Abstract
GSK1322322 is a potent inhibitor of peptide deformylase, an essential bacterial enzyme required for protein maturation. GSK1322322 is active against community-acquired skin and respiratory tract pathogens, including methicillin-resistant Staphylococcus aureus, multidrug-resistant Streptococcus pneumoniae, and atypical pathogens. This phase I, randomized, double-blind, placebo-controlled, 2-part, single-dose, dose escalation study (first time in humans) evaluated the safety, tolerability, and pharmacokinetics of GSK1322322 (powder-in-bottle formulation) in healthy volunteers. In part A, dose escalation included GSK1322322 doses of 100, 200, 400, 800, and 1,500 mg under fasting conditions and 800 mg administered with a high-fat meal. In part B, higher doses of GSK1322322 (2,000, 3,000, and 4,000 mg) were evaluated under fasting conditions. Of the 39 volunteers enrolled in the study, 29 and 10 volunteers were treated with GSK1322322 and placebo, respectively. Upon single-dose administration, GSK1322322 was absorbed rapidly, with median times to maximum plasma concentration (Tmax) ranging from 0.5 to 1.0 h. The maximum observed plasma concentration (Cmax) and exposure (area under the concentration-time curve [AUC]) of GSK1322322 were greater than dose proportional between 100 and 1,500 mg and less than dose proportional between 1,500 and 4,000 mg. Administration of the drug with a high-fat meal reduced the rate of absorption (reduced Cmax and delayed Tmax) without affecting the extent of absorption (no effect on AUC). GSK1322322 was generally well tolerated, with all adverse events being mild to moderate in intensity during both parts of the study. The most frequently reported adverse event was headache. Data from this study support further evaluation of GSK1322322.
INTRODUCTION
The emergence and spread of pathogenic bacteria resistant to many antibiotics have created the need for novel therapeutic agents (1). Epidemic antibiotic resistance has been described for numerous pathogens, including, but not limited to, a global spread of methicillin-resistant Staphylococcus aureus (MRSA) infection and drug resistance among common respiratory pathogens including Streptococcus pneumoniae (2, 3). Most of the antibiotics under development are improved derivatives of the marketed products, which are generally only partially effective against existing resistance mechanisms (4). GSK1322322, first in a new class of antibiotics, is a potent inhibitor of peptide deformylase (PDF) (5). Peptide deformylase, an essential bacterial enzyme required for protein maturation, is a clinically unexploited target (6, 7). GSK1322322 is a member of a novel hydrazinopyrimidine class of PDF inhibitors discovered through a combination of structure-based drug design and iterative medicinal chemistry (8). GSK1322322 protein binding is estimated to be <69% on the basis of in vitro study results (data not shown). GSK1322322 shows no cross-resistance with agents in current use and is fully active against pathogens resistant to multiple classes of existing antibiotics, including beta-lactams, macrolides, and quinolones (9).
GSK1322322 is active against community-acquired skin and respiratory tract pathogens, including MRSA, multidrug-resistant S. pneumoniae, and atypical pathogens (5, 9, 10). GSK1322322 exhibits a potent sub-MIC effect for most strains of S. aureus, inhibiting growth in vitro for 6 to 8 h at concentrations well below the MIC (11, 12). The potent in vivo activity of GSK1322322 against rodent respiratory tract infection and skin and soft tissue infection models has been demonstrated (5, 9). The favorable MIC and animal data coupled with the safety profile of GSK1322322 observed to date support further clinical development of GSK1322322 in target patient populations.
In this 2-part, phase I study, GSK1322322 was first administered in humans to evaluate its safety, tolerability, and single-dose pharmacokinetics (PK) with dose escalation from 100 to 1,500 mg in healthy volunteers (10). The safety, tolerability, and PK of higher doses (2,000 to 4,000 mg) were also assessed. Additionally, because GSK1322322 has pH-dependent solubility, the effect of a high-fat meal on the PK of GSK1322322 was evaluated.
MATERIALS AND METHODS
Study design and population.
