Abstract
Background: Replacement of caloric beverages with noncaloric beverages may be a simple strategy for promoting modest weight reduction; however, the effectiveness of this strategy is not known.
Objective: We compared the replacement of caloric beverages with water or diet beverages (DBs) as a method of weight loss over 6 mo in adults and attention controls (ACs).
Design: Overweight and obese adults [n = 318; BMI (in kg/m2): 36.3 ± 5.9; 84% female; age (mean ± SD): 42 ± 10.7 y; 54% black] substituted noncaloric beverages (water or DBs) for caloric beverages (≥200 kcal/d) or made dietary changes of their choosing (AC) for 6 mo.
Results: In an intent-to-treat analysis, a significant reduction in weight and waist circumference and an improvement in systolic blood pressure were observed from 0 to 6 mo. Mean (±SEM) weight losses at 6 mo were −2.5 ± 0.45% in the DB group, −2.03 ± 0.40% in the Water group, and −1.76 ± 0.35% in the AC group; there were no significant differences between groups. The chance of achieving a 5% weight loss at 6 mo was greater in the DB group than in the AC group (OR: 2.29; 95% CI: 1.05, 5.01; P = 0.04). A significant reduction in fasting glucose at 6 mo (P = 0.019) and improved hydration at 3 (P = 0.0017) and 6 (P = 0.049) mo was observed in the Water group relative to the AC group. In a combined analysis, participants assigned to beverage replacement were 2 times as likely to have achieved a 5% weight loss (OR: 2.07; 95% CI: 1.02, 4.22; P = 0.04) than were the AC participants.
Conclusions: Replacement of caloric beverages with noncaloric beverages as a weight-loss strategy resulted in average weight losses of 2% to 2.5%. This strategy could have public health significance and is a simple, straightforward message. This trial was registered at clinicaltrials.gov as NCT01017783.
INTRODUCTION
Increased consumption of SSBs5 is frequently linked to increasing cardiometabolic problems worldwide (1, 2). In the United States, the percentage of kilocalories coming from caloric beverages has almost doubled from 1965 to 2002, reaching 21.0% in 2002 (1), and the intake of SSBs has been associated with negative health risks, including obesity (2, 3) and the metabolic syndrome (4, 5). Post hoc analysis of data from the PREMIER trial showed that a decrease of 1 serving per day of SSBs was associated with a 0.49-kg decrease in weight at 6 mo (6). Whereas many have argued that a simple reduction in consumption of SSBs may have positive effects on weight, others have noted the absence of any randomized trials specifically addressing this topic (3, 4). The current study was designed to address this gap in the literature.
Replacing specific foods or beverages that provide a substantial portion of daily calories may provide a useful strategy for modest weight reduction or weight-gain prevention (7). Beverages may be ideal targets (8, 9); however, substitution of noncaloric beverages for caloric beverages will be beneficial only if compensation, or consuming the calories from other foods, does not occur. Furthermore, it is not known whether replacing SSBs with water or DBs, both noncaloric alternatives, confer a similar advantage (10). DB consumption has been associated with some health risks (4, 11, 12), including the metabolic syndrome and type 2 diabetes. Yet, for the purposes of weight loss, replacing SSBs with DBs might promote greater adherence because of the availability of a variety of flavors and properties (eg, carbonated, caffeinated, and sweetened) similar to the SSBs, which leads to greater adherence. On the other hand, observational evidence suggests that drinking water is associated with weight loss and a reduction in caloric intake (13–16). In the laboratory setting, outcomes of consuming a preload amount of water before meal ingestion has led to varying results by age, with no effect on meal consumption among younger adults (17) but less energy consumed by middle-aged and older adults (18, 19).
Few randomized trials have examined the effects of changing beverage intake alone with a specific purpose to promote modest weight loss in adults (20–24). Published studies have examined children (20, 22, 23), both artificially sweetened foods and beverages (17), or premeal water consumption (24). None have examined the replacement of caloric beverages with either water or DBs as a simple calorie-reduction method (25, 26). This is the first randomized controlled trial to use noncaloric beverage substitution alone as the primary weight-loss strategy in overweight adults and to examine 2 noncaloric alternatives.
The primary hypothesis of the CHOICE clinical trial was that participants assigned to the beverage substitution groups would achieve greater weight loss at 6 mo than would participants in the AC group (DB group > AC and Water group > AC group). Secondary outcomes were to compare the noncaloric beverage groups with the control group on criterion measures of weight loss, waist circumference, BP, glucose, and osmolality as a marker of hydration from 0 to 3 and 0 to 6 mo.
SUBJECTS AND METHODS
Participants
We recruited, enrolled, and followed participants between May 2008 and January 2010 at UNC–Chapel Hill, North Carolina. Eligible participants (n = 318) were overweight and obese [BMI (kg/m2): 25–49.9] adults aged 18–65 y who reported consuming ≥280 kcal/d of caloric beverages (including SSBs, juice, juice drinks, sweetened coffee and tea, sweetened milk, sports drinks, and alcohol, excluding white milk) and were willing to make a dietary substitution recommended by the study. Participants were screened at baseline with the use of a beverage telephone screener modified to inquire over the past week and expanded on certain beverage categories from the beverage questions on the Block food-frequency questionnaire (27, 28). This screener assessed beverage intake over the past week to determine the average number of kilocalories derived from caloric beverages per day. Participants were excluded for a recent weight loss of >5%, participation in other weight-loss or physical-activity research, lactation, recent or planned pregnancy, thyroid medication use, diabetes mellitus treated with oral medication or insulin, cancer in the prior 5 y, history of myocardial infarction or heart surgery, current psychiatric treatment, psychiatric hospitalization in past year, alcohol dependence assessed with the Rapid Alcohol Problems Screen (29), plans to move or unable to attend monthly group meetings, and inadequate means to transport “study supplies” (beverages). The Physical Activity Readiness Questionnaire was administered to screen for readiness to safely engage in exercise (30). Participants reporting heart problems, frequent chest pains, or faintness or dizziness on the Physical Activity Readiness Questionnaire were excluded, and other medical conditions required physician's consent to participate. This study was approved by and was in accordance with the ethical standards of the UNC at Chapel Hill Institutional Review Board. Written informed consent was obtained from all participants.
