Abstract
The main source of calcium carbonate (CaCO3) in the ocean comes from the shells of calcifying planktonic organisms, but substantial amounts of CaCO3 are also produced in fish intestines. The precipitation of CaCO3 assists fish in intestinal water absorption and aids in whole body Ca2+ homeostasis. Here we report that the product formed in the intestinal lumen of the gilt-head seabream, Sparus aurata, is an amorphous calcium carbonate (ACC) phase. With FTIR spectroscopy and SEM imaging, our study shows that the fish-derived carbonates from S. aurata are maintained as a stable amorphous phase throughout the intestinal tract. Moreover, intestinal deposits contained up to 54 mol% Mg2+, the highest concentration yet reported in biogenic ACC. Mg is most likely responsible for stabilizing this inherently unstable mineral. The fish carbonates also displayed initial rapid dissolution when exposed to seawater, exhibiting a significant increase in carbonate concentration.
Elemental production and cycling in our oceans are among the most important processes in the support of marine life. Elements such as Ca2+ and Mg2+, in addition to their substantial contribution to marine sediments, are used biologically in vital cellular processes and in the mineralization of skeletons and shells, made of calcium carbonate (CaCO3)1. Oceanic production of CaCO3 is usually attributed to the shells of marine planktonic organisms, specifically coccolithophores, foraminifera and pteropods2,3. However, a lesser-known source of biogenic carbonate originates from the intestinal tract of bony fishes4,5.
As part of a unique strategy for osmoregulation in a hyperosmotic environment, marine fish (as well as seawater-acclimated freshwater fish) actively drink the surrounding seawater to stay hydrated. The influx of divalent ions from seawater (10 mM Ca2+, 53 mM Mg2+, 27 mM SO42-)6 is, however, poorly absorbed by the teleost intestine and poses a challenge for the animal's osmotic regulation. In direct response, epithelial cells lining the intestine secrete significant concentrations of bicarbonate (30–100 mM HCO3−)7, effectively creating alkaline conditions (~pH 8 to 9), which lead to CaCO3 pellet formations, enveloped in a mucus sheath8. The chemical reaction producing calcium carbonate9 in the fish's intestine is illustrated by Wilson et al. (2002)10, and summarized here:
While the Ca2+ originates from imbibed seawater, the HCO3− source is produced from endogenous metabolic CO2 hydration within the intestinal epithelia10,11,12,13. The resulting precipitation of CaCO3 is said to facilitate water absorption10,12,13, by lowering the luminal fluid osmotic pressure, as well as aiding in Ca2+ homeostasis14, by removing excess Ca2+ entry into the body. Little is known, however, about the mechanism of intestinal CaCO3 formation or what occurs after the mineral deposits are excreted to the surrounding seawater.
Though previous studies have identified the intestinal deposits as Mg-calcite5,15,16, the widespread distribution of amorphous phases in biological systems17,18,19,20,21 led us to investigate this possibility in fish-gut carbonates. Amorphous calcium carbonate (ACC) is a unique mineral form produced either as a transient precursor to a final crystallized (calcite or aragonite) structure or as a stable form during the lifetime of the organism22,23. Among the advantages of biological utilization of ACC, is its isotropic structure lacking a preferred growth direction, which allows the material to take on any shape as it forms24. Furthermore, ACC is highly soluble and able to integrate significant amounts of additional elements for stabilization, which makes this phase appropriate for temporary storage of ions25,26. Our current work examines the composition, structure, and solubility of the intestinal carbonates collected from fresh unpreserved gilt-head seabream, Sparus aurata, intestinal tracts.