This was a randomized, double-blind, placebo-controlled, single-dose, sequential-cohort, dose escalation trial of healthy volunteers (study identifier PDF111341). Adults aged 18 to 65 years who were in generally good health with no clinically relevant abnormalities as determined by medical history, physical examination, laboratory tests, and cardiac monitoring were eligible for the trial. Volunteers had a body mass index of 18 to 30 kg/m2, inclusive. Volunteers were excluded from the study if they met one of the following criteria: a positive prestudy drug/alcohol screen; positive hepatitis B virus surface antigen or hepatitis C virus antibody result within 3 months of screening; positive test for HIV antibody; use of any investigational drug within 30 days, 5 half-lives, or twice the duration of the biological effect of the investigational drug (whichever is longer) before the day of dosing; or exposure to >4 new chemical entities within 12 months before the day of dosing. All volunteers provided written informed consent. The study was approved by an institutional review board and was conducted in accordance with good clinical practices.
Overall, 9 cohorts were planned for this 2-part study. Part A was planned with 6 cohorts: 5 cohorts to study the single-dose safety, tolerability, and PK with dose escalation from 100 to 1,500 mg (i.e., cohorts A to E) under fasting conditions and 1 cohort (i.e., cohort G) to assess the effect of a high-fat meal on PK parameters with the selected 800-mg GSK1322322 dose (based on safety and tolerability at previous doses and consideration of the expected increase in GSK1322322 exposures). The study was designed to administer GSK1322322 to 2 volunteers and placebo to 1 volunteer at each dose level of 100, 200, and 400 mg (i.e., cohorts A, B, and C, respectively). For the 800- and 1,500-mg doses (i.e., cohorts D and E, respectively) and the 800-mg dose with a high-fat meal (cohort G), each cohort was designed for 6 volunteers to receive GSK1322322 and 2 volunteers to receive placebo. During part B of the study, the safety, tolerability, and PK of higher doses of GSK1322322 (2,000, 3,000, and 4,000 mg) were evaluated in 3 cohorts (i.e., cohorts F1, F2, and F3) with the same 4 volunteers (i.e., 3 for GSK1322322 and 1 for placebo) in a crossover design separated by ≥1 week.
Volunteers were admitted to the unit the day before drug administration and discharged after all study procedures were completed on day 2. Volunteers were administered a powder-in-bottle oral formulation as a suspension of GSK1322322 or microcrystalline cellulose for placebo. Study drug and placebo were administered orally after an overnight fast of ≥10 h. Volunteers in cohort G were administered study medication with a high-fat meal (53% calories from fat), which included 2 slices of toasted white bread with 2 tsp of butter, 2 eggs fried in butter, 2 slices of bacon, 4 oz of hash-browned potatoes, and 8 oz of whole milk (32.1 g of protein, 70.2 g of carbohydrate, and 51.1 g of fat). Volunteers returned for a follow-up visit 12 to 18 days after their single dose of study medication. In addition, volunteers in part B also returned for a follow-up visit 7 days after their last dose of study medication.
Pharmacokinetic assessments.
Blood samples to measure plasma GSK1322322 concentrations were collected predose (within 15 min before dosing) and for a 48-h period (i.e., at 0.25, 0.5, 1, 1.5, 2, 4, 6, 8, 12, 18, 24, 36, and 48 h) postdose for GSK1322322 administered at 800, 1,500, 2,000, 3,000, and 4,000 mg. For lower doses of GSK1322322 (i.e., 100 to 400 mg), blood samples were collected only up to 24 h postdose. Volunteers emptied their bladder 20 min before dosing, and 20-ml urine samples were collected at baseline (0 h) for reference and during 2 specified time intervals (0 to 12 h and 12 to 24 h postdose).
Plasma and urine GSK1322322 concentrations were determined by Worldwide Bioanalysis (GlaxoSmithKline, King of Prussia, PA), using high-performance liquid chromatography with tandem mass spectrometry with a validated concentration range of 5 to 5,000 ng/ml GSK1322322 in human plasma. The assay for GSK1322322 concentration in human urine was validated over a range of 0.5 to 500 μg/ml. Pharmacokinetic analyses of plasma GSK1322322 concentration-time data were conducted by using noncompartmental Model 200 (for extravascular administration) of WinNonlin, version 5.2 (Pharsight Corporation, St. Louis, MO). Plasma PK parameters assessed included the area under the plasma concentration-time curve (AUC), maximum observed plasma concentration (Cmax), time to maximum plasma concentration (Tmax), and terminal elimination half-life (t1/2). For urine PK analysis, the total amount of GSK1322322 excreted (Ae) and renal clearance (CLR) were assessed. From the GSK1322322 urine data, the Ae within 24 h postdose was determined following a single dose, which was calculated as the product of the concentration in urine and the urine weight (assuming a urine density of 1 g/ml). The CLR was calculated as follows: CLR = Ae0–24/AUC0–24 (where Ae0–24 [Ae from 0 to 24 h] = Ae0–12 + Ae12–24).