Study design
This study was a 3-arm, single-center, single-blind randomized clinical trial. Participants were assigned to 1 of 2 intervention groups or to an AC group. Participants were oriented and enrolled by research staff. Participants consented to “making a dietary substitution that would enable them to reduce their caloric intake using food or beverage items commonly available at a grocery store.” After the participants’ specific substitution group was revealed, the substitution for the other study group was not revealed, and the controls were not informed of either substitution until all cohorts were completed. Eligible participants were randomly assigned as cohorts after baseline measures by using a computer-generated random-numbers method by the project coordinator with allocation concealed from the participants and investigators until randomization was revealed to the study participants at the initial group session. To limit seasonal effects on beverage consumption, the study was conducted in 5 cohorts, with the intervention beginning at different times of the year.
Interventions
Both of the intervention groups received noncaloric beverages [water or noncaloric sweetened (“diet”) beverages] and monthly group behavioral counseling to promote adherence to beverage substitution. The recommendations for each of these groups were identical except for the substituted beverage. Participants were encouraged to replace ≥2 servings (≥200 kcal) per day of caloric beverages with either water or DBs. On the basis of previous research (20), four 355–500-mL (12–16 oz) single-serving beverages per person per day were provided to ensure availability, with 2 additional servings per day to account for family members’ occasional consumption, although this was discouraged. Participants in the Water group could choose any combination of bottled still and nonsweetened sparkling water. Similarly, participants in the DB group were provided any combination of noncaloric sweetened beverages of their choice, including carbonated, noncarbonated, noncaffeinated, and caffeinated beverages. Both the Water and DB groups were given beverages at their monthly treatment group meeting.
The AC group was designed to equate treatment contact time and attention, monthly weigh-ins, and weekly monitoring to facilitate study of the additional benefit of beverage change. This group, called “Healthy Choices,” attended monthly group sessions of identical length to the beverage groups. They were weighed and given general weight-loss information (eg, instructed to read product labels, increase vegetable consumption, control portions, and increase physical activity); they were not given weight-loss calorie-reduction or physical activity goals. They were not encouraged to change beverage intake (beverages were not mentioned during the lessons or group sessions) and were not provided with beverages. All study groups had access to a group-specific study website, where they recorded the beverages (water and DB only) they consumed, reported their weekly weight, received feedback on progress, viewed tips, and linked to group-specific resources.
Measurements
Measurements were taken after a 12-h fast at baseline, 3 mo, and 6 mo. Body weight was measured, while the subjects were wearing a hospital gown and no shoes, with a calibrated digital scale (Tanita BWB 800). Height was measured at baseline by using a wall-mounted stadiometer (Perspective Enterprises Inc). Waist circumference was measured at the iliac crest (31). Resting BP was measured while the subjects were seated by using a GE Dinamap ProCare 100 after a 5-min rest; the average of 2 measurements was used (32). Fasting blood samples were collected by venipuncture according to a standard protocol. Participants provided urine samples in sterile containers for measurement of urine osmolality. All samples were analyzed at the UNC McLendon Clinical Laboratories.
Dietary intake data were collected and analyzed by using Nutrition Data System for Research software version 2008 (University of Minnesota). Two unannounced 24-h dietary recalls were administered over the telephone (one weekday and one weekend day) at each assessment period (33) by trained interviewers at the Nutrition Epidemiology Core of the UNC Clinical Nutrition Research Center (grant: DK56350). Energy expenditure was measured by using a telephone-administered 7-d physical activity recall (34) conducted after the first 24-h dietary recall.
Statistical analysis
Analysis of primary and secondary outcomes was performed by using SAS version 9.2 (SAS Institute Inc), with a type 1 error rate of 0.05 (2-tailed). For dietary data, in cases where 1 d of dietary recalls was provided, the actual data were used. Main effects of time, treatment group, and treatment-by-time interaction were examined in separate mixed-effect models for each outcome by using the unstructured dependence structure (35). For continuous variables, the Markov Monte Carlo method was used to impute missing data by generating a total of 10 imputations. Results from the imputations were combined by using PROC MIANALYZE in SAS.
Because the caloric values of the substitutions in the 2 intervention arms were equivalent and would promote equal weight loss under conditions of perfect adherence, we powered this study to compare the AC group with each of the intervention groups separately. We reasoned that adults consuming ≥280 kcal/d from beverages might realistically reduce consumption from 100 to ≥280 kcal/d, resulting in a total reduction in calories ranging from 700 to 1960 kcal/wk This range of calorie reductions would theoretically result in weight losses from 0.4 to 1.0 kg every month. Over a 6-mo period, average weight losses from 2.4 to 6 kg could be achieved with these reductions in SSB intake. With 100 participants per arm and α set at 0.05, we had 90% power to detect a difference of 1.8 kg with an SD of 3.4 kg and 25% attrition.
As a secondary comparison, the beverage groups were combined to examine the hypothesis that a reduction in caloric beverage consumption, regardless of the replacement type, would result in a greater likelihood of achieving a 5% weight loss than would the control (AC). For the binary outcome of achieving the 5% weight loss, multiple logistic regression was used, conservatively assuming that participants with missing data did not achieve this goal.