Results
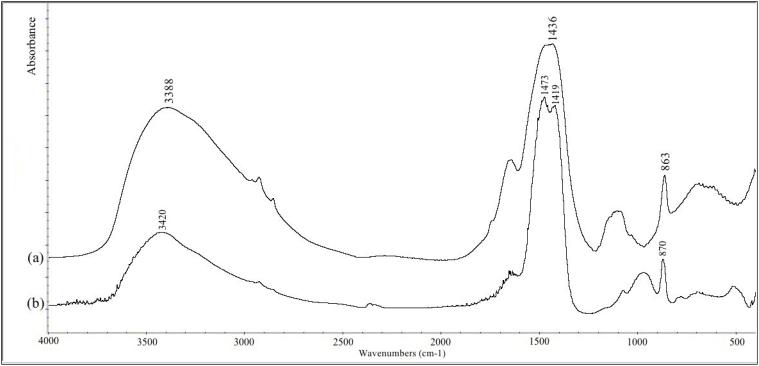
The FTIR spectrum of a fresh extracted fish-gut carbonate sample from S. aurata, (Fig. 1), shows a pronounced similarity to a synthetic amorphous calcium carbonate (ACC) standard. The characteristic peak absorptions are a double peak around 1450 cm−1, a relatively broad peak around 873 cm−1 and the absence of a peak at 712 cm−1. The presence of a peak at 712 cm−1 would be an indication of calcite. Note that the broad peak in the synthetic ACC standard spectrum around 1000 cm−1 is probably due to the stabilizing additives (phosphate). It is interesting that in the fish-gut ACC no additional peaks are present that might indicate the presence of a stabilizing agent.
Figure 1. Infrared spectra of (a) mineral deposit from the hind-gut, and (b) an amorphous calcium carbonate standard.
The large water absorption peak around 3400 cm−1, the broad partially split peak around 1440 cm−1, the peak at 863 cm−1 and the absence of a peak at 712 cm−1 all indicate that the mineral phase in the fish hind-gut is ACC. Note that the slight shits in peak maxima and the extent of splitting of the main peak around 1440 cm−1 show that the standard is probably more disordered than the fish ACC. The additional peaks in both spectra are due to additives that are probably involved in stabilizing the ACC or associated organic matter.
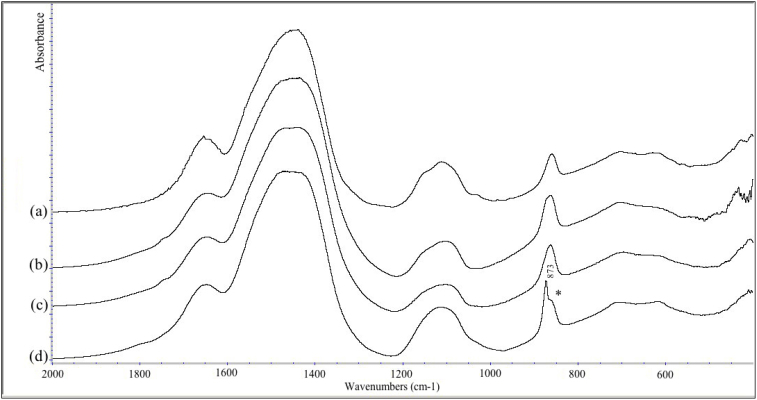
Figure 2 shows the analyses of fish-gut samples from the foregut, mid-gut, hind-gut and rectum. All the samples were composed of ACC. Interestingly, early stages of crystallization were present in the posterior regions, hind-gut and rectum of one fish (starred peak in Fig. 2). This is inferred from the sharpening up of the 873 cm−1 peak.
Figure 2. Infrared spectra of (a) the foregut, (b) the midgut and (c) the hindgut mineral deposits from one fish, and (d) the hindgut mineral deposits from another fish.
The peaks characteristic of ACC are present in (a), (b) and (c). In (d) the 860 cm−1 peak is split with a sharper peak at 873 cm−1 indicative of the presence of some crystalline calcite in addition to the ACC.
Upon dissection, a rapid assessment of the intestinal fluid pH revealed it was highly alkaline, consistent with findings from other physiological studies on marine fish4,7,10. ICP-AES results show near identical concentrations (mg/L) of Ca2+ and Mg2+ in the fish-gut samples. Magnesium content of fish carbonates in the intestinal tract was found to be 50.3 mol% (n = 4), with a maximum of 54.0 mol%.
