Abstract
Rodent malaria species Plasmodium yoelii and P. chabaudi have been widely used to validate vaccine approaches targeting blood-stage merozoite antigens. However, increasing data suggest the P. berghei rodent malaria may be able to circumvent vaccine-induced anti-merozoite responses. Here we confirm a failure to protect against P. berghei, despite successful antibody induction against leading merozoite antigens using protein-in-adjuvant or viral vectored vaccine delivery. No subunit vaccine approach showed efficacy in mice following immunization and challenge with the wild-type P. berghei strains ANKA or NK65, or against a chimeric parasite line encoding a merozoite antigen from P. falciparum. Protection was not improved in knockout mice lacking the inhibitory Fc receptor CD32b, nor against a Δsmac P. berghei parasite line with a non-sequestering phenotype. An improved understanding of the mechanisms responsible for protection, or failure of protection, against P. berghei merozoites could guide the development of an efficacious vaccine against P. falciparum.
Despite the deployment of effective control and prevention strategies, Plasmodium falciparum malaria remains a huge burden on global public health1. The development of a highly effective vaccine is still necessary if ambitions for local elimination, and ultimate eradication, are to be realized. Promising results in Phase IIb field trials with the leading candidate vaccine, RTS,S, raised hopes that this formulation may be an effective contributor to malaria control public health measures2. However, more recent results in a Phase III trial, especially in the target infant age group, have revealed relatively low levels of efficacy against clinical and severe malaria3. Further work is vital in order to develop and establish a highly effective vaccine for use in the field4, and the continuing deaths of nearly a million children each year demonstrates the urgency with which this work needs to take place1.
Numerous vaccine strategies have been proposed to tackle the complex lifecycle of the Plasmodium parasite. Leading vaccines in clinical development primarily target the sporozoite and/or liver-stages of parasite development5. However significant efforts have also been made to develop vaccines against the pathogenic blood-stage infection6 – especially given this is the lifecycle stage of the parasite against which natural immunity is acquired following repeated exposure7. Numerous blood-stage candidate vaccines have now been tested in over 40 Phase I/II clinical trials6, but the field has faced much disappointment without a single formulation demonstrating significant efficacy in the primary endpoint of a Phase IIa/b clinical trial. A substantial number of these vaccines have targeted the leading merozoite antigens merozoite surface protein 1 (MSP1) and apical membrane antigen 1 (AMA1)8,9,10,11 – targets historically identified as associated with naturally-acquired immunity, and important for the invasion process of new host red blood cells12,13. Most recently, a Phase IIb field trial of a protein vaccine, based on the 42 kDa C-terminus of MSP1 (MSP142) in Kenya showed no efficacy9, but encouragingly strain-specific efficacy was reported in the secondary analysis of a mono-valent vaccine based on the 3D7 allele of AMA1 tested in Malian children10. Intriguingly, a previous Phase IIa controlled human malaria infection (CHMI) study of the same vaccine in US adult volunteers showed no significant efficacy against challenge with the homologous 3D7 parasite clone14. It remains to be established whether future AMA1 vaccine candidates can overcome the significant hurdles surrounding the problems of antigen polymorphism15.
Vaccine research relies heavily on animal models to screen putative candidates, and the MSP1 and AMA1 targets have been previously validated in this manner as protective determinants of vaccine-induced antibodies12,13. In particular, rodent malaria species and some chimeric rodent/human malaria models have been widely used to assess the merits of various vaccine delivery platforms and candidate antigens16,17. The P. yoelii and P. chabaudi rodent malaria species have both been commonly utilized to demonstrate protection against blood-stage parasitemia by vaccines targeting the orthologous MSP1 and AMA1 antigens18,19,20,21,22. In the case of MSP1, the 19 kDa C-terminus of MSP1 (MSP119) has been found to be critical for the induction of antibody-mediated protection23, whilst the neighbouring 33 kDa region (MSP133) appears important for T cell help24,25. Numerous subunit vaccines therefore rely on the use of the entire C-terminal MSP142 region24, whilst some protein-in-adjuvant vaccines can induce antibody-mediated protection when based on MSP119 alone20.
P. berghei represents a third rodent malaria species that has been widely used for the assessment of pre-erythrocytic26,27 and transmission-blocking28,29 subunit vaccine efficacy. However, despite the widespread use of this model, a search of the literature revealed only three English-language publications that have demonstrated protection against blood-stage P. berghei by subunit vaccination30,31,32. Subunit vaccines that are reported to have been successful in protecting against blood-stage P. berghei strain NK65 in BALB/c mice include P. berghei NK65 merozoite surface protein 9 (PbMSP9) N-terminus recombinant protein formulated in alum30; a recombinant Salmonella vaccine encoding regions of blocks 3 and 4 of PbMSP1 ANKA strain31; and a recombinant protein against PbMSP119 in alum32. The recombinant Salmonella vaccine additionally demonstrated limited protection against P. berghei NK65 strain in outbred CD1 mice31. A further paper published in Chinese describes induction of partial protection against blood-stage P. berghei following immunization with recombinant PbAMA1 in Freund's adjuvant33. Despite the limited number of studies demonstrating protection against blood-stage P. berghei malaria following subunit vaccination, it is this species of rodent malaria that forms the basis of currently available chimeric parasites, designed for testing and optimizing P. falciparum vaccines against the blood-stage in mice34,35.
It is possible that additional laboratories have tested blood-stage subunit vaccines targeting P. berghei but publication bias has resulted in a lack of published descriptions of failures to achieve protection. However, more recently, two publications have reported failed protection by subunit vaccines targeting PbMSP1 and PbAMA136,37. A head-to-head comparison of blood-stage vaccine efficacy against P. berghei ANKA or P. yoelii 17XL using baculoviral-based vaccines (BBV) targeting P. berghei or P. yoelii MSP119 and AMA1 found that BBV vaccines could induce complete or partial protection against P. yoelii but not against P. berghei in BALB/c mice36. In the second study, DNA vaccines expressing PbMSP142 were tested with and without fusion to a putative molecular adjuvant (complement protein C3d) in BALB/c mice37. In this study both adjuvanted and control vaccines induced anti-PbMSP142 antibody responses, but neither of the two vaccines protected against P. berghei blood-stage challenge.
In agreement with these studies, here we report on similar difficulties relating to the induction of protective immunity against P. berghei using viral vectored vaccines, as well as recombinant protein-in-adjuvant, targeting well-studied merozoite antigens which have previously been successful in protecting against P. yoelii and P. chabaudi18,19,20,21,24,38,39. Protection was not achieved against either the ANKA or NK65 P. berghei strains, nor in a range of inbred mouse strains following active subunit immunization. Similarly, neither active immunization with vaccines against PfMSP1 nor passive transfer of anti-PfMSP119 IgG from rabbits afforded protection in mice against a P. berghei parasite line chimeric for P. falciparum MSP119. An improved understanding of protective immune mechanisms against P. berghei merozoites is therefore still needed, and could help to guide the development of an effective vaccine against P. falciparum.
Results
P. berghei MSP119 protein vaccine production and immunogenicity
Recombinant PyMSP119 protein-in-adjuvant vaccines are known to provide high levels of protection against P. yoelii blood-stage parasite challenge20,39 and protection against P. chabaudi can be similarly induced using vaccines encoding the orthologous antigen (PcMSP119)21. In order to assess whether similar vaccines could protect against P. berghei, we generated a comparable protein subunit vaccine. Recombinant PbMSP119 protein from the ANKA strain was produced and purified from E. coli as a soluble GST-fusion protein as described in Methods.
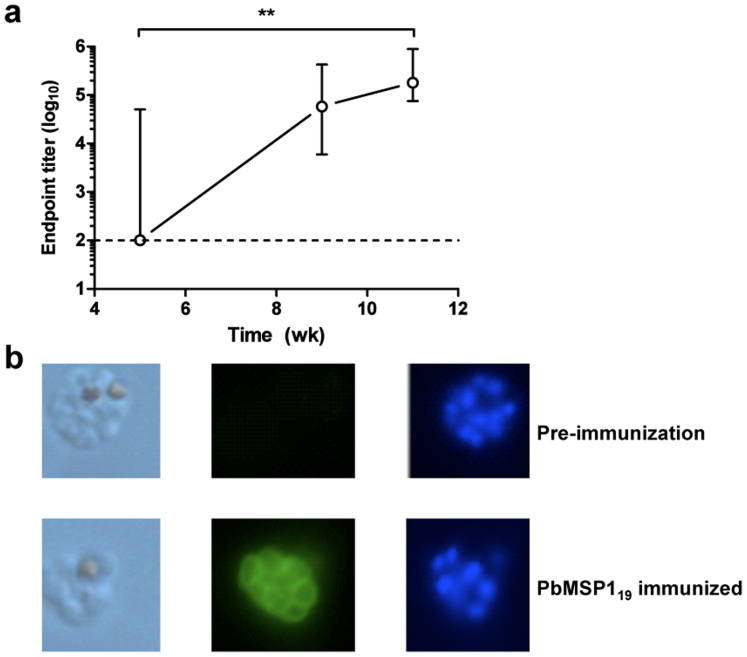
To test the immunogenicity of GST-PbMSP119 protein, BALB/c mice were immunized using a regime that has been shown to induce extremely high levels of protective efficacy against P. yoelii pRBC challenge following PyMSP119 immunization20. Five doses of 20 μg GST-PbMSP119 protein were administered using CFA (at week 0), IFA (weeks 3, 6, 8) and PBS (week 9). Sera were collected at regular intervals (weeks 5, 9, 11) and assessed by ELISA. The total IgG titer against GST-PbMSP119 increased significantly over time, as further doses of protein were administered (Figure 1a). As the ELISA assay was unable to distinguish between anti-GST and anti-PbMSP119 responses, P. berghei immunofluorescence assays (IFA) were also performed using sera collected from mice two weeks following the final immunization of five doses of GST-PbMSP119 protein (week 11). The use of these immunized sera in IFA against P. berghei ANKA thin-blood smears confirmed the presence of IgG capable of binding to native PbMSP119 protein (Figure 1b).
Figure 1. Immunogenicity of GST-PbMSP119 immunization.