RESULTS
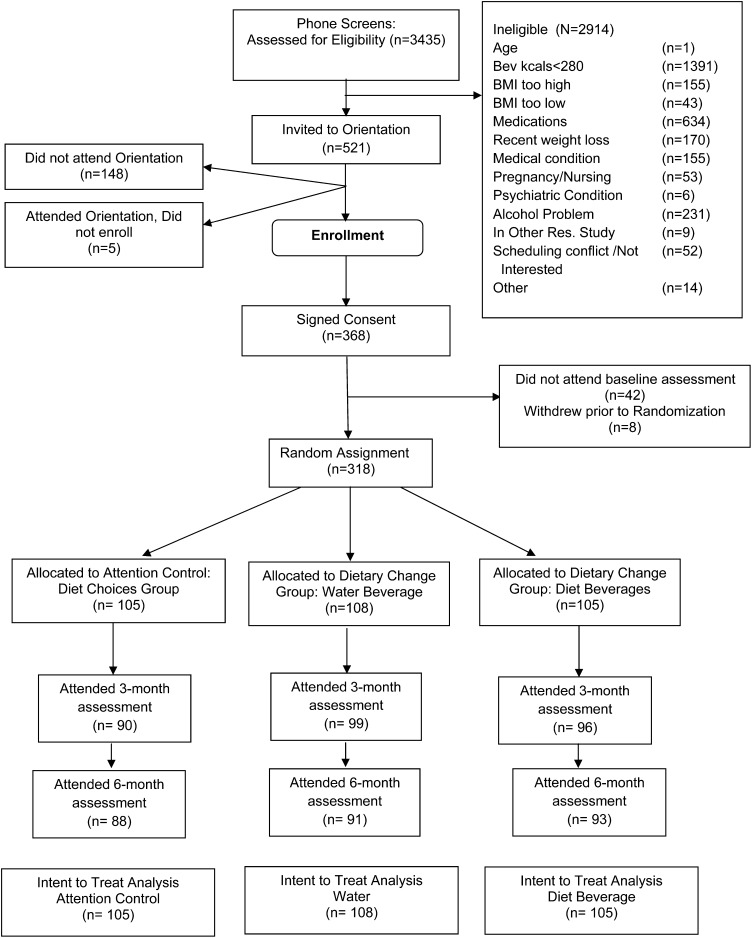
Of the 3435 persons who were assessed for eligibility, 2914 were ineligible and 318 underwent randomization (Figure 1): 105 were allocated to the AC group, 105 to the DB group, and 108 to the Water group. Participants were obese [BMI (kg/m2): 36.3 ± 5.9], 84% female, aged 42 ± 10.7 y, and of different ethnicities (54% black, 40% white, and 6% other race). No differences in baseline characteristics between the 3 groups were observed (Table 1; P > 0.05). At 3 mo, 86% (AC group), 92% (Water group), and 91% (DB group) and at 6 mo 84% (AC group), 84% (Water group), and 89% (DB group) attended the in-person measurement visit; retention rates did not differ between groups. Baseline variables—including age, race, marital status, BMI, education level, and employment status—were not significantly associated with retention of participants.
FIGURE 1.
Eligibility, enrollment, randomization, and follow-up of study participants—consort diagram. Res., research.
TABLE 1.
Baseline characteristics of the study population in the Choosing Healthy Options Consciously Everyday trial1
| Characteristic | Attentioncontrol(n = 105) | Diet beverageIntervention(n = 105) | Waterintervention(n = 108) |
| Age (y) | 41.56 ± 10.42 | 41.20 ± 11.2 | 43.2 ± 10.6 |
| Sex [n (%)] | |||
| Female | 90 (85.7) | 82 (78.1) | 96 (88.9) |
| Male | 15 (14.3) | 23 (21.9) | 12 (11.1) |
| Education [n (%)]3 | |||
| High school or less | 13 (12.5) | 7 (6.7) | 8 (7.4) |
| Some college | 42 (40.4) | 36 (34.3) | 45 (41.7) |
| College graduate or beyond | 49 (47.1) | 62 (59) | 55 (50.9) |
| Race or ethnic group [n (%)]4 | |||
| White | 45 (42.9) | 47 (44.8) | 36 (33.3) |
| Black | 51 (48.6) | 53 (50.5) | 67 (62) |
| Other | 9 (8.5) | 5 (4.8) | 5 (4.6) |
| Relation status [n (%)] | |||
| Married or living with partner | 54 (51.4) | 61 (58.1) | 58 (53.7) |
| Divorced, separated, widowed | 22 (21) | 16 (15.2) | 26 (24.1) |
| Never married | 29 (27.6) | 28 (26.7) | 24 (22.2) |
| Smoking status [n (%)] | |||
| Current smoker | 8 (7.6) | 11 (10.5) | 8 (7.4) |
| Former smoker | 25 (23.8) | 29 (27.6) | 27 (25) |
| Medications [n (%)] | |||
| Blood pressure | 30 (28.6) | 21 (20.0) | 21 (19.6) |
| Lipid lowering | 4 (3.8) | 7 (6.7) | 2 (1.9) |
| Diabetes, oral | 2 (1.9) | 1 (1.0) | 2 (1.9) |
| BMI5 | 36.8 ± 6.2 | 36.1 ± 6.2 | 35.8 + 5.2 |
Chi-square analysis showed no between-group differences.
Mean ± SD (all such values).
n = 104 in the AC group, because of 1 missing value for education.
Self-reported by the study participants.
Calculated as weight (in kg) divided by the square of height (in m).
Adherence to intervention and behaviors
The DB and Water groups attended significantly more of the 6 monthly group sessions than did the AC group: the DB group attended 5.4 ± 1.3 sessions (P < 0.001), the Water group attended 5.2 ± 1.3 sessions (P = 0.001), and the AC group attended 4.4 ± 1.8 sessions. A similar pattern of logins to the study website was observed between groups, with a significant difference observed only between the Water and AC groups (P < 0.05): 23.8 ± 23.9 logins in the DB group, 26.8 ± 37.5 in the Water group, and 16.9 ± 10.4 in the AC group over 6 mo.