Safety assessments.
Safety was assessed by the evaluation of reported and observed adverse events (AEs), vital sign measurements, electrocardiograms (ECGs), and clinical laboratory tests (i.e., chemistry, hematology, and urinalysis). Twelve-lead ECGs were obtained at screening, the day before dosing, and on the day of dosing (i.e., predose and 1, 2, 3, 6, 12, and 24 h [day 2] postdose). The ECGs were centrally read by Quintiles Cardiac Safety Services (Mumbai, India). Holter monitoring was performed for 24 h at screening and on the day of dosing (predose until 12 h postdose). To investigate a finding observed in preclinical studies, changes in circulating marginal zone B cells were analyzed by flow cytometry throughout the study. Lymphocytes were identified by a combination of light scatter properties and intensity of staining for CD45 (high CD45, low side scatter). In this population, B cells were identified by antigen density for CD20 (strong CD20 staining). Marginal zone B cell subsets were identified by gating for CD23−, CD27+, immunoglobulin D-positive (IgD+), and IgM− cells.
Statistical analyses.
Baseline and demographic characteristics, safety data, and PK parameters were summarized by using descriptive statistics. All PK parameters were loge transformed, with the exception of Tmax. The dose proportionality of GSK1322322 PK parameters (AUC and Cmax) was assessed by using the power model y = α × doseβ (where y denotes the PK parameter being analyzed, α depends on volunteer and random error, and the exponent β was estimated by regressing the loge-transformed PK parameter on the loge-transformed dose). Dose proportionality required β to be at unity for dose-dependent parameters, while the corresponding 90% confidence interval (CI) was used to quantify the degree of nonproportionality. An advanced compartment and transit (ACAT) model using GastroPlus software (Simulations Plus, Inc., Lancaster, CA) was explored over the clinically relevant doses tested. This model incorporates physicochemical (i.e., solubility and permeability), physiological (i.e., regional pH and transit time along the gastrointestinal tract), and PK (i.e., clearance and volume of distribution) parameters and other factors (such as dose and P-glycoprotein [Pgp] substrate data) to predict exposure.
The impact of food on the rate and extent of absorption of GSK1322322 was estimated with analysis of variance using SAS PROC MIXED (SAS Institute, Cary, NC). The ratio of geometric least-squares means and their associated 90% CIs were estimated for the fed and fasted conditions at the GSK1322322 800-mg dose. The Tmax of GSK1322322 was analyzed nonparametrically by using the Wilcoxon method. The point estimates and 90% CIs for the median differences (i.e., fed versus fasted conditions at the GSK1322322 800-mg dose) were computed.
RESULTS
Of the 39 volunteers enrolled in the study, 33 volunteers were included in part A, and 6 volunteers were included in part B. All volunteers in part A completed the study as planned. Of the 33 volunteers included in part A, 9 volunteers were randomized to receive placebo; 2 volunteers per cohort were included in cohorts A, B, and C; and 6 volunteers per cohort were included in cohorts D, E, and G. In part B of the study, 1 volunteer was randomized to receive placebo, and 5 volunteers were randomized to receive GSK1322322. However, 2 volunteers in the GSK1322322 treatment group were prematurely withdrawn in part B: 1 volunteer had an elevated alanine aminotransferase (ALT) level on day 11 (protocol-defined stopping criterion), and another volunteer was no longer able to complete all treatments because of a study delay. Overall, the volunteers enrolled in this study had a mean age of 31 years, and the majority of volunteers were white (92%) and male (97%).
Pharmacokinetics.