BALB/c mice (n = 5) were immunized with five doses of 20 μg GST-PbMSP119 protein. Doses were administered at week 0 s.c. in CFA, at week 3 s.c. in IFA, at weeks 6 and 8 i.p. in IFA, and at week 9 i.p. in PBS. (a) Total IgG responses against recombinant GST-PbMSP119 protein were measured by ELISA in the serum of mice taken at weeks 5, 9 and 11. Median and range of the titers are shown. The dashed line indicates the lower limit of detection. ** P < 0.01 by Friedman test with Dunn's correction for comparison of all time-points. (b) P. berghei ANKA schizonts were fixed with 4% formaldehyde and permeabilized with 0.1% NP40. Pooled sera from week 11 in (A) were tested at a dilution of 1:100. Naïve pre-immunization serum was used as a negative control. Bound antibodies were detected using goat anti-mouse IgG conjugated to Alexa Fluor 488. Images show left to right the visible schizonts, followed by fluorescence (green) and DAPI staining (blue).
P. berghei blood-stage viral vector vaccine production and immunogenicity
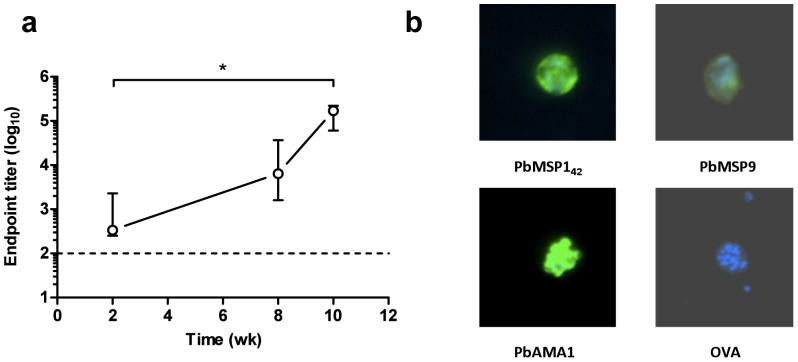
Similar to protein vaccines, AdHu5-MVA viral vector prime-boost regimes targeting PyMSP142 have been demonstrated to induce protection against blood-stage P. yoelii challenge18. Recombinant AdHu5 and MVA viruses encoding PbMSP142 from the ANKA strain were produced in order to determine the immunogenicity and protective efficacy of viral vectors against P. berghei. Mice were immunized with AdHu5 expressing PbMSP142 and eight weeks later were boosted with MVA expressing the same antigen. This regime induced PbMSP119-specific total IgG, and titers significantly increased following the MVA boost (Figure 2a). Although this ELISA used the same GST-PbMSP119 protein as above, the entirety of the measured response following viral vector immunization will be directed to the PbMSP119 moiety. Moreover, similar to the protein vaccine, endpoint titers here as determined by ELISA were also high, thus confirming the strong immunogenicity of the viral subunit vaccine delivery platform.
Figure 2. Immunogenicity of AdHu5-MVA viral vector vaccines.
(a) BALB/c mice (n = 5) were immunized i.d. with 1 × 1010 vp AdHu5 and boosted eight weeks later i.d. with 1 × 107 pfu MVA expressing PbMSP142. Total IgG responses against recombinant GST-PbMSP119 protein were measured by ELISA in the serum of mice taken at weeks 2, 8 and 10. Median and range of the titers are shown. The dashed line indicates the lower limit of detection. * P < 0.05 by Friedman test with Dunn's correction for comparison of all time-points. (b) P. berghei ANKA schizonts were fixed with 4% formaldehyde and permeabilized with 0.1% NP40. Sera from mice immunized with 1 × 1010 vp AdHu5 and boosted with 1 × 107 pfu MVA expressing the indicated antigens were used at a dilution of 1:100. Bound antibodies were detected using goat anti-mouse IgG conjugated to Alexa Fluor 488. Images show fluorescence (green) and DAPI staining (blue).
The viral vectored vaccine platform is suited to the purposes of antigen screening40, and thus whilst generating vectors encoding PbMSP142, similar recombinant AdHu5 and MVA vaccines were also generated encoding the PbAMA1 and PbMSP9 antigens, for which protection data have previously been reported in the P. berghei model30,33. These vectors were also used to immunize groups of BALB/c mice in a similar manner to those encoding PbMSP142. Recombinant PbAMA1 or PbMSP9 antigens were not available to test the induction of antibodies by ELISA, and therefore the induction of antibodies capable of recognizing native parasite antigen by vaccination was instead confirmed by IFA. Sera from AdHu5-MVA immunized BALB/c mice were assessed for binding to P. berghei ANKA mature schizonts (Figure 2b), which were the homologous parasite for PbMSP142 and PbAMA1, but heterologous for PbMSP9 (where the antigen sequence was based on the NK65 strain of P. berghei). However, these data demonstrated that the vaccines against all three antigens were immunogenic, and confirmed that IgG antibodies capable of binding to native antigen in schizonts were produced following AdHu5-MVA immunization with PbAMA1 and PbMSP9, but not following immunization with vectors expressing a control antigen (OVA).
Protective efficacy of MSP1 vaccines against blood-stage P. berghei parasites
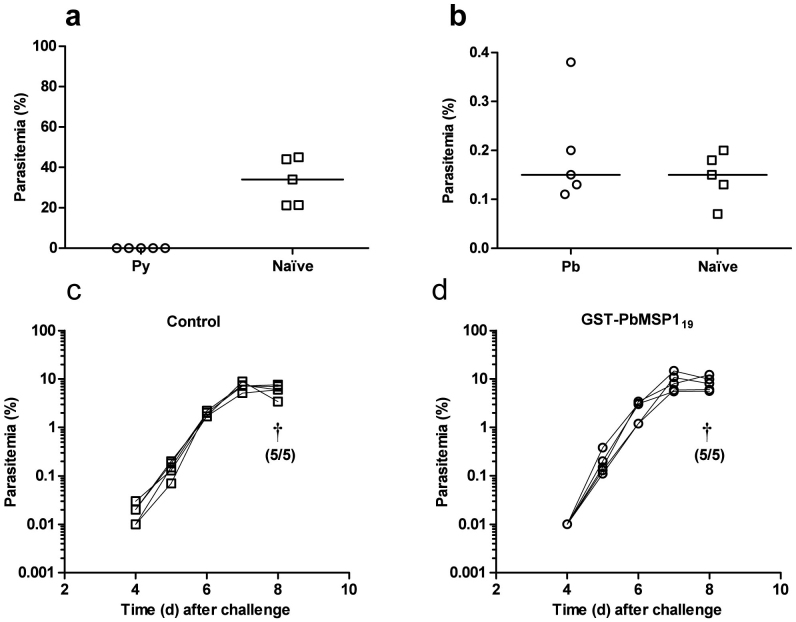
Mice can be sterilely protected against blood-stage P. yoelii challenge using a schedule of five immunizations of PyMSP119 (His6-tagged protein produced in Saccharomyces cerevisiae in Freund's adjuvant20). In order to determine if similar protection could be seen in the P. berghei model, mice were immunized as above with either the GST-PbMSP119 protein vaccine or a previously described PyMSP119 vaccine, termed PyMSP119-IMX108 41. Subsequently, in a head-to-head experiment, the mice were challenged with homologous strain blood-stage parasites (P. berghei ANKA or P. yoelii YM respectively). However, only PyMSP119 immunized mice were protected against a corresponding blood-stage Plasmodium infection. Sterile protection against P. yoelii was seen in all immunized mice (Figure 3a) whilst PbMSP119 immunized mice showed no protection against P. berghei either at the onset of patent infection (Figure 3b), or throughout the course of the infection (Figure 3c,d). As before (Figure 1a), the PbMSP119 immunized mice achieved high antibody titres to their respective MSP119 protein, and the PyMSP119 immunized mice also developed high endpoint titers prior to challenge [ELISA assay against GST-PyMSP119 protein showed a median endpoint of 1.3 × 106 with a range of 0.42 × 106–1.9 × 106]. Thus despite induction of IgG antibodies against both MSP119 antigens by the protein–in-adjuvant vaccines, protective efficacy was only achieved against P. yoelii.
Figure 3. Efficacy of P. yoelii and P. berghei MSP119 protein immunization.
BALB/c mice (n = 5/group) were immunized with five doses of 20 μg GST-PbMSP119 protein (Pb) or a recombinant PyMSP119 protein fused to IMX108 (Py) as described in Figure 1. Ten days following the final immunization Pb vaccinated mice and naïve non-immunized controls were challenged i.v. with 5 × 102 P. berghei ANKA pRBC. Similarly Py vaccinated mice and naïve non-immunized controls were challenged i.v. with 1 × 104 P. yoelii YM pRBC, followed by daily monitoring for parasitemia. (a) Py vaccinated mice were sterilely protected (monitored out to day 21). Day 4 parasitemias for individual control and vaccinated mice with medians are shown. (b) Day 5 parasitemias for individual Pb vaccinated mice and controls are shown with medians, as well as time courses of parasitemia for individual (c) control and (d) Pb vaccinated mice. † indicates that mice were culled, and the number culled from the total number in the group is shown in brackets.
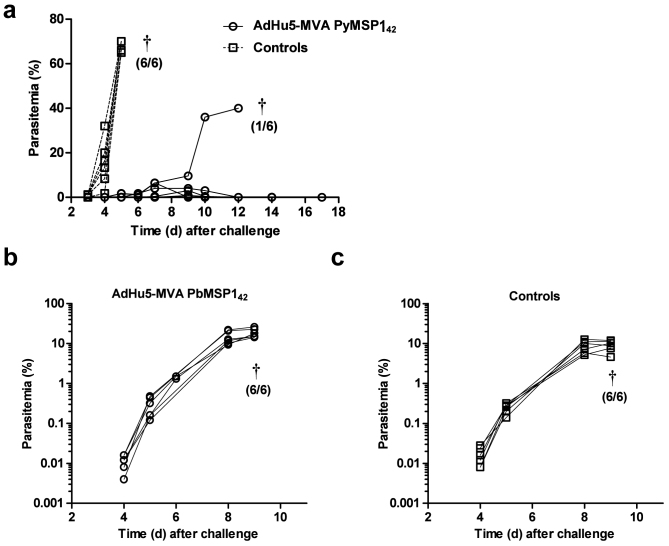
Viral vectored vaccines are capable of inducing antibodies against encoded transgenes, but induce qualitatively different types of responses as well as cellular immunity18,39. We have previously reported that significant protection against P. yoelii pRBC challenge can be achieved using a schedule of just two immunizations in an AdHu5-MVA PyMSP142 heterologous prime-boost regime, without a need for additional adjuvant18. Protection following pRBC challenge is dependent on the induction of high-titer anti-PyMSP119 antibody responses18,20. In order to determine if similar protection could be seen with P. berghei MSP142, BALB/c mice were immunized with AdHu5-MVA vaccines as described earlier. As expected, both AdHu5-MVA PbMSP142 and AdHu5-MVA PyMSP142 immunized mice achieved high antibody titres to their respective MSP119 proteins (data not shown), however only AdHu5-MVA PyMSP142 immunized mice were protected against a homologous blood-stage Plasmodium infection. Protection against P. yoelii was seen in AdHu5-MVA PyMSP142 immunized mice (Figure 4a), with delayed onset parasitemia and 83% survival – comparable to previously published data for the same regime using slightly higher viral vaccine doses18. In contrast, AdHu5-MVA PbMSP142 immunized mice showed no protection against P. berghei ANKA infection (Figure 4b,c), identical to the results obtained with protein-in-adjuvant immunized animals (Figure 3c,d).