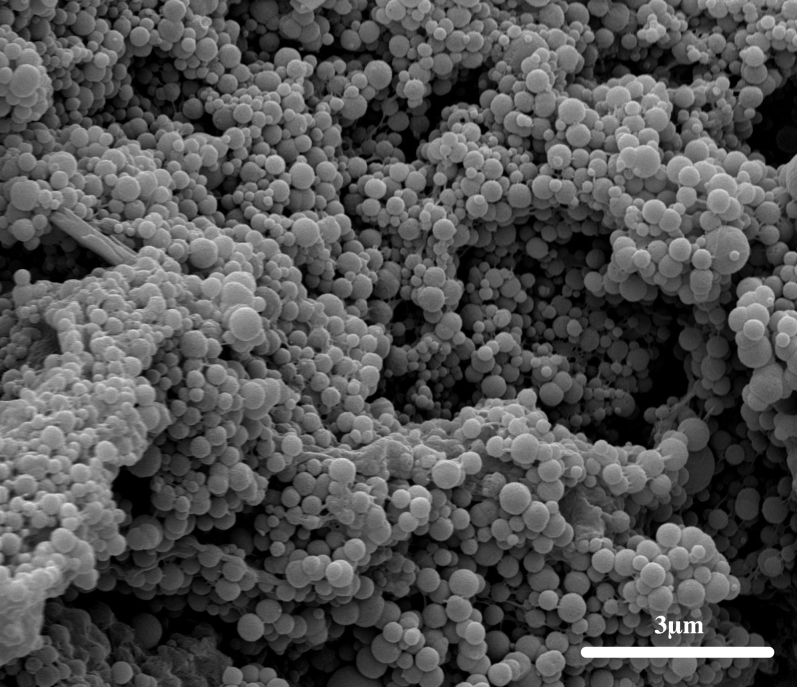
The Scanning Electron Microscope (SEM) image of fish-gut excretions revealed smooth, round aggregates of ACC, about 200–500 nm in diameter (Fig. 3). This spherical appearance is characteristic of most amorphous mineral phases25, including ACC27.
Figure 3. SEM image of ACC aggregates from the intestinal tract of S. aurata.
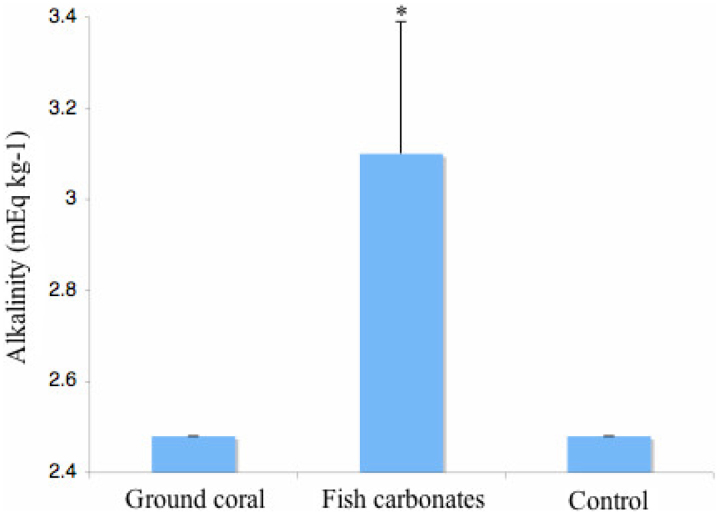
Fish carbonates incubated for one hour in seawater significantly increased the alkalinity value (p < 0.0001, n = 7) as compared to the control (seawater only) and ground coral treatment (Fig. 4). In particular, [CO32−] was almost 2-fold greater in samples containing fish-derived ACC (see SI appendix, Table S1).
Figure 4. Alkalinity results after a 1-hour incubation in ambient (pH 8.1) seawater.