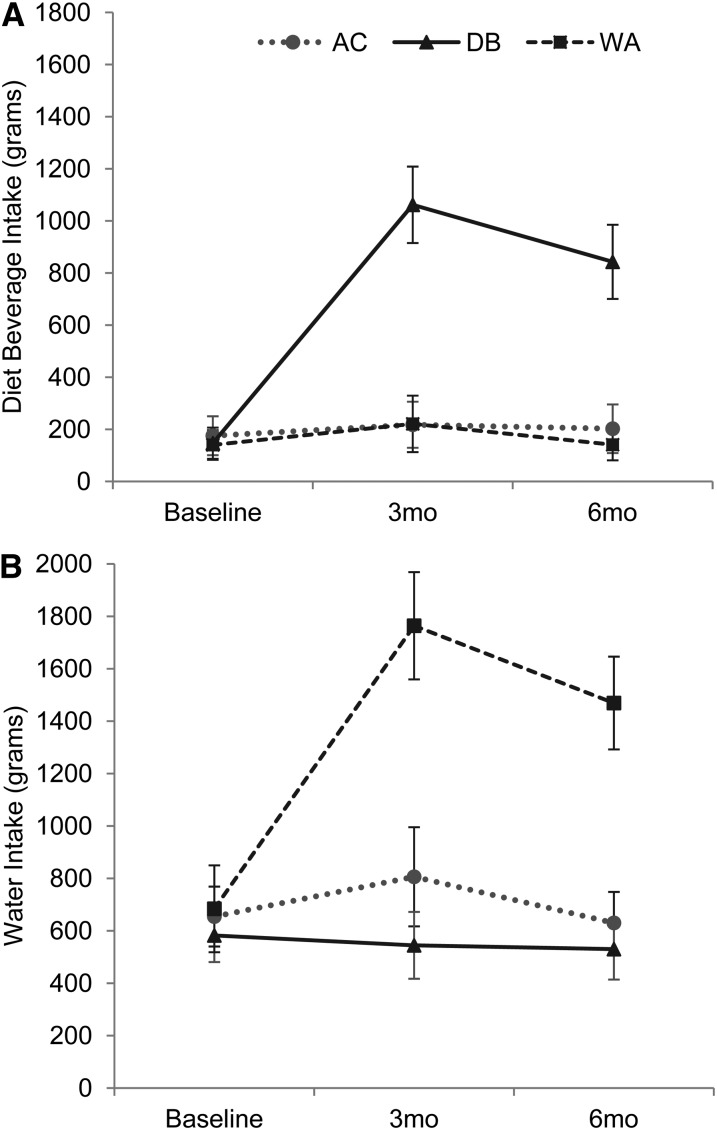
Participants in both the DB and Water groups had significantly higher reductions in caloric beverages (kcal/d) compared with the AC group at 3 and 6 mo, respectively (Table 2) : −112.81 kcal/d (95% CI: −127.87, −97.75) and −106.64 kcal/d (95% CI: −121.0, −92.28) for the AC group, −271.08 kcal/d (95% CI: −300.53, −241.63) and −259.83 kcal/d (95% CI: −288.31, −231.35) for the DB group, and –197.99 kcal/d (95% CI: −219.95, −176.03) and −187.40 kcal/d (95% CI: −208.38, −166.42) for the Water group. Intakes of the noncaloric beverage substitutions (ie, water and DBs) were in the expected direction based on intervention assignment (Figure 2). In comparison with baseline, water consumption increased significantly in the Water group at 3 mo (1080.11 g/d; 95% CI: 1054.78, 1105.44; P < 0.0001) and at 6 mo (785.21 g/d; 95% CI: 761.89, 808.53; P < 0.0001). These increases were significantly higher than changes in water consumption in the AC group of 151.78 g/d (95% CI: 130.55, 173.00; P < 0.0001) from 0 to 3 mo and of −24.17 g/d (−95% CI: −40.28, −8.26; P < 0.0001) from 0 to 6 mo. Water consumption decreased in the DB group from baseline to 3 mo (−37.81 g/d; 95% CI: −53.65, −21.97) and was significantly lower than in the Water group at 3 (P < 0.001) and 6 (P < 0.0001) mo. Changes in water consumption in the DB group were significantly lower than changes in the AC group at 3 mo (P = 0.048) but not at 6 mo (change of −52.17 g/d; 95% CI: −67.20, −37.15; P = 0.43). In comparison with baseline, the Water group consumed approximately an additional 1.0 L (∼34 oz) of water daily at 3 mo and an additional 0.8 L (∼27 oz) of water daily at 6 mo. Participants in the DB group had a significantly higher DB intake than did both the AC and Water groups at both 3 (P < 0.0001) and 6 (P < 0.0001) mo, but DB intake did not differ significantly between the Water and AC groups at either time (P = 0.97 and 0.35, respectively). In comparison with baseline, DB consumption increased in the DB group at 3 and 6 mo, respectively, by 914.81 g/d (95% CI: 899.36, 930.27; P < 0.0001) and 695.99 g/d (95% CI: 680.94, 711.05; P < 0.0001). These changes reflect a daily increase in DB intake of ∼0.9 L (30 oz) at 3 mo and of 0.7 L (24 oz) at 6 mo. Consumption of DBs in the AC group remained unchanged, increasing nonsignificantly at 3 mo by 42.18 g/d (95% CI: 30.94, 53.41) and at 6 mo by 26.98 g/d (95% CI: 15.34, 38.63). A similar nonsignificant increase in DB intake was observed in the Water group of 81.03 g/d (95% CI: 69.27, 92.80] at 3 mo and of 0.98 g/d [95% CI: −6.93, 8.90) at 6 mo. Physical activity and energy intake from caloric beverages and food are shown in Table 2. There were no between-group differences in energy intake from food. All groups showed statistically significant reductions in food energy intake from 0 to 6 mo (time: P < 0.0001). Participants made little change to intentional physical activity with no time or group × time effects (P > 0.05).
TABLE 2.
Anthropometric, diet, physical activity, and cardiometabolic changes in the AC, DB, and WA groups1
|
P value |
||||||||
| Assessment period2 |
Time |
Group × time interaction |
||||||
| Outcome variable and group | Baseline | 3 mo | 6 mo | 3 mo vs baseline | 6 mo vs baseline | Group | Baseline to 3 mo | Baseline to 6 mo |
| Weight (kg) | ||||||||
| AC | 102.6 (99.1, 106.1) | 101.1 (97.7, 104.6) | 100.7 (97.2, 104.2) | 0.0027 | <0.0001 | |||
| DB | 100.9 (97.1, 104.7) | 99.0 (95.3, 102.7) | 98.3 (94.5, 102.1) | <0.0001 | <0.0001 | 0.3501 | 0.2902 | 0.2361 |
| WA | 98.4 (95.2, 101.6) | 97.2 (94.0, 100.4) | 96.5 (93.3, 99.7) | <0.0001 | <0.0001 | 0.0967 | 0.5524 | 0.9949 |
| Waist circumference (cm) | ||||||||
| AC | 116.5 (113.9, 119.2) | 116.8 (114.1, 119.5) | 115.9 (113.2, 118.7) | 0.0928 | 0.0107 | |||
| DB | 115.5 (112.5, 118.5) | 114.9 (112.0, 117.8) | 113.4 (110.5, 116.4) | 0.4165 | 0.0103 | 0.2169 | 0.4333 | 0.2089 |