Figure 4. Efficacy of AdHu5-MVA PbMSP142 and PyMSP142 immunization.
BALB/c mice (n = 6/group) were immunized i.d. with 1 × 1010 vp AdHu5 and boosted eight weeks later i.d. with 1 × 107 pfu MVA expressing (a) PyMSP142 or (b) PbMSP142. Two weeks following the final immunization vaccinated mice and non-immunized controls were challenged with (a) 1 × 104 P. yoelii YM or (b,c) 5 × 102 P. berghei ANKA pRBC and monitored for parasitemia. Lines represent individual mice. † indicates that mice were culled and the number culled from the total number in the group is shown in brackets.
Protective efficacy of AMA1 and MSP9 vaccines against blood-stage P. berghei parasites
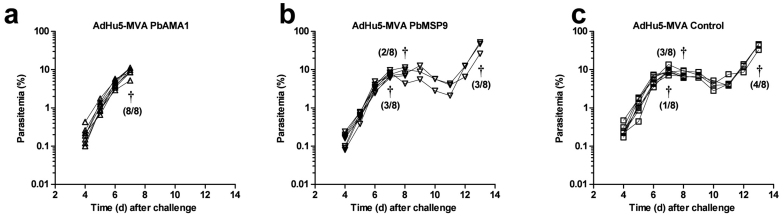
Despite the lack of efficacy with vaccines encoding the C-terminal regions of PbMSP1, there have been reports that immunization against PbAMA1 and PbMSP9 can elicit protection against P. berghei at the blood-stage30,33. We therefore assessed the efficacy of the viral vector vaccines targeting PbAMA1 and PbMSP9 in BALB/c mice. Mice were immunized with AdHu5 expressing PbAMA1, PbMSP9 or no malaria antigen and then boosted with MVA expressing the same antigen. Two weeks later mice were challenged with 5 × 102 P. berghei ANKA pRBC and monitored daily. Similar to PbMSP142, no significant efficacy was observed with either of these vaccines compared to control immunized mice (Figure 5). By day 7 post-challenge, 8/8 PbAMA1 immunized mice were culled (Figure 5a), compared to 3/8 PbMSP9 (Figure 5b) immunized and 1/8 control (Figure 5c) immunized mice. This was not due to a difference in parasitemia between groups, but rather reflected symptomatic illness which was pre-defined as the humane endpoint. All remaining mice reached this point by day 13 post-infection. Overall, these data show an absence of efficacy of both the PbMSP9 and PbAMA1 antigens, delivered by AdHu5-MVA, against blood-stage P. berghei ANKA infection.
Figure 5. Efficacy of AdHu5-MVA PbAMA1 and PbMSP9 immunization against P. berghei ANKA pRBC infection.
BALB/c mice (n = 8/group) were immunized i.d. with 1 × 1010 vp AdHu5 and boosted thirteen weeks later with 1 × 107 pfu MVA encoding either PbAMA1, PbMSP9 or no malaria antigen (Control). Two weeks post-boost mice were challenged with 5 × 102 P. berghei ANKA pRBC and monitored for parasitemia. Lines represent individual mice. † indicates that mice were culled and the number culled from the total number in the group is shown in brackets.
Published studies demonstrating protection at the blood-stage against P. berghei were all noted to have employed an NK65 strain challenge30,31,32. Biological differences between P. berghei strains have been observed, such as differences in virulence, sensitivity to drugs, and preference for invasion of normocytes or reticulocytes42,43. The AdHu5-MVA vaccines encoding PbMSP142, PbAMA1 and PbAMA1, or a co-administered mixture of all three, were therefore additionally tested against challenge with P. berghei NK65 pRBC. Also, given the PbMSP9 immunogen was originally based on the NK65 strain sequence, this experiment allowed for a homologous parasite challenge, in comparison to heterologous challenge as in the previous experiment. However, as seen with P. berghei ANKA, no protective efficacy was observed in any of the vaccinated mice (Supplemental Figure 1).
Protective efficacy of MSP1 and AMA1 vaccines against P. berghei sporozoite challenge
In previous studies with the AdHu5-MVA vectors encoding PyMSP142, we reported improved efficacy and survival outcome against P. yoelii strain YM sporozoite challenge in comparison to pRBC challenge. In these studies, vaccinated mice showed complete survival against a challenge dose of both 50 and 250 sporozoites24.
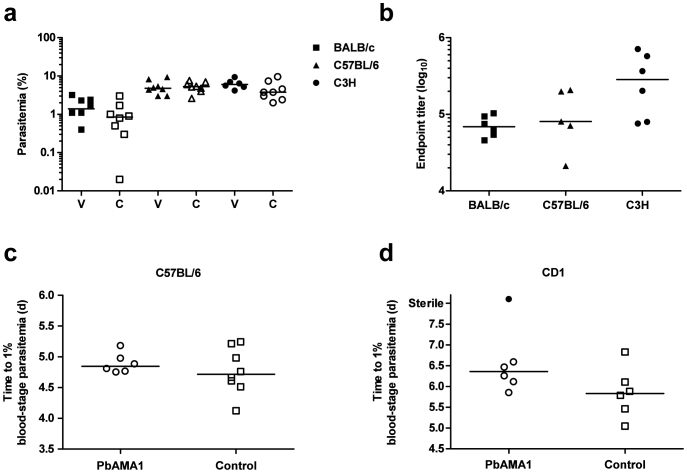
In the case of PbMSP142, BALB/c mice, immunized as before, were challenged with 500 sporozoites 14 days following the MVA boost. However, similar to the pRBC challenge studies, no efficacy was observed in vaccinees as compared to non-immunized controls (Figure 6a). To confirm that this lack of efficacy was not specific to the BALB/c strain, we also tested this regime in C3H and C57BL/6 mice. Despite achieving higher anti-PbMSP119 antibody titers in C3H mice (Figure 6b) we still observed no efficacy following sporozoite challenge (Figure 6a).
Figure 6. Efficacy of AdHu5-MVA PbMSP142 and PbAMA1 immunization against P. berghei ANKA sporozoite infection.
(a) Mice (n = 6-8/group) were immunized i.d. with 1 × 1010 vp AdHu5 and boosted eight weeks later i.d. with 1 × 107 pfu MVA expressing PbMSP142. Two weeks following the final immunization vaccinated mice and naïve non-immunized controls were challenged with 5 × 102 P. berghei ANKA sporozoites. Each mouse was monitored for the development of blood-stage infection. Parasitemia for individual vaccinated (V) and non-immunized control (C) mice and medians are shown at day 7 following challenge of three different inbred mouse strains (BALB/c, C57BL/6 and C3H). (b) Total IgG responses against recombinant GST-PbMSP-119 protein were measured by ELISA in the serum of mice taken pre-challenge at week 2 post-boost immunization. Individual titers and medians are shown. (c) C57BL/6 and (d) CD1 mice (n = 6–8/group) were immunized i.m. with 8 × 109 vp AdHu5 and boosted eight weeks later i.m. with 1 × 107 pfu MVA expressing PbAMA1. Two weeks following the final immunization vaccinated mice and non-malaria antigen vector-immunized controls were challenged with 2000 P. berghei ANKA sporozoites. Median and individual time to 1% parasitemia is shown. One CD1 mouse showed sterile protection and did not develop patent blood-stage infection.
In addition to its well-known blood-stage role, AMA1 is also known to be expressed on sporozoites44, and DNA and viral vectored vaccines encoding P. falciparum AMA1 have shown some potential pre-erythrocytic efficacy in humans against controlled infection delivered by mosquito bites8,45. We therefore additionally tested AdHu5-MVA PbAMA1 vaccines against sporozoite challenge in C57BL/6 (Figure 6c) as well as outbred CD1 mice (Figure 6d). Following challenge with 2000 sporozoites, there was no significant protection observed in either of the mouse strains, although there was a single PbAMA1 vaccinated animal on the outbred CD1 background that did not become parasitemic, indicating that on rare occasions PbAMA1 may be able to impart some protective efficacy in this model. However, given the extremely low level of efficacy afforded, the potential mechanism(s) of protection were not further explored.
Protective efficacy of P. falciparum MSP1 vaccines against a chimeric P. berghei parasite
Novel chimeric parasite lines have also been developed to enable functional assessments of immune responses to P. falciparum antigens in small mammals16. The chimeric P. berghei parasite model Pb-PfM19 was developed to enable the testing of vaccines targeting the P. falciparum MSP119 antigen in mice34. Antibody-mediated protection against Pb-PfM19 in mice has been demonstrated following passive transfer of monoclonal or polyclonal antibody into mice or following infection with Pb-PfM19 and drug-cure34,46,47. We have previously reported the preclinical development of human and simian adenoviral as well as MVA vectored vaccines encoding PfMSP1-based transgenes termed PfM115 and PfM128 48. These vectors induced anti-PfMSP119 IgG responses in mice and rabbits that showed functional growth inhibitory activity (GIA) against P. falciparum parasites in vitro18,40,48,49. A chimpanzee adenovirus 63 (ChAd63) and MVA encoding PfM128 have also been shown to immunogenic for PfMSP119 antibody induction in Phase I/IIa clinical trials8,50. In a parallel series of experiments to those reported above using wild-type P. berghei parasites, we also tested the protective efficacy of PfMSP1-based vaccines following active immunization of mice. In agreement with data using the PbMSP1-based vaccines, no protective efficacy or effect of the vaccine on blood-stage parasite growth was conferred following AdHu5-MVA immunization with the PfM115 immunogen, following challenge with 104 pRBCs of the chimeric parasite (Table 1). We observed the same results in both BALB/c and C57BL/6 mice when the experiment was repeated using a lower challenge dose of 500 pRBCs as employed in another published study47. We also tested a recombinant PfMSP119-based protein vaccine, previously reported41, administered in Freund's adjuvant and similarly observed no efficacy in BALB/c mice. Overall, these data confirmed that active immunization of inbred mouse strains with subunit vaccines encoding the PfMSP119 antigen failed to elicit efficacy against a pRBC challenge with the chimeric Pb-PfM19 parasite.
