Abstract
Background
Even after the recent approval of newer oral anticoagulants for clinical use, the vitamin K antagonist phenprocoumon remains an important treatment option for many patients. In order to quantify the hitherto “accepted” risks of phenprocoumon treatment, we analyzed adverse drug reactions (ADRs) that led to hospitalization on the internal medicine wards of four German pharmacovigilance centers.
Methods
We prospectively analyzed ADRs leading to hospitalization on the internal medicine wards of the hospitals belonging to the German Network of Regional Pharmacovigilance Centers (Rostock, Greifswald, Jena, and the Sophien- und Hufeland-Klinikum in Weimar) in the years 2000 to 2008.
Results
The 851 patients hospitalized for a phenprocoumon-associated ADR accounted for 12.4% of the 6887 ADR-related hospitalizations in the period of the study. 723 (85%) were admitted for a hemorrhage, usually in the gastrointestinal tract (482 patients); 8 patients died as a consequence of hemorrhage associated with phenprocoumon exposure. Using drug utilization data for the catchment areas of the participating hospitals, we calculate a rate of 5 to 7 hemorrhages leading to hospitalization in an internal medicine ward per 1000 patient-years under phenprocoumon treatment. One-third of the patients who had a hemorrhage were taking other interacting drugs, mainly inhibitors of platelet aggregation and non-steroidal anti-inflammatory drugs. Among the patients who were taking phenprocoumon because of a history of thromboembolic events or for atrial fibrillation, 60% to 70% of those who had hemorrhages had an international normalized ratio (INR) that was above the upper limit of the therapeutic range. Phenprocoumon-associated impairment of liver function arose in 23 patients (2.7%).
Conclusion
In this study, about one-eighth of all ADR-related admissions to hospital internal medicine wards were associated with phenprocoumon. There is a need for a comparative risk-benefit assessment of phenprocoumon and the newer oral anticoagulants under real-life conditions.
Vitamin K antagonists (VKA) have been used for many years to prevent and to treat thromboembolic diseases. In Germany, the active substance predominantly used in vitamin K antagonist preparations is phenprocoumon. According to a recent estimate, approximately 1 million patients in this country are being treated with phenprocoumon (1). Because of its narrow therapeutic range, the wide variety of interactions, and the associated risk of bleeding, phenprocoumon is one of the medications most frequently reported to cause adverse drug reactions (ADRs) associated with severe complications (e.g., hospital admission, death) (2). As a consequence of this risk profile, a further ca. 1 million patients in whom antithrombotic treatment is indicated are not prescribed VKA (1).
New oral anticoagulants (NOACs) have been approved for use in recent years, among them the factor Xa inhibitors apixaban and rivaroxaban and the thrombin inhibitor dabigatran etexilate. Notwithstanding the advantages of these substances over VKA—e.g., a lower rate of intracerebral hemorrhage, no need for regular coagulation testing, lack of interactions with foodstuffs containing vitamin K—some critical aspects have to be discussed. Especially in the case of life-threatening bleeding, the lack of routine laboratory data on coagulation and the lack of antidotes of NOACs represent significant clinical problems (3). Particularly in view of the limited transferability of the approval studies (exclusion of multimorbid patients, imperfect INR adjustment in control patients treated with warfarin [4)]) and the lack of long-term data, the Drug Commission of the German Medical Association currently sees no advantage of dabigatran or rivaroxaban treatment for patients whose INR is well controlled on phenprocoumon (5). Furthermore, for some indications (e.g., heart valve replacement) phenprocoumon will remain the only approved oral anticoagulant for the foreseeable future.
Since phenprocoumon will continue to play an important role, two crucial, practice-related questions have to be answered:
What level of risk has been and will be tolerated in patients taking phenprocoumon?
What types of problems, e.g., pharmacodynamic interactions, arise and may also need to be considered when using NOACs?
With the aim of illustrating the level of risk tolerated to date, we carried out the first German prospective multicenter longitudinal survey to analyze the frequency and attendant circumstances of severe bleeding and liver disorders during phenprocoumon treatment under real life conditions.
Method
Acquisition and analysis of data on adverse drug reactions
In the framework of the German Network of Regional Pharmacovigilance Centers, all non-elective admissions to the departments of internal medicine of the four participating centers (Rostock: University Hospital and Klinikum Südstadt; Greifswald: University Hospital; Jena: University Hospital; Weimar: Sophien- und Hufeland-Klinikum) were investigated for the presence of an ADR (2) (www.pharmacoepi.de). The survey was approved by the relevant ethics committees.
Patients with ADRs were identified by regular questioning of physicians and examination of admission books using screening criteria. After review of the patient’s medical records and discussion of the suspicion of an ADR with the treating physicians, the causal association between medication and ADR was assessed according to a standard algorithm (e1) (see eBox for details). For the purposes of this article we evaluated all hospital admissions between 1 January 2000 and 31 December 2008 for which a causal association was considered at least “possible.”
eBox. Description of the German Network of Regional Pharmacovigilance Centers and details of the method.
Under the aegis of the Network of Regional Pharmacovigilance Centers we analyzed the adverse drug reactions (ADRs) that led to admission for inpatient treatment in departments of internal medicine in the period from 2000 to June 2011 (2, 7, e5–e7 [www.pharmacoepi.de]). Cases in which ADRs were suspected were recorded in full detail (2) at four centers: Rostock (University Hospital and Klinikum Südstadt), Greifswald (University Hospital), Jena (University Hospital), and Weimar (Sophien- und Hufeland-Klinikum). Quality assurance of the suspected ADRs was carried out in Wuppertal, technical implementation and biometric analysis in Munich. The detailed recording of ADRs, together with drug utilization data, permitted estimation of incidence of ADRs requiring admission for inpatient treatment in internal medicine facilities.
Patients with ADR were identified by research assistants who regularly contacted the treating physicians; in addition, admission books or any electronic documentation of admissions were inspected. For more reliable identification of ADRs we used trigger criteria, including risk factors for an ADR (e.g., renal function disorder) and diagnoses frequently assessed as ADRs (e.g., gastrointestinal bleeding and hypoglycemia). After detailed inspection of the patient’s medical record, each suspected ADR was discussed with the treating physicians and documented in an ACCESS-based database. Together with exhaustive documentation of the ADR that led to hospital admission according to the Medical Dictionary for Regulatory Activities (MedDRA [e8]), we recorded demographic data, previous illnesses, and medications taken.
The clinical course (ADR outcome) was assessed as laid down in the ICH Guideline (e9), and the ADR severity was estimated according to a standardized algorithm (e10). The ADR causality of all drugs taken was evaluated according to the algorithm described by Bégaud et al. (e1). The temporal and pharmacological plausibilities were considered and weighed against alternative causes, e.g., comorbidities. The causal association between drug and ADR was then classified as “very likely,” “likely,” “possible,” or “dubious.” After quality assurance the documented ADRs were forwarded to the Federal Institute for Drugs and Medical Devices.