Discussion
In order to test for the possibility of encountering amorphous material in the intestinal deposits of fish, careful preparation protocols are necessary. Weiner et al. (2003) suggest preparation methods that limit the samples' exposure to air, and to other dehydrating factors, as not all biogenic ACC phases remain stable after extraction. Moreover, heat, age and even contact with water may also induce ACC to crystallize28. In this study, we used only freshly extracted mineral that was washed for a few seconds in distilled water, then acetone for less than a minute and finally ethanol. The sample was immediately dried for less than a minute under a heat lamp, and analysed within another few minutes.
Using the gentle extraction procedure described in the methods section, the following FTIR analysis and SEM image (Fig. 1 and 3) unequivocally confirm the precipitates formed in the intestine of Sparus aurata are an amorphous phase. In addition to the results represented in Fig. 1, we were also able to identify other excreted fish carbonates as ACC, for example, the threespot dascyllus (Dascyllys trimaculatus) and giant frogfish (Antennarius commersoni) from the Gulf of Eilat (SI appendix, Fig S1−S2). These findings suggest many, if not all, other marine fish utilize this amorphous pathway in the precipitation of intestinal CaCO3. However, additional work should be conducted to further investigate the consistency of this conclusion among other species.
Remarkably, this is not the only report of piscine use of an amorphous phase. In a bone mineralization study, Mahamid et al. (2008) discovered an amorphous calcium phosphate phase in developing fin bones of the zebrafish, which crystallizes with time as the bone matures29. Other animals, such as sea urchins, also take advantage of this amorphous precursor pathway, where the most soluble mineral phase is precipitated first, in accordance with the Ostwald-Lussac law30. This strategy allows the animal to regulate phase stability and provides complete control over rate and location of crystalline transformation, and later, of polymorph selectivity. The specific pathway taken by the minerals formed in fish intestines are considered here as we address the question of whether teleost ACC deposits are inclined to transform into a more crystalline form, or are intentionally maintained as a stable phase.
When examining the phase progression of S. aurata fish-derived pellets along the intestinal lumen, we encountered a nearly identical occurrence of ACC (Fig. 2). FTIR spectra obtained from mineral deposits of the anterior intestine mirror the structure of those collected from the mid-section and the posterior end. Despite evidence of initial transformation occurring in some precipitates in the hid-gut and rectum, all of the deposits residing in the lumen exhibited an entirely amorphous character. This consistency suggests the ACC pellets contain stabilizing elements that permit them to pass through the fish gut virtually unaltered.
When organisms stabilize amorphous phases, magnesium ions19, as well as organic macromolecules and proteins, are commonly involved. Of all the known biogenic ACC phases, Weiner et al. (2003) report that most contain moderate amounts of magnesium as well as additional stabilizers, such as glutamic acid or phosphate rich macromolecules25. Evidently, magnesium concentrations found in intestinal deposits from Sparus aurata (max. 54 mol%) are the highest yet reported in CaCO3 biominerals. The data presented here corroborate recently published findings of curiously high Mg2+ levels in fish-produced carbonates of other species15,16. The reason for such significant Mg2+ content in these piscine intestinal deposits may contribute to their ability to remain an amorphous phase throughout their duration in the fish gut.
Factors such as high pH and high Mg ion presence have been linked to favorable crystal formation and stabilization, particularly of ACC23,31. Coincidently, these conditions characterize the chemical environment of the teleost intestinal fluid, with concentrations of Mg2+ reaching 208 mM8,32, and therefore are more than likely to promote the formation of an amorphous carbonate mineral. With regard to the mineral's stability, Politi et al. (2010) demonstrated that a high Mg ion presence in solution not only allows for greater incorporation into the ACC mineral, but found that Mg2+ interferes with the host CaCO3 structure, ultimately preventing further crystallization. This mechanism perhaps explains the remarkable stabilizing property of biogenic high Mg-containing ACC, which, like the ACC found in a lobster's cuticle, can even delay transformation in vitro26.