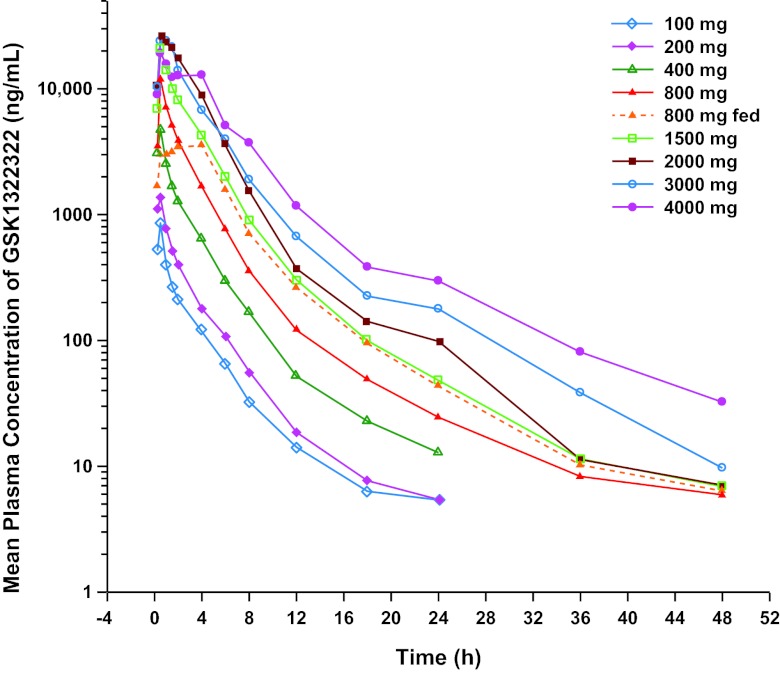
GSK1322322 administered at 100 to 4,000 mg to volunteers in the fasted state was readily absorbed; median Tmax in healthy volunteers was achieved at between 0.5 and 1.0 h across doses (Table 1). GSK1322322 was readily eliminated, with mean t1/2 values of 5.6 to 9.3 h. The mean Cmax and AUC increased with increasing doses (Fig. 1). Low to moderate between-volunteer variability was associated with these PK parameters. Results of the dose proportionality assessment indicated that after a single oral dose of GSK1322322, Cmax and AUC of GSK1322322 were greater than dose proportional between 100 and 1,500 mg and less than dose proportional between 1,500 and 4,000 mg (Table 2). However, because of the small number of volunteers, especially for doses from 100 to 400 mg (n = 2 per cohort) and from 2,000 to 4,000 mg (n = 3 per cohort), these data need to be interpreted with caution. At the projected clinically relevant dose range (800 to 1,500 mg, where n = 6 per cohort), when the dose approximately doubled from 800 to 1,500 mg, Cmax and AUC approximately doubled. The predicted bioavailabilities of the oral 100-, 400-, 800-, and 1,500-mg doses of GSK1322322 based on the ACAT model were 64%, 77%, 80%, and 82%, respectively, suggesting an increase in oral bioavailability with increasing dose.
Table 1.
Plasma pharmacokinetic parameters of GSK1322322
| Parametera | Value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Part A |
Part B |
||||||||
| Cohort A, 100 mg (n = 2) | Cohort B, 200 mg (n = 2) | Cohort C, 400 mg (n = 2) | Cohort D, 800 mg (n = 6) | Cohort E, 1,500 mg (n = 6) | Cohort G,b 800 mg (n = 6) | Cohort F1, 2,000 mg (n = 3) | Cohort F2, 3,000 mg (n = 3) | Cohort F3, 4,000 mg (n = 3) | |
| Mean AUC0–24 (μg · h/ml) (CVb [%]) | 1.6 (25) | 2.7 (12) | 8.7 (7) | 22.2 (17) | 47.4 (17) | 22.4 (11) | 75.4 (65) | 81.1 (15) | 88.7 (34) |
| Mean AUC0–∞ (μg · h/ml) (CVb [%]) | 1.6 (26) | 2.8 (11) | 8.9 (6) | 22.5 (17) | 47.9 (17) | 22.8 (11) | 76.2 (64) | 82.5 (15) | 92.0 (32) |
| Mean AUC0–t (μg · h/ml) (CVb [%]) | 1.5 (26) | 2.7 (12) | 8.7 (7) | 22.4 (17) | 47.8 (17) | 22.8 (11) | 76.1 (64) | 82.4 (15) | 91.6 (32) |
| Mean Cmax (μg/ml) (CVb [%]) | 0.9 (3) | 1.4 (39) | 4.7 (26) | 11.6 (25) | 20.1 (36) | 4.1 (14) | 24.8 (46) | 29.6 (14) | 22.2 (24) |
| Median Tmax (h) (range) | 0.5 (0.5–0.5) | 0.4 (0.25–0.5) | 0.4 (0.25–0.5) | 0.5 (0.5–0.5) | 0.5 (0.5–0.5) | 3.0 (0.5–4.0) | 0.5 (0.5–1.5) | 1.0 (0.5–1.5) | 0.5 (0.5–1.0) |
| Mean t1/2 (h) (CVb [%]) | 6.1 (11) | 6.9 (25) | 6.1 (18) | 9.3 (36) | 6.3 (45) | 6.8 (18) | 5.6 (25) | 6.2 (21) | 7.3 (32) |
CVb, between-volunteer coefficient of variation.