For the purposes of this study, bleeding events and liver damage were identified in the database by means of the standardized MedDRA queries “Haemorrhage terms (excl laboratory terms)” and “Possible drug related hepatic disorders—comprehensive search.”
Evaluations of drug-drug interactions (DDIs) were based on the summary of product characteristics for Marcumar (the phenprocoumon-containing reference preparation), refined by extended searches (Micromedex, PubMed). The interacting substances were divided into three types (Table 1):
Table 1. Drug classes interacting with phenprocoumon causing an increased risk of bleeding*.
| DDI type | Mechanism of elevation of risk of (gastrointestinal) bleeding | Drug class | Drug (class) |
| I | Inhibition of coagulation and/or direct mucosal lesion (pharmacodynamic interaction, no effect on INR) | Antithrombotic drugs | e.g., ASA (low dose), clopidogrel, heparin |
| Non-steroidal anti-inflammatory drugs | e.g., ASA (analgesic dose), diclofenac, ibuprofen, phenylbutazone, coxibs, oxicams | ||
| Selective serotonin reuptake inhibitors | e.g., citalopram, sertraline | ||
| Other antidepressants | Venlafaxine | ||
| Antimetabolites | Methotrexate | ||
| Bisphosphonates | e.g., alendronate | ||
| Iron containing preparations | Iron | ||
| II | Influence on coagulation factors (pharmacodynamic interaction, possible effect on INR) | Thyroid hormones | e.g., levothyroxine |
| Antibiotics | Selected cephalosporins (cefazolin, cefpodoxime, cefotaxime, ceftibuten) | ||
| III | Increase in phenprocoumon plasma level (pharmacokinetic interaction, possible increase in INR) | Antiarrhythmics | Amiodarone |
| Quinidine | |||
| Propafenone | |||
| Antibiotics | Macrolides (e.g., erythromycin, clarithromycin); not: azithromycin | ||
| Imidazole derivatives (e.g., metronidazole) | |||
| Cotrimoxazole | |||
| Antimycotics | Imidazole derivatives (e.g., ketoconazole) | ||
| Triazole derivatives (e.g., fluconazole, itraconazole) | |||
| Cancer drugs | Antiestrogen (tamoxifen) | ||
| Antimetabolite (capecitabine) | |||
| Tricyclic antidepressants | Imipramine, trimipramine, clomipramine, opipramol, amitriptyline, nortriptyline, doxepine | ||
| Fibrates | e.g., fenofibrate | ||
| Immunosuppressants | Leflunomide | ||
| Opioids | Tramadol | ||
| Uricostatics | Allopurinol | ||
| Others | Disulfiram |
*Data based on: summary of product characteristics for Marcumar, Micromedex; DDI, drug-drug interaction; INR: international normalized ratio; ASA: acetylsalicylic acid
Type I: substances that have a negative pharmacodynamic action on coagulation or mucosal membrane lesions (as preferred sites of gastrointestinal bleeding) without affecting the INR
Type II: substances that exert a pharmacodynamic effect on the coagulation factors (and thus on the INR)
Type III: pharmacokinetically interactive substances that, for example, raise the INR and therefore the risk of bleeding by inhibiting the metabolization of phenprocoumon.
In cases with two or more mechanisms of interaction, we assigned the active substance to the principal mechanism.
In identifying liver damage and classifying the type of damage we followed the recommendation of the US Food and Drug Administration (FDA) and took account of alanine aminotransferase (ALAT) and alkaline phosphatase (AP) (6) (eTable 1). In cases of liver disorder all co-medications were assessed for causality, leading to the classification of some other drugs as co-causes of ADR.
eTable 1. Criteria for classification of liver injuries*.
| Type of damage | ALAT-ULN | AP-ULN | (ALAT-ULN)/(AP-ULN) |
| Hepatitic | ≥ 3 | – | ≥ 5 |
| Cholestatic | – | ≥ 2 | ≤ 2 |
| Mixed type | ≥ 3 | ≥ 2 | >2 to <5 |
*cited in (6);
ALAT: alanine aminotransferase; AP: alkaline phosphatase; ULN: upper limit of normal
Statistical analysis and estimation of the incidence of adverse drug reactions
Metric variables were expressed as mean values and standard deviation if distributed normally, otherwise as median values (first quartile–third quartile). For categorical variables we calculated absolute values and percentages. The INR level used for analysis was that recorded at hospital admission. Categorically recorded INR levels (e.g., >6) were assigned numerical values (e.g., 6.1) for purposes of calculation.
The participating hospitals have a unique position characterized by a well-defined hospital catchment area and the absence of other hospitals with internal medicine emergency departments in this area (2). Drug consumption data from data processing centers for pharmacies were available only for Greifswald, Rostock, and Weimar and only for the years 2003, 2004, 2006, and 2008. Therefore no ADR incidence could be calculated for 2000 to 2002, 2005, or 2007. Each hospital’s catchment area was defined as the postal codes contributing 70–75% of the patients admitted for nonelective reasons (e2). The annual incidence was calculated as the ratio of the number of patients with ADR to the number of patients treated with phenprocoumon from the catchment areas of the study centers, stratified by year. Asymmetric 95% Clopper–Pearson confidence intervals (CI) of incidence were calculated (e3). The statistical software SAS 9.2 (SAS Institute Inc., Cary, NC, USA) was used for all analyses, and the Venn diagram was produced using the Venn Diagram Plotter (http://omics.pnl.gov/software/VennDiagramPlotter.php).
Results
Over the 9-year study period 6887 patients (ca. 3.25% of all admissions to departments of internal medicine [7]) were admitted to the internal medicine wards of the four participating centers for treatment of an at least “possible” ADR. Eight hundred fifty-one of these 6887 patients (12.4%) were shown to have a phenprocoumon-associated adverse effect. Bleeding or liver disorder occurred in 723 (85.0%) and 23 (2.7%) of patients with phenprocoumon-associated ADR, respectively (eTable 2). Cases of phenprocoumon-associated bleeding and liver disorder comprised 10.5% and 0.3%, respectively, of all ADR-related hospital admissions.
eTable 2. Characteristics of patients with ADR-related hospital admission.
| All patients with ADR | Patients with phenprocoumon- associated ADR | Patients with phenprocoumon- associated bleeding | Patients with phenprocoumon- associated liver disorder | |
| N | 6887 | 851 | 723* | 23* |
| Women (n [%]) | 4111 (59.7%) | 438 (51.5%) | 354 (49.0%) | 17 (73.9%) |
| Age in years (n = 6882) mean ± SD) | 70.2±15.7 | 71.7±11.0 | 71.9±10.8 | 60.2±14.3 |
| Number of diseases (median [Q1–Q3]) | 5.0 (3.0–6.0) | 5.0 (4.0–7.0) | 5.0 (3.0–7.0) | 4.0 (2.0–6.0) |
| Number of medications (median [Q1–Q3]) | 6.0 (4.0–9.0) | 7.0 (5.0–9.0) | 7.0 (5.0–9.0) | 4.0 (2.0–5.0) |
| Number of suspected medications (median [Q1–Q3]) | 2.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 2.0 (1.0–3.0) |
| Length of hospital stay in days (n = 6810) (median [Q1–Q3]) | 8.0 (5.0–13.0) | 9.0 (6.0–14.0) | 9.0 (6.0–14.0) | 13.0 (8.0–22.0) |
*One patient had simultaneous liver disorder and bleeding and was counted in both groups.
