016/0006-291x(84)90424-8. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L., Rees A. R., Gregoriou M., Kris R., Schlessinger J., Orci L. Subcellular distribution of the external and internal domains of the EGF receptor in A-431 cells. Exp Cell Res. 1986 Oct;166(2):312–326. doi: 10.1016/0014-4827(86)90479-9. [DOI] [PubMed] [Google Scholar]
- Cirillo D. M., Gaudino G., Naldini L., Comoglio P. M. Receptor for bombesin with associated tyrosine kinase activity. Mol Cell Biol. 1986 Dec;6(12):4641–4649. doi: 10.1128/mcb.6.12.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Collins M. K., Sinnett-Smith J. W., Rozengurt E. Platelet-derived growth factor treatment decreases the affinity of the epidermal growth factor receptors of Swiss 3T3 cells. J Biol Chem. 1983 Oct 10;258(19):11689–11693. [PubMed] [Google Scholar]
- Comoglio P. M., Di Renzo M. F., Tarone G., Giancotti F. G., Naldini L., Marchisio P. C. Detection of phosphotyrosine-containing proteins in the detergent-insoluble fraction of RSV-transformed fibroblasts by azobenzene phosphonate antibodies. EMBO J. 1984 Mar;3(3):483–489. doi: 10.1002/j.1460-2075.1984.tb01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Bowen-Pope D. F., Raines E., Ross R., Hunter T. Similar effects of platelet-derived growth factor and epidermal growth factor on the phosphorylation of tyrosine in cellular proteins. Cell. 1982 Nov;31(1):263–273. doi: 10.1016/0092-8674(82)90426-3. [DOI] [PubMed] [Google Scholar]
- Davis R. J., Czech M. P. Tumor-promoting phorbol diesters mediate phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1984 Jul 10;259(13):8545–8549. [PubMed] [Google Scholar]
- Downward J., Parker P., Waterfield M. D. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984 Oct 4;311(5985):483–485. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- Downward J., Waterfield M. D., Parker P. J. Autophosphorylation and protein kinase C phosphorylation of the epidermal growth factor receptor. Effect on tyrosine kinase activity and ligand binding affinity. J Biol Chem. 1985 Nov 25;260(27):14538–14546. [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Tremble P. M., Williams L. T. Evidence for the platelet-derived growth factor-stimulated tyrosine phosphorylation of the platelet-derived growth factor receptor in vivo. Immunopurification using a monoclonal antibody to phosphotyrosine. J Biol Chem. 1984 Jun 25;259(12):7909–7915. [PubMed] [Google Scholar]
- Friedman B., Frackelton A. R., Jr, Ross A. H., Connors J. M., Fujiki H., Sugimura T., Rosner M. R. Tumor promoters block tyrosine-specific phosphorylation of the epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1984 May;81(10):3034–3038. doi: 10.1073/pnas.81.10.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Chemical and biological properties of a growth factor from human-cultured osteosarcoma cells: resemblance with platelet-derived growth factor. J Cell Physiol. 1980 Nov;105(2):235–246. doi: 10.1002/jcp.1041050207. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981 Jun;24(3):741–752. doi: 10.1016/0092-8674(81)90100-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Hunter T., Ling N., Cooper J. A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984 Oct 4;311(5985):480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- King A. C., Cuatrecasas P. Resolution of high and low affinity epidermal growth factor receptors. Inhibition of high affinity component by low temperature, cycloheximide, and phorbol esters. J Biol Chem. 1982 Mar 25;257(6):3053–3060. [PubMed] [Google Scholar]
- King C. S., Cooper J. A. Effects of protein kinase C activation after epidermal growth factor binding on epidermal growth factor receptor phosphorylation. J Biol Chem. 1986 Aug 5;261(22):10073–10078. [PubMed] [Google Scholar]