Cohort was fed a high-fat meal.
Fig 1.
Mean concentration-time profile of GSK1322322.
Table 2.
Dose proportionality assessment of GSK1322322 pharmacokinetic parameters
| Parameter | Adjusted mean slope value (90% CI) for GSK1322322 dose |
||
|---|---|---|---|
| 100–1,500 mg | 1,500–4,000 mg | All doses | |
| AUC0–24 (μg · h/ml) | 1.31 (1.23, 1.40) | 0.64 (0.27, 1.01) | 1.22 (1.09, 1.35) |
| AUC0–∞ (μg · h/ml) | 1.31 (1.23, 1.39) | 0.66 (0.30, 1.02) | 1.22 (1.10, 1.35) |
| AUC0–t (μg · h/ml) | 1.32 (1.24, 1.40) | 0.66 (0.30, 1.02) | 1.23 (1.10, 1.36) |
| Cmax (μg/ml) | 1.23 (1.09, 1.37) | 0.16 (−1.29, 1.62) | 1.04 (0.87, 1.22) |
When GSK1322322 was administered with a high-fat meal at a dose of 800 mg, Cmax was reduced by 65% (4.1 versus 11.6 μg/ml), and Tmax was delayed by 2.5 h (3.0 versus 0.5 h); however, AUC was unchanged (i.e., AUC0–∞ of 22.8 versus 22.5 μg · h/ml) compared with the fasted state. When comparing AUC values (i.e., AUC0–24, AUC0–∞, and AUC0–t) of GSK1322322 at 800 mg in the fed versus fasted state, the point estimates were close to 1, and the 90% CI included 1, indicating that a high-fat meal had no effect on the systemic exposure of GSK1322322 (Table 3). A similar t1/2 was observed between the fasted state and the fed state. Low and moderate within-volunteer variabilities were associated with these PK parameters.
Table 3.
Food effect assessed by comparing GSK1322322 pharmacokinetic parameters for cohort Ga versus cohort Db
| Parameter | Value |
||
|---|---|---|---|
| Point estimate | 90% CI | CVw (%)c | |
| AUC0–24 (μg · h/ml) | 1.01 | 0.88, 1.17 | 13.23 |
| AUC0–∞ (μg · h/ml) | 1.01 | 0.87, 1.17 | 13.25 |
| AUC0–t (μg · h/ml) | 1.01 | 0.88, 1.17 | 13.23 |
| Cmax (μg/ml) | 0.35 | 0.29, 0.43 | 18.73 |
| Tmax (h) | 2.5d | 1.0, 3.5 | |
| t1/2 (h) | 0.73 | 0.55, 0.98 | 26.19 |
An 800-mg dose under the fed condition.
An 800-mg dose under the fasted condition.
CVw, within-volunteer coefficient of variation.
Estimated median difference for Tmax only.
Urine PK was assessed at 100-, 400-, 1,500-, and 4,000-mg dose levels only. The amount of GSK1322322 excreted in the urine within 24 h postdose (Ae0–24) increased as the dose increased (Table 4). On the basis of the mean Ae0–24, the fraction of intact GSK1322322 recovered in the urine 24 h postdose ranged from 14% to 18% of the total administered dose. The mean renal clearance of GSK1322322 ranged from 5.4 to 11.5 liters/h for doses of 100, 400, 1,500, and 4,000 mg. Between-volunteer variability in urine PK parameters was low to moderate after single-dose administration of GSK1322322.
Table 4.