ADR: adverse drug reaction
Patients who suffered other phenprocoumon-associated ADR principally had anemia or pronounced elevation of INR without clinically confirmed bleeding. In the majority (57.9%) of patients with phenprocoumon-associated adverse effects, atrial fibrillation was stated as the indication for phenprocoumon, while thrombosis/pulmonary embolism accounted for 18.0% (Table 2, eTable 3).
Table 2. Demographic and morbidity-related characteristics of patients with phenprocoumon-associated adverse drug reactions (n = 851 patients).
| Age (years) | Women (n [%]) | Number of diseases (median [Q1–Q3]) | Number of medications (median [Q1–Q3]) | Atrial fibrillation | Thrombosis/ pulmonary embolism | Heart valve replacement*1 | Other indications*2 |
| 71.7 ± 11.0 | 438 (51.5%) | 5.0 (4.0–7.0) | 7.0 (5.0–9.0) | 493 (57.9%) | 153 (18.0%) | 21 (2.5%) | 184 (21.6%) |
*1All patients with heart valve replacement (with or without atrial fibrillation)
*2In most of these cases coronary heart disease, status post myocardial infarction, and heart valve disease were documented
eTable 3. Indications for anticoagulation with phenprocoumon and other risk factors for thromboembolic complications in n = 851 patients with phenprocoumon-associated adverse drug reactions.
| Principal indication | N (%) | Further indications or risk factors (n [%]) | ||||||||
| Heart failure/cardiomyopathy | Hypertension | >75 years | Diabetes mellitus | Stroke/TIA | Valve defect | CHD/MI | Arterial sclerosis (e.g., PAOD) | Others | ||
| Atrial fibrillation | 493 (57.9) | 104 (21.1) | 400 (81.1) | 252 (51.1) | 55 (11.2) | 58 (11.8) | 53 (10.8) | 208 (42.2) | 48 (9.7) | 1 (0.2) |
| Thrombosis/pulmonary embolism | 153 (18.0) | 15 (9.8) | 89 (58.2) | 52 (34.0) | 18 (11.8) | 7 (4.6) | 3 (2.0) | 38 (24.8) | 9 (5.9) | 2 (1.3) |
| Heart valve replacement*1 | 21 (2.5) | 5 (23.8) | 16 (76.2) | 5 (23.8) | 5 (23.8) | 1 (4.8) | 17 (81.0) | 14 (66.7) | 1 (4.8) | 0 |
| Other indications*2 | 184 (21.6) | 54 (29.3) | 149 (81.0) | 54 (29.3) | 20 (10.9) | 32 (17.4) | 48 (26.1) | 79 (42.9) | 43 (23.4) | 1 (0.5) |
*1All patients with heart valve replacement (independent of presence of atrial fibrillation)
*2 In most of these cases coronary heart disease (CHD), status post myocardial infarction (MI), and heart valve disease were documented.
PAOD: peripheral arterial occlusive disease; TIA: transitory ischemic attack
Bleeding during treatment with phenprocoumon
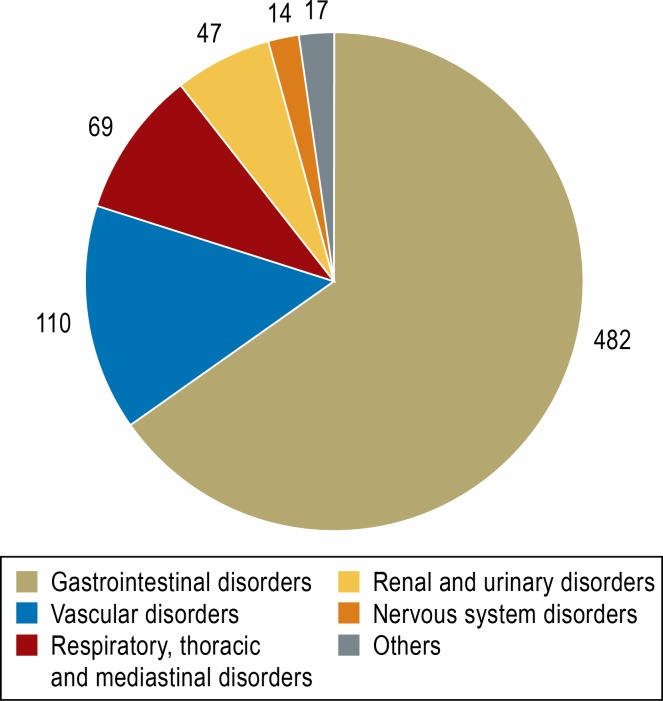
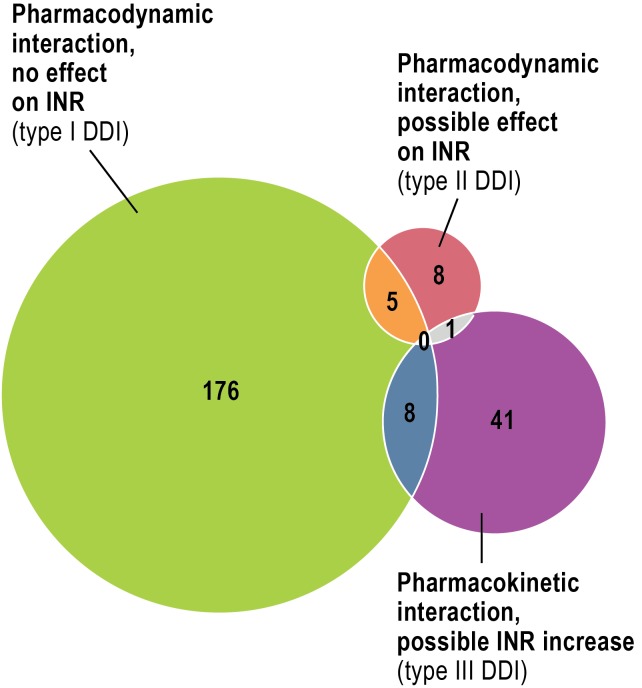
The patients with phenprocoumon-associated bleeding (n = 723) had an average age of 72 ± 11 years, and the numbers of men and women did not differ significantly (eTable 2). Intensive care was necessary in 13.3% of patients, and eight patients died of bleeding-related complications. Most instances of bleeding (66.7%) occurred in the gastrointestinal tract (Figure 1), while the category “vascular disorder” was made up predominantly of unspecified (in some cases multiple) hemorrhages (n = 84) and hematomas (n = 23). Clinically significant interactions (Table 1) were identified in 239 patients (33.1%). Most were pharmacodynamic interactions that did not affect the INR—largely platelet aggreation inhibitors (PAI, mostly low-dose acetylsalicylic acid [ASA], n = 66) and non-steroidal anti-inflammatory drugs (NSAIDs, mostly diclofenac, n = 39) (Figure 2, Table 3). Seven patients were taking a PAI and an NSAID together with phenprocoumon, and six patients were receiving dual antiplatelet therapy (DAPT) in addition to phenprocoumon (low-dose ASA plus clopidogrel).