Additionally, another aspect of the fish's intestinal environment, mucus production, may also play a role in mineral formation and stabilization. Early observations by Humbert et al. (1989) identify Ca2+ binding sites predominating in secreted mucus, and show minerals forming among mucus fibers within the lumen of an eel intestine8. Once formed, fish-gut precipitates remain within the mucus coating, until excreted. This configuration is very likely to promote ACC stabilization, which some studies have demonstrated can be achieved within an organic matrix25, or simply in a confined space33.
Besides establishing the seabream's intestinal carbonates as yet another example of biogenically stable ACC, the importance of identifying this mineral as an amorphous phase from that of a crystalline one concerns its exceptionally high solubility characteristics. While Morse et al. (2007), and recently Woosley et al. (2012), have demonstrated the high solubility of high Mg-calcites, which are about two times more soluble than aragonite, ACC solubility is estimated to be at least ten times higher than crystalline calcium carbonate34,35. During the incubation experiment, the phases exhibited rapid, but incongruent dissolution, consistent with previous findings where the composition of the mineral changed with the progression of the reaction in seawater16. This dissolution characteristic can be attributed to the mineral's high degree of stability from Mg ion incorporation and other possible macromolecule interactions, particularly of the mucus sheath, which may prevent the mineral from dissolving uniformly. However with exposure to seawater, crystallisation would be energetically favored28, and rapid dissolution would most likely diminish over time. The resulting alkalinity measurements (Fig. 4) demonstrate that fish-excreted minerals initially dissolve rapidly in seawater, leading to a significant increase in carbonate concentration.
This study has shown that the CaCO3 deposits produced in gilt-head seabream intestines are a stabilized amorphous phase. It can be suggested that some fish produce an ACC phase in order to store precipitated CaCO3 temporarily in the intestine, either for preventing re-entrance into the luminal solution, which could disrupt the lowered osmotic gradient needed for water absorption, or simply for low-energy packaging of unwanted ions for subsequent excretion. Further investigation is needed concerning mineral formation in the teleost intestine, the identification of additional elements responsible for phase stabilization in fish-gut ACC, as well as the prevalence of an ACC phase among other fish species.
Methods
The gilt-head seabream (Sparus aurata) specimens were acquired from a local fish farm in Eilat, Israel and maintained in a monitored flow-through seawater system at the Interuniversity Institute for Marine Science, Eilat (salinity 41ppt, 21–26°C). Fish were fed every other day with processed, calcium-free meat-based pellets, but food was withheld 48 h before experimentation. The tanks were cleaned everyday to make sure that no external source of calcium carbonate was available to the fish. The mean body length of the seabream used in the study was 20–28 cm (n = 31).
Collection and preparation of fish-gut carbonates
Excreted fish carbonates were collected from the bottom of the holding tank using 10 mL pipettes. For pre-excreted carbonates, fish were euthanized using clove oil, dissected, and the white pellets were collected directly from the intestinal tract. Following collection, fish carbonate samples were treated using an extra gentle washing protocol: the carbonates were dipped in double distilled water (DDW), the excess liquid wicked away, then washed immediately with 100% acetone, and finally 100% ethanol. The whole process took about 3 minutes. The samples were analysed immediately using Fourier Transform Infrared (FTIR) spectroscopy. Some samples were also preserved in ethanol or air dried at room temperature and placed in sealed 3 mL vials.
FTIR spectroscopy analysis
FTIR spectroscopy was performed immediately after dissection of the fish gut, along with a rapid assessment of intestinal fluid pH using 7.2–9.7 ranged special indicator pH paper test strips (Macherey-Nagel).
A small amount (approximately 100 micrograms) of the freshly extracted and rapidly washed (a few seconds in distilled water, then 100% ethanol and finally acetone) carbonate samples were dried under a heat lamp for less than a minute, powdered in an agate mortar and pestle and then mixed with approximately 25 mg FTIR grade KBr (Sigma). The mixture was pressed into transparent pellets. The pellet was immediately analysed using an Is5 Nicolet FTIR spectrometer at 4 cm−1 resolution. All the analyses were repeated on 3 different fish.