- Krupp M. N., Connolly D. T., Lane M. D. Synthesis, turnover, and down-regulation of epidermal growth factor receptors in human A431 epidermoid carcinoma cells and skin fibroblasts. J Biol Chem. 1982 Oct 10;257(19):11489–11496. [PubMed] [Google Scholar]
- Pandiella A., Malgaroli A., Meldolesi J., Vicentini L. M. EGF raises cytosolic Ca2+ in A431 and Swiss 3T3 cells by a dual mechanism. Redistribution from intracellular stores and stimulated influx. Exp Cell Res. 1987 May;170(1):175–185. doi: 10.1016/0014-4827(87)90127-3. [DOI] [PubMed] [Google Scholar]
- Ross A. H., Baltimore D., Eisen H. N. Phosphotyrosine-containing proteins isolated by affinity chromatography with antibodies to a synthetic hapten. Nature. 1981 Dec 17;294(5842):654–656. doi: 10.1038/294654a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Allosteric regulation of the epidermal growth factor receptor kinase. J Cell Biol. 1986 Dec;103(6 Pt 1):2067–2072. doi: 10.1083/jcb.103.6.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnett-Smith J. W., Rozengurt E. Diacylglycerol treatment rapidly decreases the affinity of the epidermal growth factor receptors of Swiss 3T3 cells. J Cell Physiol. 1985 Jul;124(1):81–86. doi: 10.1002/jcp.1041240114. [DOI] [PubMed] [Google Scholar]
- Stoscheck C. M., Carpenter G. Characterization of the metabolic turnover of epidermal growth factor receptor protein in A-431 cells. J Cell Physiol. 1984 Sep;120(3):296–302. doi: 10.1002/jcp.1041200306. [DOI] [PubMed] [Google Scholar]
- Sturani E., Vicentini L. M., Zippel R., Toschi L., Pandiella-Alonso A., Comoglio P. M., Meldolesi J. PDGF-induced receptor phosphorylation and phosphoinositide hydrolysis are unaffected by protein kinase C activation in mouse Swiss 3T3 and human skin fibroblasts. Biochem Biophys Res Commun. 1986 May 29;137(1):343–350. doi: 10.1016/0006-291x(86)91216-7. [DOI] [PubMed] [Google Scholar]
- Sunada H., Magun B. E., Mendelsohn J., MacLeod C. L. Monoclonal antibody against epidermal growth factor receptor is internalized without stimulating receptor phosphorylation. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3825–3829. doi: 10.1073/pnas.83.11.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Sweatt J. D., Carpenter G. Epidermal growth factor (EGF) stimulates inositol trisphosphate formation in cells which overexpress the EGF receptor. Biochem Biophys Res Commun. 1987 Feb 13;142(3):688–695. doi: 10.1016/0006-291x(87)91469-0. [DOI] [PubMed] [Google Scholar]
- Whiteley B., Glaser L. Epidermal growth factor (EGF) promotes phosphorylation at threonine-654 of the EGF receptor: possible role of protein kinase C in homologous regulation of the EGF receptor. J Cell Biol. 1986 Oct;103(4):1355–1362. doi: 10.1083/jcb.103.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann M. M., Fox C. F. Identification of epidermal growth factor receptors in a hyperproducing human epidermoid carcinoma cell line. J Biol Chem. 1979 Sep 10;254(17):8083–8086. [PubMed] [Google Scholar]
- Wrann M., Fox C. F., Ross R. Modulation of epidermal growth factor receptors on 3T3 cells by platelet-derived growth factor. Science. 1980 Dec 19;210(4476):1363–1365. doi: 10.1126/science.6254158. [DOI] [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J. W., Rozengurt E. Early events elicited by bombesin and structurally related peptides in quiescent Swiss 3T3 cells. I. Activation of protein kinase C and inhibition of epidermal growth factor binding. J Cell Biol. 1986 Jun;102(6):2211–2222. doi: 10.1083/jcb.102.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippel R., Sturani E., Toschi L., Naldini L., Alberghina L., Comoglio P. M. In vivo phosphorylation and dephosphorylation of the platelet-derived growth factor receptor studied by immunoblot analysis with phosphotyrosine antibodies. Biochim Biophys Acta. 1986 Mar 19;881(1):54–61. doi: 10.1016/0304-4165(86)90096-6. [DOI] [PubMed] [Google Scholar]