Figure 1.
Sites of phenprocoumon-associated bleeding
(organ systems according to the Medical Dictionary for Regulatory Activities, MedDRA; multiple answers possible)
Figure 2.
Number of patients with drug-drug interaction (DDI) and distribution of DDI
(INR, international normalized ratio)
Table 3. Number of patients with clinically relevant drug-drug interactions (DDIs) stratified by type of DDI (see Table 1) and drug class (multiple answers possible*1).
| DDI type | Drug class (n) | Top three drugs*2 (n) |
| I | Antithrombotic drugs (n=106) | Acetylsalicylic acid (low dose) (n=66), clopidogrel (n=25), enoxaparin (n=20) |
| Non-steroidal anti-inflammatory drugs (n=86) | Diclofenac (n=39), ibuprofen (n=15), acetylsalicylic acid (analgesic dose) (n=11) | |
| Selective serotonin reuptake inhibitors (n=5) | Citalopram (n=3), sertraline (n=1), escitalopram (n=1) | |
| Other antidepressants (n=1) | Venlafaxine (n=1) | |
| Antimetabolites (n=3) | Methotrexate (n=3) | |
| Bisphosphonates (n=0) | ||
| Iron containing preparations (n=1) | Iron glycine sulfate (n=1) | |
| II | Thyroid hormones (n=14) | Levothyroxine sodium (n=11), combination of levothyroxine und liothyronine (n=2), levothyroxine, combination (n=1) |
| Antibiotics (n=0) | ||
| III | Antiarrhythmics (n=10) | Amiodarone (n=10) |
| Antibiotics (n=3) | Clarithromycin (n=2), metronidazole (n=1) | |
| Antimycotics (n=0) | ||
| Cancer drugs (n=1) | Antiestrogen (tamoxifen) (n=1) | |
| Tricyclic antidepressants (n=3) | Amitriptyline (n=2), trimipramine (n=1) | |
| Fibrates (n=6) | Bezafibrate (n=4), fenofibrate (n=2) | |
| Immunosuppressants (n=1) | Leflunomide (n=1) | |
| Opioids (n=4) | Tramadol (n=3), tramadol, combinations (n=1) | |
| Uricostatics (n=27) | Allopurinol (n=27) | |
| Others (n = 0) |
*1Patients with more than one interacting medication are counted for each interacting medication
*2Top three substances: the three most frequently occurring drugs in the respective drug class
Type I: Inhibition of coagulation and/or direct mucosal lesions (pharmacodynamic interaction, no effect on INR)
Type II: Influence on coagulation factors (pharmacodynamic interaction, possible effect on INR)
Type III: Increase in phenprocoumon plasma level (pharmacokinetic interaction, possible increase in INR)
INR: international normalized ratio
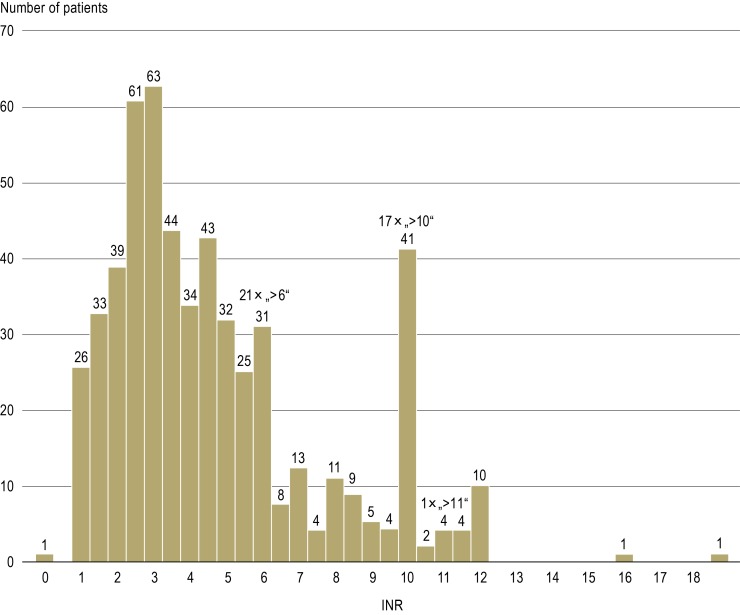
In 549 patients (75.9%) with phenprocoumon-associated bleeding the INR was documented on the day of admission to hospital. The INR was over 3.0 in 345 (62.8%) of these patients, over 4.0 in 47.5%, over 5.0 in 33.0%, and over 6.0 in 26.0% (eFigure 1). Among the patients with the principal indications of atrial fibrillation and thrombosis/pulmonary embolism, the proportion of those whose INR lay above the upper limit of the target range was between 60% and 70% (Table 4). Patients who were taking pharmacokinetically interacting drugs (type III) displayed distinctly elevated INR (average 4.3) (eTable 4).
eFigure 1.
Distribution of INR at the time of hospital admission in patients with phenprocoumon-associated bleeding
INR, international normalized ratio
Table 4. Median INR values and proportion of patients with phenprocoumon-associated bleeding whose INR was above the target range (stratified by indication).
| Indication | Number of patients | INR (median [Q1–Q3]) | Target range | Patients above target range | Patients with type I DDIs | Patients with type II or III DDIs |
| AF | 319 | 3.7 (2.5–6.1) | 2–3 | 195 (61.1%) | 83 (26.0%) | 23 (7.2%) |
| Thrombosis/ pulmonary embolism | 98 | 4.4 (2.7–6.8) | 2–3 | 67 (68.4%) | 22 (22.4%) | 6 (6.1%) |
| Valve replacement | 18 | 3.4 (2.6–9.4) | N.D.* | N.D.* | 6 (33.3%) | 3 (16.7%) |
| Others | 114 | 3.7 (2.5–5.6) | N.D.* | N.D.* | 35 (30.7%) | 4 (3.5%) |
*No analysis of INR target range in the indication groups "Valve replacement" and "Others"
N.D.: not done; INR: international normalized ratio; AF: atrial fibrillation
Type I: Inhibition of coagulation and/or direct mucosal lesions (pharmacodynamic interaction, no effect on INR)
Type II: Influence on coagulation factors (pharmacodynamic interaction, possible effect on INR)
Type III: Increase in phenprocoumon plasma level (pharmacokinetic interaction, possible increase in INR)
eTable 4. INR values of patients with phenprocoumon-associated bleeding, stratified by type of drug-drug interaction (DDI) (only clinically relevant DDIs were included)4.