GSK1322322 urine pharmacokinetic parameters
| Parameter | Mean value (% CVb)a for GSK1322322 dose |
|||
|---|---|---|---|---|
| 100 mg (n = 2) | 400 mg (n = 2) | 1,500 mg (n = 6) | 4,000 mg (n = 3) | |
| Ae0–12 (μg) | 17,191 (10) | 66,241 (13) | 242,639 (68) | 506,163 (32) |
| Ae12–24 (μg) | 692 (22) | 3,128 (18) | 12,750 (46) | 40,528 (21) |
| Ae0–24 (μg) | 17,900 (9) | 69,371 (13) | 257,779 (63) | 549,774 (28) |
| CLR (liters/h) | 11.5 (16) | 7.9 (20) | 5.4 (68) | 6.2 (8) |
CVb, between-volunteer coefficient of variation.
Safety.
The most frequently reported AEs in the study (both parts A and B) included headache (n = 14), musculoskeletal pain (n = 3), dizziness (n = 2), diarrhea (n = 2), and oropharyngeal pain (n = 2). All other AEs were reported for only 1 volunteer each. All AEs were mild or moderate in intensity. The most frequently reported AEs in part B of the study were headache (n = 2) and musculoskeletal pain (n = 2). No serious AEs were reported in both parts of the study; however, 1 volunteer in part B (GSK1322322 2,000-mg group) withdrew from the study because of an AE that met protocol-defined volunteer stopping criteria. This volunteer was withdrawn from the study 9 days after dose administration because of elevated ALT levels (i.e., ≥3 times the upper limit of normal; value, 102 IU/liter) that resolved in 49 days and was considered by the investigator to be mild and related to the study drug.
No significant trends or changes from baseline in vital signs, chemistry, and hematology data were observed. In part A, no effect on cardiac repolarization as measured by QTc interval duration (>450 ms) for doses of 100 to 1,500 mg was observed. Although most volunteers in part B did not have QTcB values that were increased by >30 ms from baseline, 1 volunteer (GSK1322322 2,000-mg group) had a maximum change from the baseline QTcB value of 31 ms. However, this volunteer had high QTcB values at screening and on the day of dosing. There was no change in marginal zone B cells over time in parts A and B of the study.
DISCUSSION
In this study, we evaluated the safety, tolerability, and PK of GSK1322322, an antibacterial with a novel mechanism of action, at doses of 100 to 4,000 mg. GSK1322322 was generally well tolerated, with no serious AEs leading to withdrawal during the study. One volunteer in part B experienced a reversible elevation in ALT levels, which was considered by the investigator to be mild and study drug related, and was withdrawn from the study. Because polymorphisms in genes that encode drug-metabolizing enzymes have been associated with elevated levels of liver enzymes after treatment with antibacterial agents (13, 14), an exploratory pharmacogenetic experiment was conducted to determine if this volunteer carried any functional variants in genes involved in the metabolism and disposition of GSK1322322 (data not shown). While this volunteer did not carry any known variants implicated in GSK1322322 exposure, additional pharmacogenetic investigation may be warranted if elevations in ALT levels are observed in future patients treated with GSK1322322.
In this study, GSK1322322 PK characteristics were favorable, with sufficient systemic exposure (AUC) projected to have clinical efficacy (15) and minimal between-volunteer variability. The initial GSK1322322 dose selection (100 to 1,500 mg) for part A of this study was based on animal models simulating the human serum concentrations necessary for potent antibacterial activity (data not shown). Results from a study evaluating the in vivo efficacy of GSK1322322 against MRSA in a subcutaneous abscess model using a computer-controlled infusion system to re-create the phase I human exposure profiles in rats demonstrated that GSK1322322 at both 1,000- and 1,500-mg doses was highly efficacious against all 3 S. aureus isolates tested. As a result of the favorable safety data from part A of the study and additional preclinical safety assessments, higher doses of GSK1322322 (2,000 to 4,000 mg) were selected for evaluation in part B of this study.