| WA | 115.1 (112.6, 117.6) | 114.4 (111.9, 116.9) | 113.1 (110.5, 115.8) | 0.2882 | 0.0143 | 0.1611 | 0.3315 | 0.2198 |
| Systolic BP (mm Hg) | ||||||||
| AC (n = 75) | 124.3 (120.9, 127.7) | 122.1 (118.3, 125.9) | 122.4 (119.5, 125.4) | 0.803 | 0.0008 | |||
| DB (n = 84) | 125.9 (122.9, 128.8) | 121.9 (119.2, 124.6) | 122.1 (119.4, 124.7) | 0.0014 | 0.0025 | 0.8353 | 0.3912 | 0.2404 |
| WA (n = 87) | 125.2 (122.1, 128.3) | 120.7 (117.4, 125.0) | 120.6 (117.9, 123.4) | 0.0002 | 0.0006 | 0.3682 | 0.1894 | 0.13373 |
| Diastolic BP (mm Hg) | ||||||||
| AC | 80.6 (78.5, 82.8) | 78.6 (76.0, 81.2) | 81.1 (78.9, 83.2) | 0.7053 | 0.1404 | |||
| DB | 82.1 (80.1, 84.0) | 81.0 (79.3, 82.7) | 81.4 (79.3, 83.4) | 0.2818 | 0.4155 | 0.8869 | 0.3193 | 0.3707 |
| WA | 80.5 (78.6, 82.5) | 79.0 (77.1, 80.9) | 79.5 (77.5, 81.5) | 0.0741 | 0.3098 | 0.3242 | 0.59253 | 0.29463 |
| Glucose (mg/dL) | ||||||||
| AC | 88.6 (86.6, 90.6) | 87.5 (85.1, 89.9) | 89.2 (87.2, 91.1) | 0.7055 | 0.0058 | |||
| DB | 91.7 (89.3, 94.1) | 90.2 (87.7, 92.7) | 89.7 (87.6, 91.9) | 0.1378 | 0.0978 | 0.7259 | 0.8563 | 0.1471 |
| WA | 93.1 (88.9, 97.2) | 90.1 (85.7, 94.6) | 89.9 (87.4, 92.3) | 0.0015 | 0.0027 | 0.6569 | 0.2143 | 0.019 |
| Urine osmolality (mOsmol/kg) | ||||||||
| AC | 673.9 (623.0, 724.9) | 690.9 (641.5, 740.3) | 706.7 (656.8, 756.6) | 0.0572 | 0.1744 | |||
| DB | 701.0 (654.5, 747.6) | 711.2 (660.0, 762.4) | 684.9 (635.7, 734.1) | 0.6819 | 0.5609 | 0.5350 | 0.8484 | 0.2070 |
| WA | 737.2 (690.0, 784.3) | 643.4 (594.8, 691.9) | 694.9 (645.9, 743.9) | <0.0001 | 0.12 | 0.7338 | 0.0017 | 0.0496 |
| Beverage (kcal) | ||||||||
| AC | 329.3 (280.2, 378.4) | 216.5 (183.0, 249.9) | 222.6 (190.6, 254.7) | 0.2733 | <0.0001 | |||
| DB | 390.4 (336.8, 444.1) | 119.3 (93.4, 145.2) | 130.6 (103.5, 157.6) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| WA | 326.6 (286.1, 367.0) | 128.6 (102.0, 155.2) | 139.2 (112.2, 166.1) | <0.0001 | <0.0001 | <0.0001 | 0.0151 | 0.02 |
| Food (kcal) | ||||||||
| AC | 1861.6 (1703.8, 2019.4) | 1501.5 (1391.4, 1611.5) | 1386.9 (1287.2, 1486.6) | 0.4775 | <0.0001 | |||
| DB | 1886.9 (1752.1, 2021.8) | 1621.3 (1512.0, 1730.7) | 1487.6 (1382.7, 1592.5) | 0.0001 | <0.0001 | 0.1677 | 0.399 | 0.5228 |
| WA | 1715.5 (1605.9, 1825.1) | 1396.9 (1317.6, 1476.2) | 1371.1 (1253.4, 1488.7) | <0.0001 | <0.0001 | 0.8436 | 0.8223 | 0.2840 |
| Physical activity (kcal) | ||||||||
| AC | 1015.6 (772.6, 1258.5) | 908.2 (679.9, 1136.5) | 1012.3 (753.8, 1270.8) | 0.8095 | 0.139 | |||
| DB | 1083.7 (828.9, 1338.6) | 1225.4 (886.2, 1564.6) | 1012.0 (730.8, 1293.2) | 0.1739 | 0.6349 | 0.0655 | 0.2256 | 0.1761 |
| WA | 753.2 (507.4, 999.0) | 909.0 (672.4, 1145.5) | 962.9 (718.3, 1207.4) | 0.5072 | 0.9811 | 0.1400 | 0.8158 | 0.2750 |
Intention-to-treat with multiple imputation repeated-measures mixed-model analysis. n = 108 for WA, n = 105 for DB, and n = 105 for AC for all analyses except those for BP and glucose. Adjustments were made to the sample to exclude for BP and diabetes medication use. For BP: n = 87 for WA, n = 84 for DB, and n = 75 for AC. For glucose: n = 106 for WA, n = 104 for DB, and n = 103 for AC. Statistically significant P values are shown in bold. AC, attention control; BP, blood pressure; DB, diet beverage; WA, water.
All values are means; 95% CIs in parentheses.
In a completer's analysis using repeated-measures mixed model, there was a significant treatment × time interaction for the comparison between the WA and AC groups (P < 0.05).
FIGURE 2.
Means (95% CIs) estimated by using an intention-to-treat analysis with multiple imputation of DB intake (A; n = 105) and WA intake (B; n = 108) during the 6-mo study. A repeated-measures mixed model was used to examine time, group, and treatment × time interaction at each time point. A: time 0–3 and 0–6 mo, P < 0.001; treatment × time at 3 and 6 mo, P < 0.001. B: time 0–3 and 0–6 mo, P < 0.001; treatment × time at 3 and 6 mo, P < 0.001. AC: n = 105. AC, attention control; DB, diet beverage; WA, water.