| Patients with INR value (n) | INR (median [Q1–Q3]) | ||
| Phenprocoumon without DDI | 356 | 3.8 (2.6–6.1) | |
| Phenprocoumon and type I DDI | 146 | 3.9 (2.4–5.7) | |
| Phenprocoumon and type II DDI | 6 | 2.9 (2.6–3.4) | |
| Phenprocoumon and type III DDI | 29 | 4.3 (3.5–6.1) | |
| Phenprocoumon and type II and/or III DDI* | 36 | 4.0 (3.0–5.8) | |
| Other combination | 11 | 4.2 (3.0–6.8) | |
| All | 549 | 3.9 (2.6–6.1) | |
*Type II and type III: All substances that affect the INR (pharmacodynamic and pharmacokinetic)
INR: international normalized ratio
Type I: Inhibition of coagulation and/or direct mucosal lesions
(pharmacodynamic interaction, no effect on INR)
Type II: Influence on coagulation factors (pharmacodynamic interaction, possible effect on INR)
Type III: Increase in phenprocoumon plasma level (pharmacokinetic interaction, possible increase in INR)
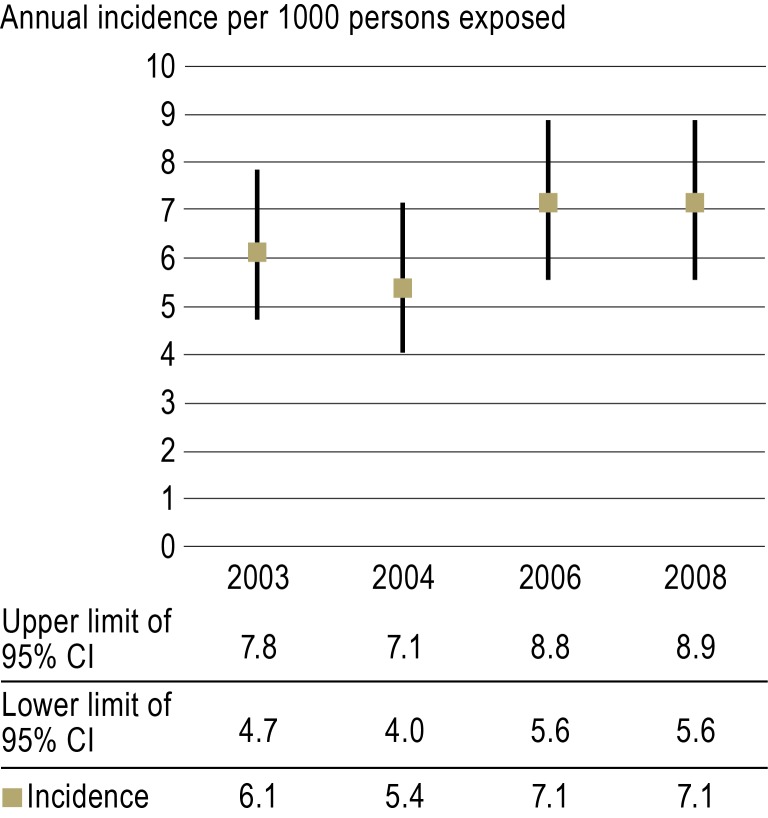
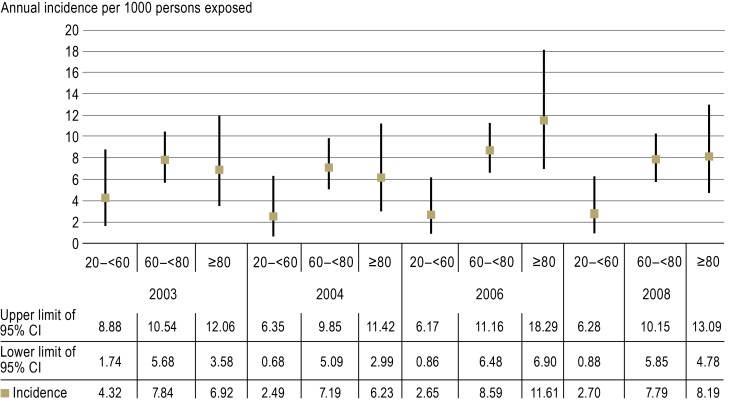
The estimates of incidence revealed that five to seven bleeding events can be expected annually per 1000 phenprocoumon-treated patients requiring admission to departments of internal medicine (Figure 3). Stratifying the patients according to age, it emerged that younger patients (20 to 59 years) were at a lower risk of phenprocoumon-related bleeding than those in older age groups (60 to 79 years and 80 years or over) (eFigure 2).
Figure 3.
Annual incidence of phenprocoumon-associated bleeding leading to admission to internal medicine wards per 1000 patients taking phenprocoumon (Greifswald, Rostock, and Weimar).
95% CI: 95% confidence interval
eFigure 2.
Annual incidence of phenprocoumon-associated bleeding leading to admission to internal medicine wards per 1000 patients taking phenprocoumon
(Greifswald, Rostock, and Weimar centers)
95% CI: 95% confidence interval
Liver disorder during treatment with phenprocoumon
The 23 patients with phenprocoumon-associated liver disorder leading to hospital admission were on average much younger than the patients with phenprocoumon-associated bleeding, and a higher proportion of them were female (eTable 2). The median treatment duration before the onset of the liver disorder was 159 days (Q1–Q3: 42–229 days; minimum: 4 days; maximum: 3730 days), and most of the patients (n = 13) showed a hepatocellular (hepatitic) pattern of damage. The median maximal increases of ALAT and AP in relation to the upper limit of normal were 15.8-fold and 1.3-fold, respectively (eTable 5). Intensive care was necessary in one patient, and permanent liver injury occurred in two patients. Phenprocoumon was discontinued in a total of 21 patients. In 13 patients other substances in addition to phenprocoumon were judged to have caused ADR.
eTable 5. Maximal changes in selected liver function parameters in the 23 patients with phenprocoumon-associated liver injury*.
| Parameter | Maximal increase (expressed as multiple of upper limit of normal) (median [Q1–Q3]) |
| Total bilirubin (n = 17) | 7.8 (2.6–19.3) |
| ALAT (n = 22) | 15.8 (4.5–30.7) |
| ASAT (n = 23) | 12.9 (3.9–26.1) |
| AP (n = 19) | 1.3 (0.8–2.0) |
| Gamma-GT (n = 23) | 5.5 (2.4–11.0) |
*Pattern of damage: hepatocellular n = 13, cholestatic n = 1, mixed type n = 1, unclassifiable n = 8 (including n = 4 patients with missing AP values and n = 4 patients with an increase of hepatic laboratory parameters below the respective values stated in eTable 1 (abnormality of liver tests) ALAT: alanine aminotransferase;
ASAT: aspartate aminotransferase; AP alkaline phosphatase; Gamma-GT: gamma-glutamyltransferase
These substances were:
Beta blockers (bisoprolol: n = 3; metoprolol: n = 1)
ACE inhibitors (ramipril: n = 2; enalapril: n = 1)
HMG-CoA reductase inhibitors (simvastatin: n = 2; pravastatin: n = 1)
Allopurinol: n = 2
Diuretics (hydrochlorothiazide: n = 1; xipamide: n = 1; torasemide: n = 1; spironolactone: n = 1; combination of hydrochlorothiazide and triamterene: n = 1 [multiple answers possible]).