Table 1. Chimeric parasite challenge experiments. Protein or viral vectored vaccines were administered as shown. Regime lists dose, route and day (d) of immunizations. Survival outcome is reported for the Pb-PfM19 chimeric parasite, and wild-type Pb-PbM19 control. n.d. = not done. N/A = not applicable.
| Vaccine | Regime | Challenge | Antigen | Mouse strain | Pb-PfM19 survival | Pb-PbM19 survival |
|---|---|---|---|---|---|---|
| PfMSP119-IMX108 | 3 × 40 μg s.c. d0, d14, d28 | d42 | PfMSP119 | BALB/c | 0/6 | n.d. |
| CFA/IFA/IFA | 104 pRBC i.v. | |||||
| AdHu5-MVA | 5 × 1010 vp i.d. d0 | d70 | PfM115 | BALB/c | 0/6 | 0/6 |
| 5 × 107 pfu i.d. d56 | 104 pRBC i.v. | |||||
| Naïve | N/A | 104 pRBC i.v. | N/A | BALB/c | 0/3 + 0/6 | 0/6 |
| AdHu5-MVA | 5 × 1010 vp i.d. d0 | d70 | PfM115 | BALB/c | 0/6 | n.d. |
| 5 × 107 pfu i.d. d56 | 5 × 102 pRBC i.v. | |||||
| AdHu5-MVA | 5 × 1010 vp i.d. d0 | d70 | PfM115 | C57BL/6 | 0/6 | n.d. |
| 5 × 107 pfu i.d. d56 | 5 × 102 pRBC i.v. | |||||
| Naïve | N/A | 5 × 102 pRBC i.v. | N/A | BALB/c | 0/6 | n.d. |
| Naïve | N/A | 5 × 102 pRBC i.v. | N/A | C57BL/6 | 0/6 | n.d. |
Protection against the chimeric parasite Pb-PfM19 by active subunit PfMSP1 vaccine immunization was not possible in our hands and has not been reported elsewhere. However, sterile protection of BALB/c mice following i.v. transfer of 900 μg purified total IgG from PfMSP142-immunized rabbits has been reported in one study47, and also in a second study using 1.5 mg purified total IgG from PfMSP119-immunized rabbits and an alternative but highly similar chimeric parasite35. We thus also investigated here whether passive transfer could protect against challenge with the Pb-PfM19 parasite. Total IgG was purified from the serum of New Zealand white rabbits that had previously been immunized with an AdHu5-MVA or ChAd63-MVA regime and vectors encoding the PfM128 antigen. These rabbits had raised antibody responses against the 3D7/MAD20/ETSR allele of PfMSP119 that were comparable to those reported in our previous studies (Supplemental Figure 2A) and homologous to the PfMSP119 strain D10 sequence in the chimeric parasite34. Rabbits were primed i.m. with 5 × 1010 vp ChAd63 or AdHu5 expressing PfM128 on day 0 and boosted i.m. with 1 × 108 pfu MVA-PfM128 on day 56. Serum was harvested two weeks later on day 70. Day 0 pre-immune IgG from the same rabbits and from a control viral vector immunized rabbit were also purified. 0.5mg IgG was transferred i.v. to BALB/c mice on days −1, 0 and +1, and on day 0 mice were challenged with 5 × 102 PbPfM19 pRBC by i.v. injection. However, no significant differences in parasitaemia were observed between any groups following challenge (Supplemental Figure 2B).
The role of FcγRIIb (CD32b) in antibody-mediated protection against P. berghei
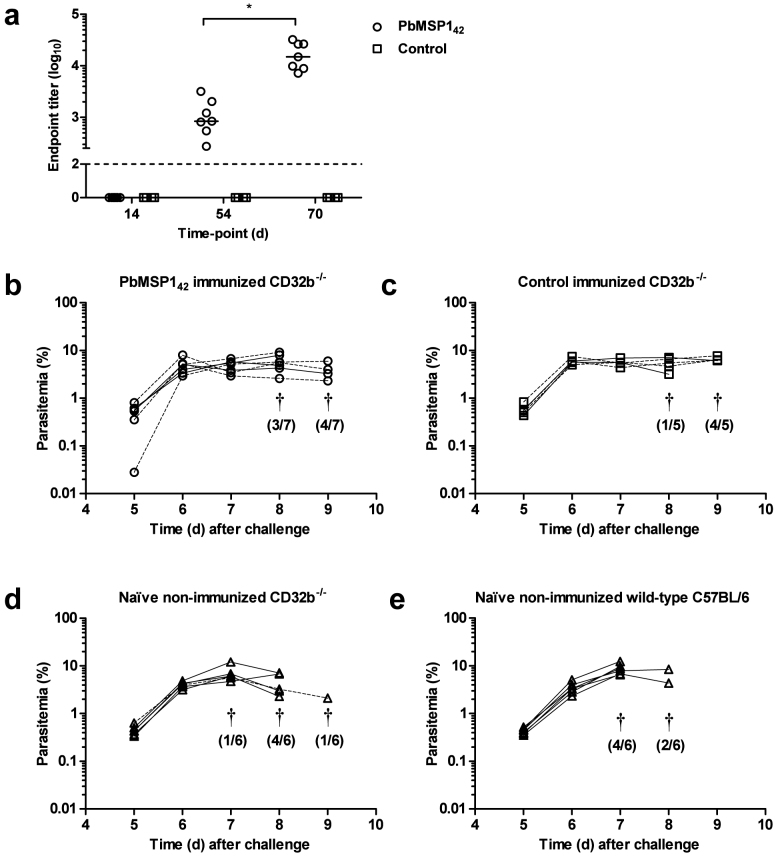
The data so far have demonstrated an inability of vaccines targeting three different merozoite antigens to protect against blood-stage P. berghei infection. Data from natural infection studies have suggested antibody Fc-mediated mechanisms are important in protection against blood-stage P. berghei. This is supported by the findings that mice lacking the common γ signalling chain, required for activatory signalling through Fc-gamma receptors (FcγR), have increased susceptibility to blood-stage P. berghei XAT infection51. Additional support is observed as human antibodies against PfMSP119 were able to protect against the chimeric Pb-PfM19 parasite only in mice transgenic for human FcγRI (CD64)46. The inhibitory effect of IgG1 binding to the inhibitory Fc receptor (CD32b/FcγRIIb) may in fact act to prevent activatory signals induced by IgG2a through other FcRs52,53. Given viral vectored vaccines are known to induce both IgG1 and IgG2a18,48,54, FcγRIIb knockout mice (CD32b−/−) were therefore used to investigate whether removing the inhibitory signals induced through FcγRIIb allowed protection against blood-stage P. berghei infection to be observed following subunit vaccination.
CD32b−/− mice on a C57BL/6 background were immunized with AdHu5-MVA vectors expressing either PbMSP142 or no antigen (vector control). As before, anti-PbMSP119 IgG responses were induced by immunization (Figure 7a). Two weeks post-boost, mice were challenged with P. berghei ANKA pRBC and monitored for parasitemia. No significant differences in parasitemia were observed in the PbMSP142 immunized mice (Figure 7b) in comparison to control immunized (Figure 7c) or naïve non-immunized mice (Figure 7d). The lack of the FcγRIIb also did not appear to influence the kinetics of the infection in naïve mice, with no significant differences observed in parasitemia between naïve non-immunized CD32b−/− (Figure 7d) and naïve non-immunized wild-type C57BL/6 mice (Figure 7e). This result suggests that the lack of vaccine efficacy in wild-type mice was not due to inhibitory signalling through FcγRIIb preventing activatory IgG2a-mediated signals.
Figure 7. Efficacy of AdHu5-MVA PbMSP142 in CD32b−/− mice.
CD32b−/− mice were immunized i.d. with 1 × 1010 vp AdHu5 and boosted eight weeks later i.d. with 1 × 107 pfu MVA encoding either no antigen (Control) or PbMSP142. (a) On days 14, 54 (pre-boost) and 70 (2 weeks post-boost) sera were collected and PbMSP119-specific total IgG measured by ELISA. Dots represent individual mice and bars show median endpoint titre. * P = 0.02 between d54 and d70 by Wilcoxon matched pairs signed rank test. The dashed line indicates the lower limit of detection. Two weeks post-boost (b) PbMSP142 immunized CD32b−/− mice and (c) control immunized mice were challenged with 5 × 102 P. berghei ANKA pRBC and monitored for parasitemia. Groups of (d) non-immunized (naïve) CD32b−/− and (e) wild-type C57BL/6 mice were also challenged. Lines represent individual mice of 5–7 mice/group (females solid lines and males dashed lines). † indicates that mice were culled. The number culled that day from the total number of mice in the group is shown in brackets.
The role of sequestration in antibody-mediated protection against P. berghei merozoites
We also tested a final hypothesis as to whether sequestration of P. berghei parasites was affecting the efficacy of the anti-merozoite vaccines. We considered that sequestration of infected erythrocytes might reduce the period of exposure of merozoites to antibody. Studies have shown sequestration of P. berghei pRBC in bone marrow, thereby bringing parasites in close proximity to immature RBCs in particular55. Such attributes of the parasite could potentially favour rapid RBC invasion by newly released merozoites, leaving minimal time in which antibodies could interfere with this process. To test this, we made use of a recently described P. berghei parasite line that cannot sequester, due to ablation of CD36 binding as a result of deletion of the smac gene56. Studies with this parasite have indeed suggested that pRBC sequestration may confer advantages to parasite growth in addition to avoidance of splenic removal56. BALB/c mice were immunized with the AdHu5-MVA vaccines encoding PbMSP142, PbAMA1 or PbMSP9, or a co-administered mixture of all three, and subsequently challenged with Δsmac pRBCs. However, similar to all the other studies, no vaccine efficacy was apparent in this case. Parasitemias were comparable between the vaccinees and controls (Supplemental Figure 3), and all mice were culled on day 10 post challenge.