Physiologic measures: weight, waist, BP, glucose, and hydration
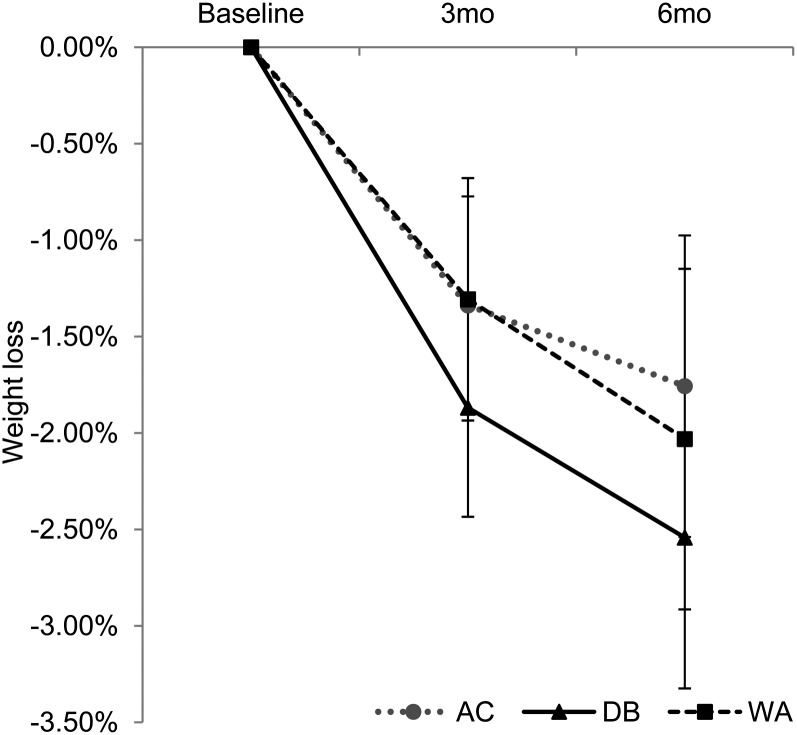
All groups lost weight at 3 and 6 mo (Table 2; all P < 0.01); neither the DB group nor the Water group differed from the AC group at either time point. The mean (±SE) percentage weight loss (Figure 3) was −1.34% ± 0.27 in the AC group, −1.87% ± 0.32 in the DB group, and −1.31% ± 0.27 in the Water group at 3 mo and was −1.76% ± 0.35 in the AC group, −2.54% ± 0.45 in the DB group, and −2.03% ± 0.40 in the Water group at 6 mo. Other physiologic comparisons between the 2 beverage groups and the AC group are shown in Table 2. Absolute weight, waist circumference, and systolic BP had decreased significantly by 6 mo in all groups, but there were no treatment × time interactions between the Water group and the AC group or between the DB group and the AC group on these outcomes at either 3 or 6 mo. The Water group showed significant improvements in fasting glucose of −3.21 mg/dL (95% CI −3.89, −2.53) compared with the AC group (0.59 mg/dL; 95% CI: 0.35, 0.83) at 6 mo; fasting glucose decreased by −1.92 mg/dL (95% CI: −2.38, −1.46) at 6 mo in the DB group, but was not different from that in the AC group. The Water group also significantly improved urine osmolality: −93.83 mOsmol/kg (95% CI: −108.26, −79.4) at 3 mo and −42.27 mOsmol/kg (95% CI: −52.16, −32.38) at 6 mo compared with the AC group at 3 mo (17.00 mOsmol/kg; 95% CI: 11.11, 22.89) and 6 mo (32.76 mOsmol/kg; 95% CI: 27.51, 38.01). Urine osmolality in the DB group was not significantly different from that in the AC group at 3 mo (10.19 mOsmol/kg; 95% CI: 4.13, 16.25) and at 6 mo (−16.11 mOsmol/kg; 95% CI: −23.94, −8.28).
FIGURE 3.
Means (95% CIs) estimated by using an intention-to-treat analysis with multiple imputation. A repeated-measures mixed model was used to examine time, group, and treatment × time interaction at each time point. A significant effect of time was observed for the DB (n = 105), WA (n = 108), and AC (n = 105) groups at 0–3 mo (P < 0.001) and 0–6 mo (P < 0.001). There were no significant treatment × time interactions between the WA and AC groups or between the DB and AC groups at any time. AC, attention control; DB, diet beverage; WA, water.
In a secondary analysis of study completers, results were consistent with the findings of the multiple imputation analysis except with respect to BP. Compared with the AC group, completers in the Water group had a significantly lower systolic BP at 6 mo (P = 0.03) and diastolic BP at both 3 (P = 0.03) and 6 (P = 0.04) mo.
Criterion measures of weight loss
Participants assigned to the DB group had a greater likelihood of achieving a 5% weight loss than did the AC group (OR: 2.29; 95% CI: 1.05, 5.01; P = 0.04), but the OR was not significantly different between the water group and the AC group (OR: 1.87; 95% CI: 0.84, 4.14; P = 0.13). To explore the hypothesis that replacing beverages (regardless of substitution) results in a significant weight loss at 6 mo, a combined analysis of participants in either beverage group was compared with the AC group. Participants assigned to the combined beverage replacement interventions were twice as likely to have achieved a 5% weight loss than were those in the AC group (OR: 2.07; 95% CI: 1.02, 4.22; P = 0.04); 19.5% (n = 42) in the beverage groups combined achieved a 5% loss compared with 10.5% (n = 11) in the AC group.
DISCUSSION
Participants in the beverage interventions significantly reduced their intake of caloric beverages and increased their consumption of the recommended noncaloric beverages in a manner consistent with intervention assignment. The average daily reduction of caloric beverages across both of the beverage groups was approximately the 2-serving/d recommendation that was prescribed (eg, −235 kcal/d at 3 mo and −225 kcal/d by 6 mo). The AC group also reduced their caloric beverage consumption by ∼1 serving/d (−112 kcal/d at 3 mo, −106 kcal/d at 6 mo), despite not being informed about the true study purpose or substitutions to that group or about the beverages being used as a weight-control strategy in the AC treatment sessions. Thus, we observed a smaller caloric difference between the 2 beverage substitution groups and the control group. All interventions groups showed statistically significant weight losses by 6 mo, but there were no differences between groups.
The DB group reported an absolute reduction in caloric beverage intake of ∼70 kcal more per day at both 3 and 6 mo compared with the Water group, although reported reductions in energy (kcal) from food averaged over the 6 mo were very similar between the DB and Water groups. This reflected better adherence to the beverage replacement prescription in the DB group, which resulted in a greater likelihood of achieving a 5% weight loss compared with the AC group at 6 mo. The greater adherence to intake of DBs over time may have been due to the variety of flavors (36) or similar properties to the caloric beverage (eg, caffeinated), which suggests that it may be easier for consumers of caloric beverages to replace their beverages with noncaloric sweetened alternatives. The pattern of weight loss in the Water group suggests a slower weight loss and may reflect that consumers of caloric beverages needed to adjust preferences for consuming nonsweetened beverages over time.
Despite similar or somewhat smaller weight losses, the water replacement groups showed statistically significant reductions in fasting glucose and improvements in hydration compared with the control AC group. The DB group also showed improvements in many of these variables by 6 mo, but the changes were not significantly different from those in the AC group. In the completer's analysis, the improvements in systolic and diastolic BP in the Water group were statistically significant from those in the AC group. Reductions in fasting glucose and BP seen in the Water group are similar to the improvements seen in other weight-loss trials at 6 mo (37, 38). These analyses should be interpreted with caution because the hydration-BP relation has not been well-studied and other mechanisms, other than the benefits of lowering body weight, are not clear (39). Future analyses will examine potential diet, physical activity, and other mechanisms that might account for these changes.