Discussion
Just over 10% of all ADR-related hospital admissions in internal medicine are due to phenprocoumon-associated bleeding. The patients in this category in the present study were multimorbid and were taking a median of seven different drugs. Between 60% and 70% of the measured INR values lay above the target range for the indication concerned. This emphasizes the problem of fluctuating INR levels, which depend on numerous factors, from adherence and nutrition to interactions.
An interaction judged to be clinically relevant was observed in around 30% of the patients who suffered bleeding: At the forefront were the well-known interactions of phenprocoumon with PAIs and NSAIDs (8–10), which can also be expected with NOACs. In an analysis of data from a statutory health insurance provider, patients who were taking phenprocoumon together with diclofenac, ibuprofen, or clopidogrel showed significantly elevated adjusted odds ratios for severe bleeding, respectively 1.60, 1.63, and 1.83 (10). PAI-related DDIs will also increase in significance with the continuing growth of dual platelet inhibition (11). In contrast, the hotly debated interaction between VKA and serotonin reuptake inhibitors (12– 14) was of lesser importance in the present study.
The apparently complicated pharmacokinetic interactions played only a subordinate role overall. Principally involved were two drugs not infrequently taken by elderly patients: amiodarone and allopurinol. It has been recommended that patients who are already on warfarin should have the warfarin dose reduced by about one third when they start taking amiodarone (15, 16). The small number of studies of allopurinol have shown variable results with regard to metabolism of VKA (17, 18). The strong interaction of VKA with cotrimoxazole (9, 19, 20), which should be strenuously avoided, was not found in the present analysis. From the clinical viewpoint, the INR should be determined soon (within around 5 days) after the commencement of comedication—particularly drugs with high interaction potential (e.g., cotrimoxazole)—in order to be able to react promptly to any increase in INR. Amiodarone is a special case: Owing to the long half-life and slow saturation, the maximal effect is usually achieved only after 2 weeks. Prophylactic reduction of the phenprocoumon dose can be contemplated; however, this runs the risk of underdosing in the short term.
One important limitation of this and other investigations of interactions is the choice of drugs to be considered as interacting. The substance about which most is known in this respect is warfarin, but owing to differences in metabolization (CYP2C9 plays only a minor role for phenprocoumon), phenprocoumon possesses a somewhat different potential for pharmacokinetic interaction (21). It was this unsatisfactory state of knowledge that led us to adopt the summary of product characteristics for Marcumar—despite the known limitations of this data source (22)—as the basis for our analysis. The database Micromedex provided supplementary and more detailed information. We deliberately excluded potentially interacting drugs and drug classes for which the data were sparse or divergent (e.g., statins and Ginkgo biloba [10, 23–25]).
It must be remembered that probabilities lie at the root of all considerations of the causes of ADR. While assessment of causality is relatively uncomplicated in patients who are taking no interacting drug other than phenprocoumon, it is more difficult to pinpoint the cause in those who, for example, are also taking low-dose ASA. Finally, other coagulation-inhibiting drugs may be wholly or partly to blame for the bleeding, but causal involvement of phenprocoumon has to be assessed as at least “possible.” The influence of further factors such as comorbidities also has to be considered. For example, infection with Helicobacter pylori may cause gastric hemorrhage even without consumption of VKA, but the intake of phenprocoumon increases the risk. Such constellations were taken into account.
The data show that five to seven bleeding events per 1000 patients treated with phenprocoumon lead to admission to internal medicine wards each year. This corresponds to an annual incidence of 0.5% to 0.7%. This study was restricted to departments of internal medicine, so cerebral hemorrhages, for example, which are treated primarily in neurological/neurosurgical facilities, were not recorded; therefore, the true risk of bleeding associated with phenprocoumon is higher. For instance, one study of data from a statutory health insurance provider (including all medical disciplines) reported an annual incidence of bleeding-related hospitalization of 2.8% in patients treated with phenprocoumon (10). Particularly noteworthy is the finding, in another report based on statutory health insurance data, of significantly elevated risk of intracerebral hemorrhage in patients treated with phenprocoumon (26): Two clinical trials showed significantly lower rates of intracerebral hemorrhage with rivaroxaban and dabigatran, respectively, than with warfarin (27, 28). Moreover, the present study covered only a limited population (the catchment areas of the four centers contained ca. 500 000 inhabitants altogether), so its findings may not apply in their entirety to Germany as a whole. Crucial parameters include the frequency of INR measurement, the quality of INR adjustment, and the number and quantity of prescribed and freely available interacting comedications, among others. To negate the effect of any anomalies in age distribution in the catchment areas, we calculated the incidence by age group (eFigure 2).
Despite the small number of cases of phenprocoumon-associated liver injuries, repeated case reports confirm the clinical relevance of hepatic ADRs (29– 31). The predominance of women among patients with phenprocoumon-associated liver disorders in our study agrees well with other researchers’ results—although contrasting findings have been reported (32). A non-dose-related immunologic mechanism was recently shown to lie behind phenprocoumon-associated liver damage (33), and both hepatocellular and cholestatic liver injuries have been described (34, 35). There is no consensus, however, regarding the assignment of cases to the different damage types (e4). The substances suspected as possible (co-)factors have also been linked to liver injuries by other investigators (36). It should be mentioned, however, that assessment of the possible causal role of a drug is frequently time-consuming because of the many other potential reasons for liver injuries (e.g., viral diseases and alcohol abuse) (37).
From a clinical viewpoint, it is important that phenprocoumon-associated liver injury may occur in patients who have already been taking the drug for a long time, and that as many medications as possible (ideally, all) should be discontinued. Cross-reactions with NOACs are unlikely, so these compounds could represent a suitable alternative in such cases.
Summary
Around an eighth of all ADR-related admissions in internal medicine were found to be associated with administration of the oral vitamin K antagonist phenprocoumon. A third of the patients with phenprocoumon-related bleeding showed relevant interactions, particularly with PAIs and NSAIDs, that could also occur with NOACs. In 60% to 70% of patients with phenprocoumon-associated bleeding the INR levels exceeded the upper limit of the therapeutic range, and in these cases NOACs might offer greater treatment safety. Not all patients could have been treated with NOACs, however, because there are known contraindications as well as warnings with regard to liver disorders and also other interactions.
In comparison with controlled clinical trials under artificial conditions (39), the lack of regular review of coagulation by the primary care physician could possibly lead to an increased risk of bleeding (e.g., because of the less stringently monitored consumption of over-the-counter NSAIDs). Studies comparing the benefits and risks of phenprocoumon and the new oral anticoagulants are urgently required.
Key Messages.
Each year, around five to seven of every 1000 patients being treated with phenprocoumon experience severe (usually gastrointestinal) bleeding that leads to admission to an internal medicine ward.
Clinically significant drug-drug interactions (DDIs) were found in about one third of the patients with phenprocoumon-associated bleeding.
Pharmacodynamic interactions were particularly important (e.g., comedication with platelet aggregation inhibitors or non-steroidal anti-inflammatory drugs).