Discussion
Here we report on the ability of subunit vaccines targeting the MSP1, AMA1 and MSP9 antigens to protect against blood-stage P. berghei parasites. Active immunization with subunit vaccines targeting these merozoite antigens has previously been reported to protect against blood-stage P. berghei infection in only four studies30,31,32,33. Following active immunization of BALB/c mice here with AdHu5-MVA vectors expressing these three P. berghei merozoite antigens, antibodies capable of binding to native parasite antigen were observed, indicating that all three antigen-delivery regimes were immunogenic. In addition, vector immunization against PbMSP142 induced PbMSP119-specific IgG that reached high endpoint titers in an ELISA assay. However, despite induction of these responses, no protection was afforded against P. berghei ANKA pRBC challenge. We observed similar results in a variety of inbred mouse strains, including C3H mice that developed the highest antibody titers. If protection is antibody-mediated in this mouse model, we cannot exclude the possibility that titers induced by viral vector immunization were too low to protect, or that the fine specificity of the antibody response was qualitatively non-protective. Also, it is possible the PbMSP1 vaccines targeted an incorrect region of the molecule; given responses against the PbMSP1 N-terminal region may be important (as reported for a recombinant Salmonella-based vaccine31). However, this is not the case in the P. yoelii and P. chabaudi models and is therefore unlikely. Delivery of the orthologous PyMSP142 or PcAMA1 antigens respectively by AdHu5-MVA is protective against blood-stage challenge in mice (shown here and in previous reports18,19). Similarly immunization with MSP119 protein in Freund's adjuvant (a regime capable of inducing extremely high titer responses) was shown here to afford sterilizing immunity against P. yoelii (in agreement with an earlier report of the same regime20), but no efficacy against either wild-type P. berghei or the chimeric Pb-PfM19 parasite.
Initial pRBC challenge experiments were performed using P. berghei ANKA. However in the published examples of protection against wild-type blood-stage P. berghei by subunit vaccination, the challenge strain was P. berghei NK65 rather than ANKA30,31,32. P. berghei NK65 infection results in a greater number of ‘latent merozoites' than ANKA, meaning merozoites are in the blood for longer (and thus potentially more accessible to antibody) before invading a new RBC57. NK65 is also known to i) have a greater predilection to invade immature RBC (reticulocytes); ii) be more likely to multiply infect the same cell; and iii) produce more ring forms early in infection43, leading to a less synchronous infection than the ANKA strain. Challenge experiments were therefore repeated using P. berghei NK65 but, despite these differences, no efficacy was seen. It cannot be excluded that the failure to protect against P. berghei NK65 may be due to differences in homology between the vaccine antigens and NK65 challenge strain (in the case of PbMSP142 and PbAMA1). However, assessment of sequence data from NK65 and ANKA P. berghei parasite lines found no differences in PbAMA1 and only one SNP in PbMSP1 (CJJ and BMF-F, unpublished observations), and in the case of PbMSP9 (based on the NK65 sequence) antibodies raised by AdHu5-MVA vaccination were capable of recognizing P. berghei ANKA schizonts in the IFA. Moreover, no protection was also observed against sporozoite challenge (bar in the case of one animal immunized with PbAMA1), despite the possibility of viral vectored immunization leading to the development of CD8+ T cells capable of targeting P. berghei liver-stage forms. Such liver-stage protection has been reported using the same vectors in the PyMSP142 model24. Overall these data are in agreement with more recently published studies showing a lack of anti-PbMSP142 and -PbAMA1 vaccine efficacy in mice36,37, and add to the growing body of evidence that P. berghei may possess mechanisms that can circumvent such responses.
Studies with a non-lethal/attenuated strain P. berghei XAT (derived from P. berghei NK65 58) have shown that protection requires IFN-γ and IgG2a59. Similarly, FcR were shown to be essential in mediating blood-stage protection against Pb XAT following infection of FcR common γ-chain knockout mice51. Protection was also reported against the Pb-PfM19 chimeric parasite using a human IgG1 mAb against PfMSP119, but only in transgenic mice expressing the human FcγRI (CD64)46, but not for an isotype switched human IgA mAb against the same epitope in human FcαRI (CD89) transgenic mice60. In agreement with this, a similar experiment with the ‘non-cytophilic' mouse IgG1 isotype failed to show protection in wild-type mice (although ‘cytophilic' IgG2a was not tested)61. We therefore tested here whether vaccine-induced protection against the merozoite antigens could be improved in mice lacking the inhibitory FcγRIIb (CD32b). This knockout would be more likely to enhance the action of mouse IgG1 (due to the greater inhibition of this isotype and low activatory-to-inhibitory (A:I) ratio62) but could still also enhance the action of vaccine-induced IgG2a and IgG2b – known to be induced by AdHu5-MVA vaccination18,48,54. Immunization of CD32b−/− mice might also be expected to enhance immune responses to the vaccine due to removal of the regulatory/inhibitory FcγR signalling at the time of immunization. This has been reported in studies for antibodies63,64 as well as tumor-specific CD8+ T cells65. Wild-type and CD32b−/− mice were not directly compared in this study, and in future it will be important to establish the impact of CD32b absence on vaccine immunogenicity. However, IgG responses were clearly primed and boosted against PbMSP119 in the knockout mice but, despite the absence of possible inhibitory FcγRIIb signalling, no vaccine efficacy was observed. No difference was also observed between naïve wild-type and knockout mice, again in contrast to the P. chabaudi infection model that observed reduced parasitemia in CD32b−/− mice66.
In contrast to subunit vaccines against the merozoite, whole parasite immunization protocols of mice can afford significant protection against subsequent parasite challenge. Such protocols may involve drug-cure, immunization with killed parasites in adjuvant or exposure to attenuated/non-lethal infection. Interpretation of such studies is complicated by the significant variability observed with each different protocol and each unique inbred mouse and parasite strain combination67. Nonetheless, following such ‘immunization' protocols mice are typically protected against homologous challenge, whilst outcome of heterologous challenge varies significantly. However, early studies clearly document reduced levels of efficacy afforded by exposure to replicating P. berghei parasites as compared to the other rodent malaria species68. In 1977 Playfair et al. also documented differences in the ease with which protection could be achieved between rodent malaria species following administration of a killed blood-stage vaccine. Whilst lysed and fixed P. yoelii pRBC induced homologous protection that was at least partly antibody-mediated, similar experiments with P. berghei pRBC led to little or no protection69. Although formalin-killed blood-stage parasites are able to protect against P. berghei this protection is incomplete and mice develop parasitemia levels of ~10%. The mechanism of this protection appears to be at least partly antibody-mediated as delay to parasitemia occurred following the transfer of immune sera to non-immunised mice70. Protective immunity in mice against blood-stage challenge could also be induced by self-limiting infections with growth-attenuated P. berghei ANKA parasites mutants lacking plasmepsin 4 71. Following challenge, the trophozoite/schizont-infected RBCs were trapped in the spleen resulting in rapid and efficient removal of parasites from the circulation. These observations indicate that the protective blood-stage immunity induced in this case was mediated against pRBC rather than merozoite invasion. Proposed reasons for the difficulty in protecting against P. berghei include sequestration of the parasite, immune suppression or evasion, and antigenic variation. P. berghei pRBC sequester in a similar way to P. falciparum, through adherence to the host receptor CD36 and such sequestration appears to be beneficial for parasite growth56. However, anti-merozoite vaccine efficacy was not improved here when tested against the Δsmac parasite line, suggesting this sequestration phenotype is unlikely to be helping P. berghei parasites circumvent such immune responses. Another possible contributor to the difficulty in protecting against blood-stage P. berghei might be the suppression of the immune system during parasite infection. Blood-stage P. berghei can rapidly suppress MHC class I and class II presentation of both malarial and other antigens by dendritic cells within 4 days of infection72. Such a phenomenon may prevent the essential development of de novo protective immune responses or the boosting of vaccine-induced antibodies. However, the vaccines tested here were ineffective against the first 4 days of parasite growth – a period in which pre-existing vaccine-induced responses are probably critical, and which probably precedes the development of either parasite-induced immunosuppression or substantial infection-induced adaptive immune responses.
Protection against a chimeric P. berghei line expressing P. falciparum MSP119 was also assessed here. Similar to other reported studies using baculoviral-based vaccines36, or PfMSP119 protein in Freund's adjuvant80, we again failed to protect against this parasite in two strains of mice following active subunit vaccine immunization. Passive transfer of IgG from PfMSP119-immunized rabbits or a human mAb has been demonstrated to protect against challenge with blood-stage Pb-PfM19 parasites46,47. However in our hands polyclonal purified IgG, from PfMSP1 immunized rabbits, did not protect against Pb-PfM19. It remains wholly possible that the antibody titers here were too low to protect, or that the antibodies were of incorrect fine specificity. Protection of mice (made semi-immune to this parasite line by drug-cure) from homologous pRBC challenge correlated with PfMSP119-specific inhibitory antibodies, but not with titers of total PfMSP-119 IgG34. However, these PfMSP1 immunogens have been previously shown to be recognized by conformational inhibitory anti-PfMSP119 mAbs and to raise IgG in rabbits that show functional activity in vitro48. Intriguingly, in both reported results to date where passive immunization was successful46,47, sterilizing immunity was observed. Partial efficacy or blood-stage control has not been reported. Successful outcome may therefore rely on an immediate clearance mechanism of the challenge inoculum rather than reduced rates of RBC invasion, though this requires further investigation. The Pb-PfM19 parasite used here also lacks the first four αα of PfMSP119 and instead expresses the first 4 αα of PbMSP11934, and this may affect the ability of IgG to inhibit MSP142 processing. Although protection against this parasite by transfer of PfMSP142-specific IgG has been observed47, the independently developed chimeric parasite developed by Cao et al.35, possesses the full length PfMSP119 sequence and may be a more suitable parasite line for future experiments.