The design of the current study differs from that of other studies in the literature, ie, previous research in adults focused on changes in beverage intake as part of overall dietary restrictions aimed to induce weight loss rather than on beverage replacement as the primary weight-loss strategy. Despite these differences, this study can be compared with others that examined the effects of beverage consumption during weight loss. In examining our findings on changes in caloric beverages overall, this study showed that a reduction in caloric beverages of ∼2 servings resulted in a 2-kg weight loss at 6 mo across the DB and Water groups, which is more than the 0.6-kg weight loss that was associated with a 200-kcal/d reduction in liquid calories in a secondary data analysis of PREMIER. This study did not show as much of an advantage to an increase in water consumption as has been reported previously in the literature (13, 24). Participants in our study reported an increased consumption of water of 1 L/d at 3 mo and of ∼0.80 L/d at 6 mo, yet they lost ∼0.25% (or 0.3 kg) more than those in the AC group at 6 mo. Stookey et al (14) reported a 2-kg greater weight loss among water consumers on a hypocaloric diet than among those who consumed less water, although participants in that study were not randomly assigned to consume more water. In the study by Dennis et al (24), middle-aged adults on a hypocaloric diet were randomly assigned to consume 500 mL (∼16 oz) of water 3 times/d, before each meal, or to follow the hypocaloric diet without premeal water consumption. Those randomly assigned to premeal water consumption lost ∼1.3% more than did those on the hypocaloric diet alone (P = 0.13). Future studies could examine whether the amount (eg, 1 compared with 1.5 L), the timing (premeal compared with not), or the other factors explain these differences. Notably, these prior studies included 8–29% nonwhite participants, whereas CHOICE included 60% nonwhite participants.
The results of this trial are encouraging despite the modest weight loss achieved. In more intensive clinic-based behavioral lifestyle modification programs (40–42), 5–10% weight losses have been observed at 6 mo. This is not surprising because such programs typically include greater caloric restriction (500–1000 kcal/d), goals for caloric expenditure, more intensive diet and activity monitoring, and frequent patient-provider contact. This intervention required minimal self-monitoring (only beverages) and included monthly treatment visits with recommendations to change one aspect of dietary behavior and produced a 2–2.5% weight loss. The importance of caloric beverages as a target for calorie reduction is noteworthy because they are typically consumed at least daily, whereas food intake types may vary. This approach is more consistent with others recommending small but potentially sustainable lifestyle changes that can be made to improve health (43–45).
The strengths of this study were that it is the first randomized trial in adults to examine a simple strategy for calorie reduction and weight control, with participants masked to the study purpose, including >50% racial and ethnic minorities, strong retention rates, 24-h dietary recalls, provision of beverages, an AC group, and objective weight and physiologic outcome measures. Importantly, the AC group was not a “no treatment control group”; this group was taught general weight-control strategies, reported weight and general behavior (not kcal) weekly, and attended 60-min monthly treatment meetings equating for contact time and other variables known to affect weight loss among motivated individuals. Limitations of the study included the potential for being underpowered, self-report measures of diet and physical activity, underrepresentation of men, a relatively short duration (6 mo) to allow benefit of a small caloric change such as beverage substitution to accrue, and lack of long-term follow-up.
On a population level, replacement of caloric beverages with noncaloric alternatives could be an important public health message. This strategy also has implications for health care settings because assessing SSB intake is feasible, and the prescriptive recommendation to replace caloric beverages with noncaloric alternatives is simple and straightforward. Replacing SSB with either DBs or water, based on the consumers’ preference and ability to adhere, appears warranted at this stage of research on the basis of these findings. Future research should examine long-term health effects of consuming either beverage as a replacement for caloric beverages before specific recommendations can be made.
Acknowledgments
The CHOICE Study would not have been possible without its participants and the UNC–Chapel Hill weight research program staff and interventionists, including Melissa Crane, Keneisha Quick, Dori Steinberg, James Smith, Noel Kulik, Steven Zablonski, and Jordan Wong.
The authors’ responsibilities were as follows—DFT: had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; DFT, JS, and BP: designed the research; DFT, KE, KP, MD, and GT-M: conducted the research; XW and EL: analyzed the data; DFT, GT-M, JS, and BP: wrote the manuscript; and DFT: had primary responsibility for the final content. All authors read and approved the final manuscript. None of the authors has or had any conflicts of interest. Nestlé Waters USA had no involvement in the study design, conduct, or preparation and review of this manuscript.
Footnotes
Abbreviations used: AC, attention control; BP, blood pressure; CHOICE, Choosing Healthy Options Consciously Everyday; DB, diet beverage; SSB, sugar-sweetened beverage; UNC, University of North Carolina.