Inductively coupled plasma mass spectrometry (ICP-AES)
Fish carbonate samples collected directly from the intestinal tract were prepared using the gentle washing method (DDW, 100% ethanol and then acetone) and ground into a fine powder. The powdered material was dissolved for 1 hour in 1% nitric acid and centrifuged for 10 minutes at 10000 rpm. The pellet contained the organic macromolecules. The supernatant was analysed with ARCOS ICP-EOP spectrometer (Spectro, Germany) for three independent repeats, using a cross-flow nebulizer.
Scanning Electron Microscopy
Image analysis was conducted on an FEI Quanta 200F Scanning Electron Microscope. Samples were removed from ethanol preservation solution and allowed to briefly air dry. They were then coated with gold using a Polaron SC7640 Sputter Coater.
Alkalinity incubation
Ambient seawater, pH 8.14, from the Gulf of Eilat was filtered (Acropak™ 1500, Supor® Membrane 0.8/0.2 μm), stabilized at 25°C, and transferred to 40 mL vials. 35 mg of fish carbonates (dried, preserved samples in 100% ethanol, following the gentle washing protocol previously described (using DDW, 100% acetone and ethanol), or ground coral skeleton (Stylophora pistillata) were added to the vials and incubated for one hour on a shaker, at 25°C. The contents of the vials were filtered (Minisart®) to remove excess solid from the water samples and measured for final pHNBS with a Metrohm pH meter (826 pH mobile, Metrohm, Germany) and glass electrode (calibrated with J.T. Baker NIST Standard Reference Buffers, Avantor Performance Materials B.V., The Netherlands). The total alkalinity (TA) was measured by an open-cell potentiometric titration (Metrohm 862 Compact Titrosampler) following the protocol from Smith and Kinsey (1978)34. Using the CO2sys program35, carbonate chemistry was calculated from measured TA and pH values, with dissociation constants of Mehrbach et al. (1973)36. A randomization ANOVA was performed comparing differences in alkalinity between treatments, given the unequal variances and small sample size, using the statistical program, R.
Author Contributions
E.F. and S.W. conducted the experiments and analyzed the data. E.F. wrote the main manuscript text and S.W. prepared figures 1, 2. All authors reviewed and edited the manuscript.
Supplementary Material
Supplementary Information: Biogenic Fish-gut Calcium Carbonate is a Stable Amorphous Phase in the Gilt-head Seabream, Sparus aurata
Acknowledgments
We are grateful to the staff of the Interuniversity Institute for Marine Sciences in Eilat for logistic help. Many thanks go to Dr. Angelo Colorni of the National Center for Mariculture, Israel Oceanographic and Limnological Research for invaluable fish health and maintenance advice. We thank Mr Assaf Gal, Weizmann Institute, for his assistance with the ICP-AES analyses, and Dr. Yakov Langzam of Bar Ilan University for assistance with the SEM imaging. This study was supported in part by an Israel Science Foundation grant to MF and by the EU FP-7 MedSeA project, and in part by a German Research Foundation, DIP grant. SW is the incumbent of the Dr. Trude Burchardt Professorial Chair of Structural Biology.
References
- Morse J. W., Arvidson R. S. & Luttge A. Calcium carbonate formation and dissolution. Chem. Rev. 107, 342–381 (2007). [DOI] [PubMed] [Google Scholar]
- Kleypas J. A. & Langdon C. Coral reefs and changing seawater chemistry. In: Coral Reefs and Climate Change: Science and Management, Coastal and Estuarine Studies (J. T. Phinney, O. Hoegh-Guldberg, J. Kleypas, W. Skirving, A. Strong Eds.), 61, American Geophysical Union, Washington, DC (2006), pp. 73–110. [Google Scholar]
- Fabry V. J., Seibel B. A., Feely R. A. & Orr J. C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 65, 414 (2008). [Google Scholar]
- Walsh P. J., Blackwelder P., Gill K. A., Danulat E. & Mommsen T. P. Carbonate deposits in marine fish intestines: a new source of biomineralization. Limnol. Oceanogr. 36, 1227–1232 (1991). [Google Scholar]
- Wilson R. W. et al. Contribution of fish to the marine inorganic carbon cycle. Science. 323, 359–362 (2009). [DOI] [PubMed] [Google Scholar]
- Brown E. et al. Seawater: its composition, properties and behaviour. Butterworth-Heinemann, Oxford. (1926).