Overall, these data suggest a failure of vaccines against commonly studied merozoite antigens to protect against P. berghei blood-stage infection, in contrast to many similar studies using P. yoelii and P. chabaudi. Although qualitative differences in the immune responses induced by the P. berghei vaccines tested here may explain the failure to protect, this remains unlikely given the success of both viral vector and protein/adjuvant vaccine platforms targeting the same antigens from other rodent malaria species and in raising IgG that is functional against P. falciparum in vitro. A fraction of parasite-derived material containing unknown antigens was recently reported to be protective in this model73, and thus vaccines targeting other blood-stage proteins may yet prove to be more effective. Despite supporting data obtained using the P. yoelii and P. chabaudi models, protein/adjuvant and viral vectored PfMSP1- and PfAMA1-based vaccines have faced significant disappointment in Phase IIa/b clinical trials to-date6,8. It may well be that the seemingly more stringent P. berghei mouse model will prove to be a better predictor of P. falciparum clinical vaccine efficacy. These observations have important implications for the development of chimeric parasite models used to test P. falciparum vaccine candidates, and the interpretation of future studies demonstrating protection in one or other mouse model16. Further studies remain warranted to establish whether or not P. falciparum does indeed share mechanisms with P. berghei that potentially enable this rodent malaria to circumvent immune responses induced by vaccines against merozoite antigens such as MSP1 and AMA1.
Methods
Viral vector vaccine generation
Recombinant human adenovirus serotype 5 (AdHu5) and modified vaccinia Ankara (MVA) viral vector vaccines expressing candidate antigens were designed according to previously published methods18. All vectors encoded the transgene of interest (listed below), with an N-terminal in-frame signal sequence from human tissue plasminogen activator (tPA). Adenoviral vaccines were grown in HEK293 cells, purified by CsCl centrifugation74 and titered by UV spectrophotometry to give units of viral particles (vp/mL)18, whilst MVA vaccines were grown in chicken embryo fibroblasts (CEFs), purified by centrifugation through a sucrose cushion75 and titered by fluorescence plaque assay using the GFP marker to give plaque-forming units (pfu/mL)18.
PbMSP9
PbMSP9 NK65 strain (GenBank AY302245) commenced at amino acid (αα) 23 (histidine) and was truncated at αα 392 (glutamic acid)30. In order to remove potential sites of N-linked glycosylation, the serine residues at αα sites 96, 155, 171, 301 and 338 were substituted with alanine. An additional serine residue at αα site 284 was substituted with alanine to remove an active serine protease site and avoid potential toxicity in vitro during virus propagation (ADD, SJD unpublished observations). The transgene was synthesized by GeneArt GmbH (Regensburg, Germany) and codon optimized for expression in mice.
PbAMA1
PbAMA1 ANKA strain (GenBank U45969) commenced at αα 21 (cysteine) and was truncated at αα 478 (glutamic acid). In order to remove potential sites of N-linked glycosylation, serine residues at αα sites 160, 233, 251, 290, 407 and 412 were substituted with alanine, and asparagine at αα site 189 was substituted with glutamine. The gene was synthesized as for PbMSP9.
PbMSP142
The 42 kDa C-terminus of P. berghei MSP1 was amplified from genomic DNA extracted from wild-type P. berghei ANKA strain (Pb-PbM19)34 using the following oligonucleotide primers (see below for description of parasites and PCR method): forward primer 5′-TCC GAA AAT GCA CAA GAA AAA AAT A-3′; reverse primer 5′-TCC CAT AAA GCT GGA AGA GCT ACA GAA-3′. Primers were designed to amplify DNA from αα site 1416 (serine) and finish prior to the GPI anchor at αα site 1776 (glycine) (PlasmoDB: PBANKA_083100).
PfMSP1
Vaccines encoding the P. falciparum MSP1 inserts termed PfM115 and PfM128 have been described previously48. Briefly, the PfM115 insert encodes a 115 kDa composite P. falciparum MSP1 antigen. From N- to C-terminus it includes the conserved blocks of MSP1 sequence (blocks 1, 3, 5 and 12) from the 3D7 clone, followed by Wellcome strain block 16 (MSP133), linked by a glycine-proline linker to 3D7 clone blocks 16 and 17 (MSP142). PfM128 encodes a 128 kDa insert as described above for PfM115, except the Wellcome strain MSP133 sequence was replaced with the sequence encoding MSP142.
PyMSP142
AdHu5 and MVA vectors encoding MSP142 from P. yoelii YM have been previously described18.
Ovalbumin and no malaria antigen controls
AdHu5 and MVA vectors encoding hen ovalbumin (OVA) have been previously described39, as have AdHu5 lacking a transgene amd MVA encoding only GFP38.
Protein vaccines
Generation of recombinant P. yoelii strain YM MSP119-glutathione S-transferase (GST) fusion protein has been described previously18. Recombinant PyMSP119 and PfMSP119 fused to IMX108 (mouse complement C4 binding protein, C4bp)41 were kindly provided by Dr F. Hill (Imaxio, France). Recombinant PbMSP119 was produced as a GST fusion protein using previously published methods18. To generate the vector, PbMSP119 (αα methionine 1669 – glycine 1776) was amplified from the PbMSP142 sequence described above by PCR and cloned into the expression vector pGEX-2T (Amersham Biosciences, Bucks., UK).
Animals and immunization studies
All procedures were performed in accordance with the terms of the UK Animals (Scientific Procedures) Act Project Licence and were approved by the University of Oxford Animal Care and Ethical Review Committee (PPL 30/2414). Six to eight week old female BALB/c (H-2d), C3H (H-2k), C57BL/6 (H-2b) and CD1 (outbred) mice were obtained from Harlan, UK, and housed in specific pathogen-free conditions. Unless otherwise stated, mice were immunized intramuscularly (i.m.) with 1 × 1010 vp AdHu5 vaccines and then boosted eight weeks later with 1 × 107 pfu MVA expressing the same antigen, as per a previously established immunization regime18,24. Protein vaccines were administered at doses of 20 μg at weeks 0 (subcutaneously, s.c.), 3 (s.c.), 6 (intraperitoneally, i.p.), 8 (i.p.) and 9 (i.p.). The adjuvant used for subcutaneous injection at week 0 was complete Freund's adjuvant (CFA) and at weeks 3, 6 and 8 was incomplete (IFA). At week 9 protein was administered in PBS. This regime was based on a published protocol20 shown to induce very high-titer antibody responses. Sera were collected from tail vein bleeds as described in Results.
Fcγ receptor IIb (CD32b) deficient mice on a C57BL/6 background (CD32b−/−)63 were provided from the Queen's Medical Centre, Nottingham, UK and bred at the Wellcome Trust Centre for Human Genetics, Oxford, UK. Genotype was confirmed using DNA extracted from tissue samples and the Expand High Fidelity PCR Kit (Roche Diagnostics, UK) and previously described reaction conditions76, except the primers were: 5′-CTC GTG CTT TAC GGT ATC GCC-3′ (Mutant); 5′-AAA CTC GAC CCC CCG TGG ATC-3′ (Common); and 5′-TTG ACT GTG GCC TTA AAC GTG TAG-3′ (Wild-type).
New Zealand white rabbits were used for all rabbit experiments. Vaccines were shipped from Oxford and the immunization of rabbits and collection of sera was performed by Agrobio, France. Rabbits were immunized i.m. on day 0 with 5 × 1010 vp AdHu5 or ChAd63 expressing PfM128 or no malaria insert (control) and then boosted i.m. on day 56 with 1 × 108 pfu MVA expressing PfM128 or GFP (control). Serum was collected pre-immunization on day 0 as well as two weeks post-boost on day 70.
Rabbit IgG purification
Polyclonal rabbit IgG was purified from serum samples by Protein G affinity chromatography using a buffer system (Immunopure, Pierce) according to manufacturer's instructions. IgG was quantified using a spectrophotometer at 280 nm. The extinction coefficient for rabbit IgG is 1.44 (i.e. 1 mg/mL of rabbit IgG has an OD of 1.44 at 280 nm). The concentration of antibody was therefore calculated as follows: Concentration of rabbit IgG (mg/mL) = (OD × dilution factor)/1.44.
Immunogenicity assays
Mouse and rabbit total IgG ELISAs were performed according to previously published methods48. Briefly, protein was coated onto ELISA plates at a concentration of 2 μg/mL in PBS. Test sera were applied in duplicate wells and serially diluted. The endpoint titers were taken as the x-axis intercept of the dilution curve at an absorbance value 3 × standard deviations greater than the OD405 nm for naïve mouse sera.
Immunofluorescence assays (IFA) were performed using methods based on those described previously48. Briefly, blood from P. berghei infected mice was cultured in vitro overnight to enable development of schizonts. Slides were prepared with a thin smear of this blood. Slides were fixed with 4% formaldehyde and 0.1% NP40 for 15 min at room temperature (RT). Mouse sera were diluted 1:100 in PBS and incubated on slides for 45 min. Slides were washed with PBS and incubated with Alexa Fluor 488 goat anti-mouse IgG for 30 min. Slides were washed in PBS, DAPI was applied, and the slides were viewed under a fluorescence microscope.
Parasites
P. berghei NK65 (MR4 Number: MRA-268) was obtained through the Malaria Research and Reference Reagent Resource Centre (MR4) and was deposited by Prof. V. Nussenzweig, New York University, USA. P. berghei strain ANKA clone 234, strain NK65 or P. yoelii strain YM parasitized red blood cell (pRBC) challenges were carried out as previously described18,77. Briefly, donor mice were infected by i.p. injection of cryopreserved infected blood stocks, before passage into experimental mice which were infected by intravenous (i.v.) injection with 500 (P. berghei) or 10,000 (P. yoelii) pRBCs unless otherwise stated. Parasitemia was monitored from day 3 post-challenge by Giemsa-stained thin blood smear and was calculated as a percentage of infected RBC. Mice were considered uninfected if no parasites were observed in 50 fields of view. For P. berghei strain ANKA 234 sporozoite challenge studies, infected Anopheles stephensi mosquitoes were prepared as previously described78. Salivary glands were collected by dissection and placed in a tissue homogenizer with RPMI 1640 (Sigma) to release the sporozoites, which were then counted using a haemocytometer. Unless otherwise stated, mice were challenged i.v. with 500 sporozoites and monitored for blood-stage infection from day 5 as for pRBC challenge. In some cases, a linear-regression model was generated to predict time until 1% parasitemia as previously described79.