For the indications “treatment of thromboembolic event” and “atrial fibrillation,” 60% to 70% of patients had INR levels above the therapeutic range.
Liver disorders (mostly hepatocellular) accounted for 2.7% of phenprocoumon-related hospital admissions.
Acknowledgments
Translated from the original German by David Roseveare.
The Network of Regional Pharmacovigilance Centers received financial support from the Federal Institute for Drugs and Medical Devices from 1996 to June 2011 (project number V-11337/68605). The authors are grateful for the help received from the following members of the Network of Regional Pharmacovigilance Centers: Dr. Karen Saljé, Kathleen Klein, Manuela Arnold, Kathleen Wergin (Greifswald); Dr. Silke Müller, Grit Haase (Rostock); Dipl.-pharm. Astrid Scheuerlein, Dorothea Gruca (Jena); Silke Surber, Dipl.-med. Kerstin Fricke, Dr. Ilselore R. Günther, Prof. Reinhard Fünfstück (Weimar); Dr. Steffen Haffner (Wuppertal).
Footnotes
Conflict of interest statement
Dr. Schmiedl has received a lecture fee from Rottapharm Madaus.
Dr. Farker has received third-party funding for research projects from Mitsubishi Pharma Deutschland GmbH and Novartis Pharma GmbH.
Prof. Thürmann has received consulting fees from Biotest Pharma AG, Fresenius Kabi, and MYR GmbH and lecture fees from BayerVital, Biotest Pharma AG, Rottapharm Madaus GmbH and from the German Institute for Quality and Efficiency in Health Care (IQWiG). Third-party funding was provided to her by Biotest Pharma AG, Stada GmbH, and Bayer Schering Pharma AG.
The remaining authors declare that no conflict of interest exists.
References
- 1.Harbrecht U. Old and new anticoagulants. Hamostaseologie. 2011;31:21–27. doi: 10.5482/ha-1149. [DOI] [PubMed] [Google Scholar]
- 2.Schneeweiss S, Hasford J, Göttler M, Hoffmann A, Riethling AK, Avorn J. Admissions caused by adverse drug events to internal medicine and emergency departments in hospitals: a longitudinal population-based study. Eur J Clin Pharmacol. 2002;58:285–291. doi: 10.1007/s00228-002-0467-0. [DOI] [PubMed] [Google Scholar]
- 3.Steiner T. Neue direkte Antikoagulantien. Was im Notfall zu beachten ist. Dtsch Arztebl. 2012;109(39):A 1928–A 1930. [Google Scholar]
- 4.Van Spall HG, Wallentin L, Yusuf S, et al. Variation in warfarin dose adjustment practice is responsible for differences in the quality of anticoagulation control between centers and countries: an analysis of patients receiving warfarin in the randomized evaluation of long-term anticoagulation therapy (RE-LY) trial. Circulation. 2012;126:309–316. doi: 10.1161/CIRCULATIONAHA.112.101808. [DOI] [PubMed] [Google Scholar]
- 5.Schott G, Bräutigam K, Ludwig WD. Orale Antikoagulation bei nicht valvulärem Vorhofflimmern. Empfehlungen zum Einsatz der neuen Antikoagulation Dabigatran (Pradaxa®) und Rivaroxaban (Xarelto®) Arzneimittelkommission der deutschen Ärzteschaft. 2012 Version 1.0. September. [Google Scholar]
- 6.Abboud G, Kaplowitz N. Drug-induced liver injury. Drug Saf. 2007;30:277–294. doi: 10.2165/00002018-200730040-00001. [DOI] [PubMed] [Google Scholar]
- 7.Rottenkolber D, Schmiedl S, Rottenkolber M, et al. Net of Regional Pharmacovigilance Centers: Adverse drug reactions in Germany: direct costs of internal medicine hospitalizations. Pharmacoepidemiol Drug Saf. 2011;20:626–634. doi: 10.1002/pds.2118. [DOI] [PubMed] [Google Scholar]
- 8.Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penning-van Beest F, Erkens J, Petersen KU, Koelz HR, Herings R. Main comedications associated with major bleeding during anticoagulant therapy with coumarins. Eur J Clin Pharmacol. 2005;61:439–444. doi: 10.1007/s00228-005-0947-0. [DOI] [PubMed] [Google Scholar]
- 10.Jobski K, Behr S, Garbe E. Drug interactions with phenprocoumon and the risk of serious haemorrhage: a nested case-control study in a large population-based German database. Eur J Clin Pharmacol. 2011;67:941–951. doi: 10.1007/s00228-011-1031-6. [DOI] [PubMed] [Google Scholar]
- 11.Nationale Versorgungsleitlinie Chronische KHK, Kapitel 11 Modul Medikamentöse Therapie, Langfassung, Dezember 2011, Version 1.0. www.versorgungsleitlinien.de/themen/khk/pdf/nvl-khk-2auflage-modul-pharmakotherapie-1.1.pdf. (last accessed on 10 December 2012)
- 12.Opatrny L, Delaney JA, Suissa S. Gastro-intestinal haemorrhage risks of selective serotonin receptor antagonist therapy: a new look. Br J Clin Pharmacol. 2008;66:76–81. doi: 10.1111/j.1365-2125.2008.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schalekamp T, Klungel OH, Souverein PC, de Boer A. Increased bleeding risk with concurrent use of selective serotonin reuptake inhibitors and coumarins. Arch Intern Med. 2008;168:180–185. doi: 10.1001/archinternmed.2007.32. [DOI] [PubMed] [Google Scholar]
- 14.Vidal X, Ibáñez L, Vendrell L, Conforti A, Laporte JR Spanish-Italian Collaborative Group for the Epidemiology of Gastrointestinal Bleeding. Risk of upper gastrointestinal bleeding and the degree of serotonin reuptake inhibition by antidepressants: a case-control study. Drug Saf. 2008;31:159–168. doi: 10.2165/00002018-200831020-00005. [DOI] [PubMed] [Google Scholar]
- 15.Kerin NZ, Blevins RD, Goldman L, Faitel K, Rubenfire M. The incidence, magnitude, and time course of the amiodarone-warfarin interaction. Arch Intern Med. 1988;148:1779–1781. [PubMed] [Google Scholar]
- 16.Sanoski CA, Bauman JL. Clinical observations with the amiodarone/warfarin interaction: dosing relationships with long-term therapy. Chest. 2002;121:19–23. doi: 10.1378/chest.121.1.19. [DOI] [PubMed] [Google Scholar]
- 17.Pond SM, Graham GG, Wade DN, Sudlow G. The effects of allopurinol and clofibrate on the elimination of coumarin anticoagulants in man. Aust N Z J Med. 1975;5:324–328. doi: 10.1111/j.1445-5994.1975.tb03266.x. [DOI] [PubMed] [Google Scholar]
- 18.Jähnchen E, Meinertz I, Gilfrich HJ. Interaction of allopurinol with phenprocoumon in man. Klin Wochenschr. 1977;55:759–761. doi: 10.1007/BF01476963. [DOI] [PubMed] [Google Scholar]