REFERENCES
- 1.Duffey KJ, Popkin BM. Shifts in patterns and consumption of beverages between 1965 and 2002. Obesity (Silver Spring) 2007;15:2739–47 [DOI] [PubMed] [Google Scholar]
- 2.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 2006;84:274–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health 2007;97:667–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D′Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007;116:480–8 [DOI] [PubMed] [Google Scholar]
- 5.Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Appel LJ, Loria C, Lin PH, Champagne CM, Elmer PJ, Ard JD, Mitchell D, Batch BC, Svetkey LP, et al. Reduction in consumption of sugar-sweetened beverages is associated with weight loss: the PREMIER trial. Am J Clin Nutr 2009;89:1299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennis EA, Flack KD, Davy BM. Beverage consumption and adult weight management: a review. Eat Behav 2009;10:237–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DellaValle DM, Roe LS, Rolls BJ. Does the consumption of caloric and non-caloric beverages with a meal affect energy intake? Appetite 2005;44:187–93 [DOI] [PubMed] [Google Scholar]
- 9.Flood JE, Roe L, Rolls BJ. The effect of increased beverage portion size on energy intake at a meal. J Am Diet Assoc 2006;106:1984–90 [DOI] [PubMed] [Google Scholar]
- 10.Nielsen SJ, Siega-Riz AM, Popkin BM. Trends in energy intake in U.S. between 1977 and 1996: similar shifts seen across age groups. Obes Res 2002;10:370–8 [DOI] [PubMed] [Google Scholar]
- 11.Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR., Jr Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009;32:688–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation 2008;117:754–61 [DOI] [PubMed] [Google Scholar]
- 13.Stookey JD, Constant F, Gardner CD, Popkin BM. Replacing sweetened caloric beverages with drinking water is associated with lower energy intake. Obesity (Silver Spring) 2007;15:3013–22 [DOI] [PubMed] [Google Scholar]
- 14.Stookey JD, Constant F, Gardner CD, Popkin BM. Drinking water is associated with weight loss in overweight dieting women independent of diet and activity. Obesity (Silver Spring) 2008;16:2481–8 [DOI] [PubMed] [Google Scholar]
- 15.Wang YC, Ludwig DS, Sonneville K, Gortmaker SL. Impact of change in sweetened caloric beverage consumption on energy intake among children and adolescents. Arch Pediatr Adolesc Med 2009;163:336–43 [DOI] [PubMed] [Google Scholar]
- 16.Daniels MC, Popkin BM. Impact of water intake on energy intake and weight status: a systematic review. Nutr Rev 2010;68:505–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolls BJ, Kim S, Fedoroff IC. Effects of drinks sweetened with sucrose or aspartame on hunger, thirst and food intake in men. Physiol Behav 1990;48:19–26 [DOI] [PubMed] [Google Scholar]
- 18.Davy BM, Dennis EA, Dengo AL, Wilson KL, Davy KP. Water consumption reduces energy intake at a breakfast meal in obese older adults. J Am Diet Assoc 2008;108:1236–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Walleghen EL, Orr JS, Gentile CL, Davy BM. Pre-meal water consumption reduces meal energy intake in older but not younger subjects. Obesity (Silver Spring) 2007;15:93–9 [DOI] [PubMed] [Google Scholar]
- 20.Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics 2006;117:673–80 [DOI] [PubMed] [Google Scholar]
- 21.Raben A, Vasilaras TH, Møller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 2002;76:721–9 [DOI] [PubMed] [Google Scholar]
- 22.James J, Thomas P, Cavan D, Kerr D. Preventing childhood obesity by reducing consumption of carbonated drinks: cluster randomised controlled trial. BMJ 2004;328:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Libuda L, Alexy U, Sichert-Hellert W, Stehle P, Karaolis-Danckert N, Buyken AE, Kersting M. Pattern of beverage consumption and long-term association with body-weight status in German adolescents—results from the DONALD study. Br J Nutr 2008;99:1370–9 [DOI] [PubMed] [Google Scholar]
- 24.Dennis EA, Dengo AL, Comber DL, Flack KD, Savla J, Davy KP, Davy BM. Water consumption increases weight loss during a hypocaloric diet intervention in middle-aged and older adults. Obesity (Silver Spring) 2010;18:300–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr 2009;89:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allison DB, Mattes RD. Nutritively sweetened beverage consumption and obesity: the need for solid evidence on a fluid issue. JAMA 2009;301:318–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Block G, Mandel R, Gold E. On food frequency questionnaires: the contribution of open-ended questions and questions on ethnic foods. Epidemiology 2004;15:216–21 [DOI] [PubMed] [Google Scholar]
- 28.Block G, Wakimoto P, Jensen C, Mandel S, Green RR. Validation of a food frequency questionnaire for Hispanics. Prev Chronic Dis 2006;3:A77. [PMC free article] [PubMed] [Google Scholar]
- 29.Cherpitel CJ. Screening for alcohol problems in the U.S. general population: comparison of the CAGE, RAPS4, and RAPS4-QF by gender, ethnicity, and service utilization. Rapid Alcohol Problems Screen. Alcohol Clin Exp Res 2002;26:1686–91 [DOI] [PubMed] [Google Scholar]
- 30.ACSM American College of Sports Medicine's guidelines for exercise testing and prescription. 6th ed New York, NY: Lippincott, Williams, & Wilkins, 2000 [Google Scholar]
- 31.Mason C, Katzmarzyk PT. Effect of the site of measurement of waist circumference on the prevalence of the metabolic syndrome. Am J Cardiol 2009;103:1716–20 [DOI] [PubMed] [Google Scholar]
- 32.National Institutes of Health, National Heart, Lung, and Blood Institute, Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure North American Association for the Study of Obesity. The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. Bethesda, MD: NIH, 2000 [Google Scholar]
- 33.Fox TA, Heimendinger J, Block G. Telephone surveys as a method for obtaining dietary information: a review. J Am Diet Assoc 1992;92:729–32 [PubMed] [Google Scholar]
- 34.Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, Paffenbarger RS Jr. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol 1985;121:91–106 [DOI] [PubMed] [Google Scholar]
- 35.Little RJA. Regression with missing X's: a review. J Am Stat Assoc 1992;87:1227–37 [Google Scholar]
- 36.Raynor HA, Jeffery RW, Phelan S, Hill JO, Wing RR. Amount of food group variety consumed in the diet and long-term weight loss maintenance. Obes Res 2005;13:883–90 [DOI] [PubMed] [Google Scholar]
- 37.Tate DF, Jackvony EH, Wing RR. Effects of Internet behavioral counseling on weight loss in adults at risk for type 2 diabetes: a randomized trial. JAMA 2003;289:1833–6 [DOI] [PubMed] [Google Scholar]
- 38.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009;360:859–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popkin BM, D'Anci KE, Rosenberg IH. Water, hydration, and health. Nutr Rev 2010;68:439–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Look AHEAD Research Group, Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med 2010;170:1566–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin PH, Svetkey LP, et al. , Writing Group of the PREMIER Collaborative Research Group Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 2003;289:2083–93 [DOI] [PubMed] [Google Scholar]
- 42.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gokee LaRose J, Tate DF, Gorin AA, Wing RR. Preventing weight gain in young adults: a randomized controlled pilot study. Am J Prev Med 2010;39:63–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Damschroder LJ, Lutes LD, Goodrich DE, Gillon L, Lowery JC. A small-change approach delivered via telephone promotes weight loss in veterans: results from the ASPIRE-VA pilot study. Patient Educ Couns 2010;79:262–6 [DOI] [PubMed] [Google Scholar]
- 45.Stroebele N, de Castro JM, Stuht J, Catenacci V, Wyatt HR, Hill JO. A small-changes approach reduces energy intake in free-living humans. J Am Coll Nutr 2009;28:63–8 [DOI] [PMC free article] [PubMed] [Google Scholar]