A chimeric parasite line known as Pb-PfM19 and a control parasite line, Pb-PbM19, were kindly provided by Dr B. S. Crabb and Dr T. F. de Koning-Ward. These chimeric parasite lines were generated using an allelic replacement approach, on a P. berghei ANKA background34. Pb-PfM19 expresses P. falciparum strain D10 MSP119 (MAD20/ETSR allele) in place of wild-type PbMSP119, and Pb-PbM19 was generated as a transfection control, replacing wild-type PbMSP119 with the identical wild-type sequence. The identity of the two parasite lines was confirmed before the studies by PCR. Infected blood at 5–10% parasitemia was harvested from BALB/c donor mice by cardiac exsanguination into 10 mM EDTA. Blood was made up to 5 mL with PBS, and white blood cells filtered out using a Plasmodipur filter (Euro-Diagnostica, The Netherlands). Erythrocytes were washed in PBS before extraction of parasite DNA using a QIAamp Blood Mini Kit (Qiagen). Parasite identity was confirmed by Expand High Fidelity PCR (Roche Diagnostics, UK) according to the manufacturer's instructions and using 4 mM MgCl2 and an annealing temperature of 52°C. Species-specific primers were used for MSP119: PfMSP119 (5′-ATG CGT AAA AAA ACA ATG TCC AGA AAA T-3′; 5′-GTT AGA GGA ACT GCA GAA AAT ACC ATC G-3′) and PbMSP119 (5′-CTG CAA ATG CTG GAT GTT TTA GAT A-3′; 5′-CAT CAT AAT ATG CAT TAG GGG TTG G-3′). Amplified DNA fragments were of the expected fragment sizes (PbMSP119 = 228 bp, PfMSP119 = 271 bp) and identities for their respective parasites (data not shown).
P. berghei ANKA parasites lacking the schizont membrane-associated cytoadherence protein (SMAC) have previously been described (Δsmac; reference 56; mutant 1160cl7; RMgm ID: RMgm-661). Δsmac parasites exhibit reduced CD36-mediated sequestration and reduced growth rates in wild-type mice.
Statistics
Data were analyzed using GraphPad Prism v5.03. Continuous outcomes in two independent groups were compared using a Mann-Whitney U test, or using the Wilcoxon matched pairs signed rank test for paired data. A Kruskal-Wallis test with post-hoc Dunn's analysis was used to compare responses between more than two groups. A Friedman test was used for paired data when there were more than two time-point observations. In all cases P ≤ 0.05 was considered significant (* P ≤ 0.05 and ** P ≤ 0.01).
Author Contributions
A.L.G., E.K.F., A.C.M., S.C.G., A.V.S.H., R.J.P. and S.J.D. conceived, planned and designed experiments, including design of viral vectors. C.J.J. and B.M.F. designed and provided the Δsmac parasites. A.G.G., E.K.F., A.R.W., A.D.D., S.C.D.C., K.B., S.B., M.D.J.D. and D.L. conducted the experiments. A.L.G., E.K.F. and S.J.D. analyzed the data. A.L.G., E.K.F. and S.J.D. wrote the paper. All authors reviewed the manuscript.
Supplementary Material
Supplemental Information
Acknowledgments
We thank Julie Furze, Mark Tunnicliff, and the Jenner Institute Vector Core Facility and Adjuvant Bank Facility (Oxford, UK) for assistance; Fergal Hill (Imaxio, France) for provision of protein vaccines; and Brendan Crabb and Tania de Koning-Ward (Australia) for provision of the chimeric parasite Pb-PfM19 and the control parasite line, Pb-PbM19. ALG was funded by a MRC Clinical Training Fellowship [Grant number G0600424]. This work was supported in part by the EMVDA (European Malaria Vaccine Development Association, a European Commission (EC) FP6-funded consortium [LSHP-CT-2007-037506]; the Wellcome Trust [084113/Z/07/Z]; and EVIMalaR, an EC FP7/2007-2013-funded programme [Grant agreement No. 242095]. RJP was supported by a Wellcome Trust project grant (082915/B/07/Z). CJJ was supported by a grant from the European Community's Seventh Framework Program (FP7/2007–2013; Grant Agreement No. 242095). BFF was supported by a grant from The Netherlands Organization for Scientific Research (ZonMW TOP Grant No. 9120_6135). ADD held a Wellcome Trust Research Training Fellowship [089455/2/09/Z]. SCG, AVSH and SJD are Jenner Investigators, and SJD is a MRC Career Development Fellow [G1000527].
Footnotes
ALG, EKF, ARW, ADD, SCdC, MDJD, ACM, SCG, AVSH, RJP and SJD are named inventors on patent applications covering malaria vectored vaccines and/or immunization regimes. KB, SB, DL, CJJ and BMF have no competing financial interests.
References
- Murray C. J. et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379 (9814), 413–431 (2012). [DOI] [PubMed] [Google Scholar]
- Alonso P. L. et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 364 (9443), 1411–1420 (2004). [DOI] [PubMed] [Google Scholar]
- Agnandji S. T. et al. A Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Infants. N Engl J Med 367 (24), 2284–2295 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily J. P. Malaria vaccine trials--beyond efficacy end points. N Engl J Med 367 (24), 2349–2351 (2012). [DOI] [PubMed] [Google Scholar]
- Hill A. V. Vaccines against malaria. Philos Trans R Soc Lond B Biol Sci 366 (1579), 2806–2814 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman A. L. & Draper S. J. Blood-stage malaria vaccines - recent progress and future challenges. Ann Trop Med Parasitol 104 (3), 189–211 (2010). [DOI] [PubMed] [Google Scholar]
- Langhorne J., Ndungu F. M., Sponaas A. M. & Marsh K. Immunity to malaria: more questions than answers. Nat Immunol 9 (7), 725–732 (2008). [DOI] [PubMed] [Google Scholar]
- Sheehy S. H. et al. ChAd63-MVA-vectored Blood-stage Malaria Vaccines Targeting MSP1 and AMA1: Assessment of Efficacy Against Mosquito Bite Challenge in Humans. Mol Ther 20 (12), 2355–2368 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogutu B. R. et al. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS ONE 4 (3), e4708 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thera M. A. et al. A field trial to assess a blood-stage malaria vaccine. N Engl J Med 365 (11), 1004–1013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara I. et al. A randomized controlled phase 2 trial of the blood stage AMA1-C1/Alhydrogel malaria vaccine in children in Mali. Vaccine 27 (23), 3090–3098 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A. The carboxy-terminus of merozoite surface protein 1: structure, specific antibodies and immunity to malaria. Parasitology 136 (12), 1445–1456 (2009). [DOI] [PubMed] [Google Scholar]
- Remarque E. J., Faber B. W., Kocken C. H. & Thomas A. W. Apical membrane antigen 1: a malaria vaccine candidate in review. Trends Parasitol 24 (2), 74–84 (2008). [DOI] [PubMed] [Google Scholar]
- Spring M. D. et al. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PLoS One 4 (4), e5254 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouattara A. et al. Molecular Basis of Allele-Specific Efficacy of a Blood-Stage Malaria Vaccine: Vaccine Development Implications. J Infect Dis 207 (3) , 511–519 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlambo G. & Kumar N. Transgenic rodent Plasmodium berghei parasites as tools for assessment of functional immunogenicity and optimization of human malaria vaccines. Eukaryot Cell 7 (11), 1875–1879 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorne J., Quin S. J. & Sanni L. A. Mouse models of blood-stage malaria infections: immune responses and cytokines involved in protection and pathology. Chem Immunol 80, 204–228 (2002). [DOI] [PubMed] [Google Scholar]
- Draper S. J. et al. Effective induction of high-titer antibodies by viral vector vaccines. Nat Med 14 (8), 819–821 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S. et al. Recombinant Viral-Vectored Vaccines Expressing Plasmodium chabaudi AS Apical Membrane Antigen 1: Mechanisms of Vaccine-Induced Blood-Stage Protection. J Immunol 188 (10), 5041–5053 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirunpetcharat C. et al. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J Immunol 159 (7), 3400–3411 (1997). [PubMed] [Google Scholar]
- Rotman H. L., Daly T. M. & Long C. A. Plasmodium: immunization with carboxyl-terminal regions of MSP-1 protects against homologous but not heterologous blood-stage parasite challenge. Exp Parasitol 91 (1), 78–85 (1999). [DOI] [PubMed] [Google Scholar]
- Narum D. L., Ogun S. A., Thomas A. W. & Holder A. A. Immunization with parasite-derived apical membrane antigen 1 or passive immunization with a specific monoclonal antibody protects BALB/c mice against lethal Plasmodium yoelii yoelii YM blood-stage infection. Infect Immun 68 (5), 2899–2906 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlborg N., Ling I. T., Howard W., Holder A. A. & Riley E. M. Protective immune responses to the 42-kilodalton (kDa) region of Plasmodium yoelii merozoite surface protein 1 are induced by the C-terminal 19-kDa region but not by the adjacent 33-kDa region. Infect Immun 70 (2), 820–825 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper S. J. et al. Recombinant viral vaccines expressing merozoite surface protein-1 induce antibody- and T cell-mediated multistage protection against malaria. Cell Host Microbe 5 (1), 95–105 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipasa J. et al. Identification of T cell epitopes on the 33-kDa fragment of Plasmodium yoelii merozoite surface protein 1 and their antibody-independent protective role in immunity to blood stage malaria. J Immunol 169 (2), 944–951 (2002). [DOI] [PubMed] [Google Scholar]
- Kaba S. A. et al. A nonadjuvanted polypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria. J Immunol 183 (11), 7268–7277 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar S. et al. Single-dose protection against Plasmodium berghei by a simian adenovirus vector using a human cytomegalovirus promoter containing intron A. J Virol. 82 (8), 3822–3833 (2008). [DOI] [PMC free article] [PubMed]
- Blagborough A. M. & Sinden R. E. Plasmodium berghei HAP2 induces strong malaria transmission-blocking immunity in vivo and in vitro. Vaccine 27 (38), 5187–5194 (2009). [DOI] [PubMed] [Google Scholar]
- Matsuoka H. et al. Induction of anti-malarial transmission blocking immunity with a recombinant ookinete surface antigen of Plasmodium berghei produced in silkworm larvae using the baculovirus expression vector system. Vaccine 14 (2), 120–126 (1996). [DOI] [PubMed] [Google Scholar]
- Lopera-Mesa T. M., Kushwaha A., Mohmmed A. & Chauhan V. S. Plasmodium berghei merozoite surface protein-9: immunogenicity and protective efficacy using a homologous challenge model. Vaccine 26 (10), 1335–1343 (2008). [DOI] [PubMed] [Google Scholar]
- Toebe C. S., Clements J. D., Cardenas L., Jennings G. J. & Wiser M. F. Evaluation of immunogenicity of an oral Salmonella vaccine expressing recombinant Plasmodium berghei merozoite surface protein-1. Am J Trop Med Hyg 56 (2), 192–199 (1997). [DOI] [PubMed] [Google Scholar]