- 19.Baillargeon J, Holmes HM, Lin YL, Raji MA, Sharma G, Kuo YF. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med. 2012;125:183–189. doi: 10.1016/j.amjmed.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schalekamp T, van Geest-Daalderop JH, Kramer MH, van Holten-Verzantvoort AT, de Boer A. Coumarin anticoagulants and co-trimoxazole: avoid the combination rather than manage the interaction. Eur J Clin Pharmacol. 2007;63:335–343. doi: 10.1007/s00228-007-0268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harder S, Thürmann P. Clinically important drug interactions with anticoagulants. An update. Clin Pharmacokinet. 1996;30:416–444. doi: 10.2165/00003088-199630060-00002. [DOI] [PubMed] [Google Scholar]
- 22.Bergk V, Haefeli WE, Gasse C, Brenner H, Martin-Facklam M. Information deficits in the summary of product characteristics preclude an optimal management of drug interactions: a comparison with evidence from the literature. Eur J Clin Pharmacol. 2005;61:327–335. doi: 10.1007/s00228-005-0943-4. [DOI] [PubMed] [Google Scholar]
- 23.Schelleman H, Bilker WB, Brensinger CM, Wan F, Yang YX, Hennessy S. Fibrate/Statin initiation in warfarin users and gastrointestinal bleeding risk. Am J Med. 2010;123:151–157. doi: 10.1016/j.amjmed.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sierpina VS, Wollschlaeger B, Blumenthal M. Ginkgo biloba. Am Fam Physician. 2003;68:923–926. [PubMed] [Google Scholar]
- 25.Jiang X, Williams KM, Liauw WS, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425–432. doi: 10.1111/j.1365-2125.2005.02322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behr S, Andersohn F, Garbe E. Risk of intracerebral hemorrhage associated with phenprocoumon exposure: a nested case-control study in a large population-based German database. Pharmacoepidemiol Drug Saf. 2010;19:722–730. doi: 10.1002/pds.1973. [DOI] [PubMed] [Google Scholar]
- 27.Patel MR, Mahaffey KW, Garg J, et al. ROCKET AF Investigators: Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 28.Connolly SJ, Ezekowitz MD, Yusuf S, et al. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 29.Cordes A, Vogt W, Dahm HH, et al. Phenprocoumon-induziertes Leberversagen. Dtsch Med Wochenschr. 2003;128:1884–1886. doi: 10.1055/s-2003-42161. [DOI] [PubMed] [Google Scholar]
- 30.Dietrich CG, Götz M, Fischbach W, et al. Schwere Hepatopathie und subakutes Leberversagen mit „Fast-track“-Leberzirrhose bei älterer Patientin. Z Gastroenterol. 2010;48:398–400. doi: 10.1055/s-0028-1109522. [DOI] [PubMed] [Google Scholar]
- 31.Pennartz C, Schrader H, Ritter PR, Tannapfel A, Schmidt WE, Meier JJ. Unklare Hepatopathie bei einem Patienten mit Vorhofflimmern. Internist (Berl) 2012;53:88–92. doi: 10.1007/s00108-011-2908-2. [DOI] [PubMed] [Google Scholar]
- 32.Bell LN, Chalasani N. Epidemiology of idiosyncratic drug-induced liver injury. Semin Liver Dis. 2009;29:337–347. doi: 10.1055/s-0029-1240002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein R. Evidence for immunological (allergic) mechanisms in a subgroup of patients with phenprocoumon-induced liver disease. Eur J Clin Pharmacol. 2009;65:1195–1201. doi: 10.1007/s00228-009-0705-9. [DOI] [PubMed] [Google Scholar]
- 34.Schimanski CC, Burg J, Möhler M, et al. Phenprocoumon-induced liver disease ranges from mild acute hepatitis to (sub-) acute liver failure. J Hepatol. 2004;41:67–74. doi: 10.1016/j.jhep.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Woolley S, Burger HR, Zellweger U. Phenprocoumon-induzierte cholestatische Hepatitis. Dtsch Med Wochenschr. 1995;120:1507–1510. doi: 10.1055/s-2008-1055506. [DOI] [PubMed] [Google Scholar]
- 36.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rochon J, Protiva P, Seeff LB, et al. Drug-Induced Liver Injury Network (DILIN): Reliability of the Roussel Uclaf Causality Assessment Method for assessing causality in drug-induced liver injury. Hepatology. 2008;48:1175–1183. doi: 10.1002/hep.22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rockey DC, Seeff LB, Rochon J, et al. US Drug-Induced Liver Injury Network: Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010;51:2117–2126. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thürmann PA. Sind Studienergebnisse pharmakologischer Interventionen auf den Alltag übertragbar? Z Evidenz Fortbild Qual Gesundh wesen. 2009;103:367–370. doi: 10.1016/j.zefq.2009.05.024. [DOI] [PubMed] [Google Scholar]
- e1.Begaud B, Evreux JC, Jouglard J, Lagier G. [Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France] Therapie. 1985;40:111–118. [PubMed] [Google Scholar]
- e2.Wennberg JE, Gittelsohn AM. U.S. Department of Health and Human Services (HRA) 80-14012. Washington: 2008. A small area approach to the analysis of health system performance. [Google Scholar]
- e3.Clopper C, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- e4.Fontana RJ, Seeff LB, Andrade RJ, et al. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010;52:730–742. doi: 10.1002/hep.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e5.Thürmann PA, Haack S, Werner U, et al. Tolerability of beta-blockers metabolized via cytochrome P450 2D6 is sex-dependent. Clin Pharmacol Ther. 2006;80:551–553. doi: 10.1016/j.clpt.2006.08.004. [DOI] [PubMed] [Google Scholar]
- e6.Schmiedl S, Szymanski J, Rottenkolber M, et al. Deutsche Pharmakovigilanz-Studiengruppe. Fingerhut - ein alter Hut? Eine Analyse stationärer Aufnahmen durch digitalisassoziierte unerwünschte Arzneimittelwirkungen. Med Klin (Munich) 2007;102:603–611. doi: 10.1007/s00063-007-1064-x. [DOI] [PubMed] [Google Scholar]
- e7.Rottenkolber D, Schmiedl S, Rottenkolber M, et al. Drug-induced blood consumption: the impact of adverse drug reactions on demand for blood components in German departments of internal medicine. Basic Clin Pharmacol Toxicol. 2012;111:240–247. doi: 10.1111/j.1742-7843.2012.00890.x. [DOI] [PubMed] [Google Scholar]
- e8.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA) Drug Saf. 1999;20:109–117. doi: 10.2165/00002018-199920020-00002. [DOI] [PubMed] [Google Scholar]
- e9.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Maintenance of the ICH Guideline on Clinical Safety Data Management: Data Elements for Transmission of Individual Case Safety Reports E2B(R2). 2001 [cited 24/09/2010] www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2B/Step4/E2B_R2__Guideline.pdf. (last accessed on 10th December 2012) [Google Scholar]
- e10.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229–2232. [PubMed] [Google Scholar]