- Grosell M., Laliberte C. N., Wood S., Jensen F. B. & Wood C. M. Intestinal HCO3- secretion in marine teleost fish: evidence for an apical rather then a basolateral Cl−/HCO3− exchanger. Fish Physiol. Biochem. 24, 81–95 (2001). [Google Scholar]
- Humbert W., Voegel J., Kirsch R. & Simonneaux V. Role of intestinal mucus in crystal biogenesis: an electron-microscopical, diffraction and X-ray microanalytical study. Cell Tissue Res. 255, 575–583 (1989). [DOI] [PubMed] [Google Scholar]
- Millero F. J. Chemical Oceanography. CRC Press, Boca Raton, Fla., ed. 3 (2006). [Google Scholar]
- Wilson R. W., Wilson J. M. & Grosell M. Intestinal bicarbonate secretion by marine teleost fish--why and how? Biochim. Biophys. Acta. 1566, 182–193 (2002). [DOI] [PubMed] [Google Scholar]
- Wilson R. W. & Grosell M. Intestinal bicarbonate secretion in marine teleost fish–source of bicarbonate, pH sensitivity, and consequences for whole animal acid-base and calcium homeostasis. Biochim. Biophys. Acta. 1618, 163–174 (2003). [DOI] [PubMed] [Google Scholar]
- Grosell M. Intestinal anion exchange in marine fish osmoregulation. J. Exp. Biol. 209, 2813–2827 (2006). [DOI] [PubMed] [Google Scholar]
- Whittamore J. M., Cooper C. A. & Wilson R. W. HCO3− secretion and CaCO3 precipitation play major roles in intestinal water absorption in marine teleost fish in vivo. Am. J. Physiol. 298, R877–R886 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes J., Power D. M. & Canário A. V. M. Parathyroid hormone-related protein-stanniocalcin antagonism in regulation of bicarbonate secretion and calcium precipitation in a marine fish intestine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R150–R158 (2010). [DOI] [PubMed] [Google Scholar]
- Perry C. T. et al. Fish as major carbonate mud producers and missing components of the tropical carbonate factory. Proc. Natl. Acad. Sci. USA 108, 3865 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woosley R. J., Millero F. J. & Grosell M. The solubility of fish-produced high magnesium calcite in seawater. J. Geophys. Res. 117, C0401822 (2012). [Google Scholar]
- Beniash E., Aizenberg J., Addadi L. & Weiner S. Amorphous calcium carbonate transforms into calcite during sea urchin larval spicule growth. Proc. R. Soc. B. 264, 461–465 (1997). [Google Scholar]
- Aizenberg J., Lambert G., Weiner S. & Addadi L. Factors involved in the formation of amorphous and crystalline calcium carbonate: a study of an ascidian skeleton. J. Am. Chem. Soc. 124, 32–39 (2002). [DOI] [PubMed] [Google Scholar]
- Raz S., Testeniere O., Hecker A., Weiner S. & Luquet G. Stable amorphous calcium carbonate is the main component of the calcium storage structures of the crustacean Orchestia cavimana. Biol. Bull. 203, 269–274 (2002). [DOI] [PubMed] [Google Scholar]
- Gago-Duport L., Briones M., Rodriguez J. & Covelo B. Amorphous calcium carbonate biomineralization in the earthworm's calciferous gland: Pathways to the formation of crystalline phases. J. Struct. Biol. 162, 422–435 (2008). [DOI] [PubMed] [Google Scholar]
- Weiner S., Mahamid J., Politi Y., Ma Y. & Addadi L. Overview of the amorphous precursor phase strategy in biomineralization. Front. Mater. Sci. 3, 104–108 (2009). [Google Scholar]
- Raz S., Hamilton P. C., Wilt F. H., Weiner S. & Addadi L. The transient phase of amorphous calcium carbonate in sea urchin larval spicules: the involvement of proteins and magnesium ions in its formation and stabilization. Adv. Funct. Mater. 13, 480–486 (2003). [Google Scholar]