- Wan Omar A. et al. A recombinant 19 kDa Plasmodium berghei merozoite surface protein 1 formulated with alum induces protective immune response in mice. Trop Biomed 24 (1), 119–126 (2007). [PubMed] [Google Scholar]
- Li S. L., Zhang D. M., Cao Y. & Pan W. Q. [Expression and immunogenicity evaluation of ectodomain and subdomains of Plasmodium berghei apical membrane antigen 1]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 25 (1), 1–5 (2007). [PubMed] [Google Scholar]
- de Koning-Ward T. F. et al. A new rodent model to assess blood stage immunity to the Plasmodium falciparum antigen merozoite surface protein 119 reveals a protective role for invasion inhibitory antibodies. J Exp Med 198 (6), 869–875 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Zhang D. & Pan W. Construction of transgenic Plasmodium berghei as a model for evaluation of blood-stage vaccine candidate of Plasmodium falciparum chimeric protein 2.9. PLoS One 4 (9), e6894 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. et al. Plasmodium berghei circumvents immune responses induced by merozoite surface protein 1- and apical membrane antigen 1-based vaccines. PLoS One 5 (10), e13727 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. et al. Differential effects of C3d on the immunogenicity of gene gun vaccines encoding Plasmodium falciparum and Plasmodium berghei MSP1(42). Vaccine 28 (28), 4515–4522 (2010). [DOI] [PubMed] [Google Scholar]
- Forbes E. K. et al. Combining Liver- and Blood-Stage Malaria Viral-Vectored Vaccines: Investigating Mechanisms of CD8+ T Cell Interference. J Immunol 187 (7), 3738–3750 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cassan S. C. et al. The requirement for potent adjuvants to enhance the immunogenicity and protective efficacy of protein vaccines can be overcome by prior immunization with a recombinant adenovirus. J Immunol 187 (5), 2602–2616 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas A. D. et al. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat Commun 2, 601 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogun S. A., Dumon-Seignovert L., Marchand J. B., Holder A. A. & Hill F. The oligomerization domain of C4-binding protein (C4bp) acts as an adjuvant, and the fusion protein comprised of the 19-kilodalton merozoite surface protein 1 fused with the murine C4bp domain protects mice against malaria. Infect Immun 76 (8), 3817–3823 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman I. W. Malaria: parasite biology, pathogenesis, and protection. (ASM, Washington, DC, 1998). [Google Scholar]
- Deharo E., Coquelin F., Chabaud A. G. & Landau I. The erythrocytic schizogony of two synchronized strains of plasmodium berghei, NK65 and ANKA, in normocytes and reticulocytes. Parasitol Res 82 (2), 178–182 (1996). [DOI] [PubMed] [Google Scholar]
- Silvie O. et al. A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. J Biol Chem 279 (10), 9490–9496 (2004). [DOI] [PubMed] [Google Scholar]
- Chuang I. et al. DNA Prime/Adenovirus Boost Malaria Vaccine Encoding P. falciparum CSP and AMA1 Induces Sterile Protection Associated with Cell-Mediated Immunity. PLoS One 8 (2), e55571 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh R. S. et al. The importance of human FcgammaRI in mediating protection to malaria. PLoS Pathog 3 (5), e72 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva S. et al. Immunogenicity and protective efficacy of Escherichia coli expressed Plasmodium falciparum merozoite surface protein-1(42) using human compatible adjuvants. Vaccine 24 (12), 2007–2016 (2006). [DOI] [PubMed] [Google Scholar]
- Goodman A. L. et al. New candidate vaccines against blood-stage Plasmodium falciparum malaria: prime-boost immunization regimens incorporating human and simian adenoviral vectors and poxviral vectors expressing an optimized antigen based on merozoite surface protein 1. Infect Immun 78 (11), 4601–4612 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas A. D. et al. Tailoring subunit vaccine immunogenicity: Maximizing antibody and T cell responses by using combinations of adenovirus, poxvirus and protein-adjuvant vaccines against Plasmodium falciparum MSP1. Vaccine 28 (44), 7167–7178 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy S. H. et al. Phase Ia Clinical Evaluation of the Plasmodium falciparum Blood-stage Antigen MSP1 in ChAd63 and MVA Vaccine Vectors. Mol Ther 19 (12), 2269–2276 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneto T. et al. A critical role of Fc receptor-mediated antibody-dependent phagocytosis in the host resistance to blood-stage Plasmodium berghei XAT infection. J Immunol 166 (10), 6236–6241 (2001). [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F. & Ravetch J. V. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 310 (5753), 1510–1512 (2005). [DOI] [PubMed] [Google Scholar]
- Woof J. M. Immunology. Tipping the scales toward more effective antibodies. Science 310 (5753), 1442–1443 (2005). [DOI] [PubMed] [Google Scholar]
- Biswas S. et al. Transgene Optimization, Immunogenicity and In Vitro Efficacy of Viral Vectored Vaccines Expressing Two Alleles of Plasmodium falciparum AMA1. PLoS One 6 (6), e20977 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. Hematopoietic tissue in malaria: facilitation of erythrocytic recycling by bone marrow in Plasmodium berghei-infected mice. J Parasitol 69 (2), 307–318 (1983). [PubMed] [Google Scholar]
- Fonager J. et al. Reduced CD36-dependent tissue sequestration of Plasmodium-infected erythrocytes is detrimental to malaria parasite growth in vivo. J Exp Med 209 (1), 93–107 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaute-Lafitte A. et al. The chemosensitivity of the rodent malarias--relationships with the biology of merozoites. Int J Parasitol 24 (7), 981–986 (1994). [DOI] [PubMed] [Google Scholar]
- Waki S., Tamura J., Imanaka M., Ishikawa S. & Suzuki M. Plasmodium berghei: isolation and maintenance of an irradiation attenuated strain in the nude mouse. Exp Parasitol 53 (3), 335–340 (1982). [DOI] [PubMed] [Google Scholar]
- Waki S., Uehara S., Kanbe K., Nariuch H. & Suzuki M. Interferon-gamma and the induction of protective IgG2a antibodies in non-lethal Plasmodium berghei infections of mice. Parasite Immunol 17 (10), 503–508 (1995). [DOI] [PubMed] [Google Scholar]
- Shi J. et al. The generation and evaluation of recombinant human IgA specific for Plasmodium falciparum merozoite surface protein 1–19 (PfMSP119). BMC Biotechnol 11, 77 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adame-Gallegos J. R., Shi J., McIntosh R. S. & Pleass R. J. The generation and evaluation of two panels of epitope-matched mouse IgG1, IgG2a, IgG2b and IgG3 antibodies specific for Plasmodium falciparum and Plasmodium yoelii merozoite surface protein 1–19 (MSP1(19)). Exp Parasitol 130 (4), 384–393 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F. & Ravetch J. V. Fcgamma receptors: old friends and new family members. Immunity 24 (1), 19–28 (2006). [DOI] [PubMed] [Google Scholar]
- Takai T., Ono M., Hikida M., Ohmori H. & Ravetch J. V. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature 379 (6563), 346–349 (1996). [DOI] [PubMed] [Google Scholar]
- Clatworthy M. R. & Smith K. G. FcgammaRIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J Exp Med 199 (5), 717–723 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalergis A. M. & Ravetch J. V. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. J Exp Med 195 (12), 1653–1659 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy M. R. et al. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcgammaRIIb reduce susceptibility to malaria. Proc Natl Acad Sci U S A 104 (17), 7169–7174 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. S. & Good M. F. Whole parasite blood stage malaria vaccines: a convergence of evidence. Hum Vaccin 6 (1), 114–123 (2010). [DOI] [PubMed] [Google Scholar]
- Cox F. E. Protective immunity between malaria parasites and piroplasms in mice. Bull World Health Organ 43 (2), 325–336 (1970). [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B. & Cottrell B. J. Protection of mice against malaria by a killed vaccine: differences in effectiveness against P. yoelii and P. berghei. Immunology 33 (4), 507–515 (1977). [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R. Host defenses in murine malaria: analysis of the mechanisms of immunity to Plasmodium berghei generated in response to immunization with formalin-killed blood-stage parasites. Infect Immun 24 (3), 707–712 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaccapelo R. et al. Plasmepsin 4-deficient Plasmodium berghei are virulence attenuated and induce protective immunity against experimental malaria. Am J Pathol 176 (1), 205–217 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundie R. J. et al. Blood-stage Plasmodium berghei infection leads to short-lived parasite-associated antigen presentation by dendritic cells. Eur J Immunol 40 (6), 1674–1681 (2010). [DOI] [PubMed] [Google Scholar]
- Bagai U., Pawar A. & Kumar V. Antibody responses to 43 and 48 kDa antigens of blood-stage Plasmodium berghei in Balb/c mice. J Parasit Dis 34 (2), 68–74 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottingham M. G. et al. Preventing spontaneous genetic rearrangements in the transgene cassettes of adenovirus vectors. Biotechnol Bioeng 109 (3), 719–728 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebanski M. et al. Protection from Plasmodium berghei infection by priming and boosting T cells to a single class I-restricted epitope with recombinant carriers suitable for human use. Eur J Immunol 28 (12), 4345–4355 (1998). [DOI] [PubMed] [Google Scholar]
- Forbes E. K. et al. T cell responses induced by adenoviral vectored vaccines can be adjuvanted by fusion of antigen to the oligomerization domain of c4b-binding protein. PLoS One 7 (9), e44943 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. C. et al. Anti-CD25 antibody enhancement of vaccine-induced immunogenicity: increased durable cellular immunity with reduced immunodominance. J Immunol 175 (11), 7264–7273 (2005). [DOI] [PubMed] [Google Scholar]
- Gilbert S. C. et al. Enhanced CD8 T cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime-boost immunisation regimes. Vaccine 20 (7–8), 1039–1045 (2002). [DOI] [PubMed] [Google Scholar]
- Reyes-Sandoval A. et al. CD8+ T effector memory cells protect against liver-stage malaria. J Immunol 187 (3), 1347–1357 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafuye-Mlwilo M. Y., Mukherjee P. & Chauhan V. S. Kinetics of humoral and memory B cell response induced by the Plasmodium falciparum 19-kilodalton merozoite surface protein 1 in mice. Infect Immun 80 (2), 633–42 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Information