- Addadi L., Raz S. & Weiner S. Taking advantage of disorder: amorphous calcium carbonate and its roles in biomineralization. Adv. Mater. 15, 959–970 (2003). [Google Scholar]
- Weiner S., Levi-Kalisman Y., Raz S. & Addadi L. Biologically formed amorphous calcium carbonate. Connect. Tissue Res. 44, 214–218 (2003). [PubMed] [Google Scholar]
- Bentov S., Weil S., Glazer L., Sagi A. & Berman A. Stabilization of amorphous calcium carbonate by phosphate rich organic matrix proteins and by single phosphoamino acids. J. Struct. Biol. 171, 207–215 (2010). [DOI] [PubMed] [Google Scholar]
- Politi Y. et al. Role of magnesium ion in the stabilization of biogenic amorphous calcium carbonate: A structure−function investigation. Chem. Mater. 22, 161–166 (2009). [Google Scholar]
- Combes C. & Rey C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta. Biomater. 9, 3362–3378 (2010). [DOI] [PubMed] [Google Scholar]
- Radha A., Forbes T. Z., Killian C. E., Gilbert P. & Navrotsky A. Transformation and crystallization energetics of synthetic and biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. USA. 107, 16438–16443 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamid J., Sharir A., Addadi L. & Weiner S. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: Indications for an amorphous precursor phase. Proc. Natl. Acad. Sci. USA. 105, 12748–12753 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M., Simkiss K. & Greaves G. N. Amorphous structure of intracellular mineral granules. Biochem. Soc. Trans. 14, 549–552 (1986). [DOI] [PubMed] [Google Scholar]
- Rodriguez-Blanco J., Shaw S., Bots P., Roncal-Herrero T. & Benning L. The role of pH and Mg on the stability and crystallization of amorphous calcium carbonate. J. Alloys Compounds. 536S, S477–S479 (2011). [Google Scholar]
- Genz J., McDonald M. D. & Grosell M. Concentration of MgSO4 in the intestinal lumen of Opsanus beta limits osmoregulation in response to acute hypersalinity stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R895–R909 (2011). [DOI] [PubMed] [Google Scholar]
- Stephens C. J., Ladden S. F., Meldrum F. C. & Christenson H. K. Amorphous calcium carbonate is stabilized in confinement. Adv. Funct. Mater. 20, 2108–2115 (2010). [Google Scholar]
- Merion O. E. et al. Solubility and bioavailability of stabilized amorphous calcium carbonate. J. Bone Miner. Res. 26, 364–372 (2011). [DOI] [PubMed] [Google Scholar]
- Brečević L. Solubility of amorphous calcium carbonate. J. Cryst. Growth. 98, 504–510 (1989). [Google Scholar]
- Smith S. V. & Kinsey D. W. Calcification and organic carbon metabolism as indicated by carbon dioxide. In: Coral Reefs: Research Methods. Monographs on Oceanographic Methodology (D. Stoddart and R. Johannes, Eds.), UNESCO, Paris (1978) pp. 469–484. [Google Scholar]
- Pierrot D., Lewis E. & Wallace D. MS Excel program developed for CO2 system calculations. ORNL/CDIAC-105. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, TN. (2006).
- Mehrbach C., Culberson C., Hawley J. & Pytkowicz R. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907 (1973). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information: Biogenic Fish-gut Calcium Carbonate is a Stable Amorphous Phase in the Gilt-head Seabream, Sparus aurata