After a single, oral dose of GSK1322322 in the powder-in-bottle formulation at 100 to 4,000 mg, the drug was readily absorbed, with median Tmax ranging from 0.5 to 1.0 h, and was readily eliminated, with mean t1/2 ranging from 5.6 to 9.3 h. Values for Cmax and AUC were greater than dose proportional for doses from 100 to 1,500 mg and less than dose proportional for doses from 2,000 to 4,000 mg. In the clinically relevant dose range of 800 to 1,500 mg (n = 6 for GSK1322322 treatment per cohort), when the dose approximately doubled, Cmax and AUC approximately doubled. GSK1322322 is a substrate of Pgp in vitro and has moderate to high passive permeability (data not shown). A potential for saturation of this efflux transporter with increasing doses may have contributed to a somewhat greater-than-proportional increase in GSK1322322 exposure between 100- and 1,500-mg doses.
At doses of 2,000 to 4,000 mg, the absorption appeared to have reached a plateau, and GSK1322322 PK appeared to be less than dose proportional between 1,500 and 4,000 mg. The decrease in bioavailability (less-than-dose-proportional increase in Cmax and AUC when dose increased) at these higher doses may be due to the limitation in solubility of the powder-in-bottle formulation at such large doses. Coadministration of GSK1322322 with food delayed the Tmax by 2.5 h and reduced the Cmax by approximately 65% without affecting the extent of absorption. This food effect needs to be interpreted cautiously, as it was a cross-volunteer comparison (different cohorts of volunteers received 800 mg GSK1322322 in fasted versus fed states) and not statistically powered to detect the effect and thus will require further evaluation as the GSK1322322 formulation is finalized.
In this phase I study, GSK1322322 was safe and well tolerated in this healthy volunteer study population. Pharmacokinetic results demonstrated that GSK1322322 has relatively favorable oral absorption and moderate variabilities around most PK parameters and is appropriate to be given twice daily or once daily based on its t1/2. Results from this single-dose, first-time-in-humans study demonstrate the potential of GSK1322322 to become the first-in-class PDF inhibitor for clinical use and support its further evaluation in clinical studies.
ACKNOWLEDGMENTS
Funding for this study was provided by GlaxoSmithKline. All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors.
We acknowledge the following individuals for their contributions: Lisa Stanton, Darlinghurst, Australia, for flow cytometry and Cheryl Friend (operations manager), Franziska Loehrer (general manager), Karen Quan (contracted trial physician), and Nicholas Buckley (principal investigator at study site), GlaxoSmithKline Medicines Research Unit, Prince of Wales Hospital, Randwick, NSW, Australia. Editorial support in the form of developing the manuscript outline and first draft, suggesting editorial revisions to drafts, assembling tables, collating author comments, copyediting, fact checking, and referencing was provided by Pratibha Hebbar, Christine Levesque, and Chris Lawrence at MedThink SciCom and was funded by GlaxoSmithKline.
All authors are employees of GlaxoSmithKline.
Footnotes
Published ahead of print 12 February 2013
REFERENCES
- 1. Schmieder R, Edwards R. 2012. Insights into antibiotic resistance through metagenomic approaches. Future Microbiol. 7:73–89 [DOI] [PubMed] [Google Scholar]
- 2. Rehm SJ, Tice A. 2010. Staphylococcus aureus: methicillin-susceptible S. aureus to methicillin-resistant S. aureus and vancomycin-resistant S. aureus. Clin. Infect. Dis. 51(Suppl 2):S176–S182 doi:10.1086/653518 [DOI] [PubMed] [Google Scholar]
- 3. Song JH, Chung DR. 2010. Respiratory infections due to drug-resistant bacteria. Infect. Dis. Clin. North Am. 24:639–653 [DOI] [PubMed] [Google Scholar]
- 4. Donadio S, Maffioli S, Monciardini P, Sosio M, Jabes D. 2010. Sources of novel antibiotics—aside the common roads. Appl. Microbiol. Biotechnol. 88:1261–1267 [DOI] [PubMed] [Google Scholar]
- 5. Lewandowski T, Peters T, Simon N, Kulkarni S. 2010. Potent activity of GSK1322322 a novel peptide deformylase inhibitor in a Haemophilus influenzae and Streptococcus pneumoniae respiratory tract infection model. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA, 12 to 15 September 2010 http://www.icaac.org/ [Google Scholar]
- 6. Apfel CM, Locher H, Evers S, Takács B, Hubschwerlen C, Pirson W, Page MGP, Keck W. 2001. Peptide deformylase as an antibacterial drug target: target validation and resistance development. Antimicrob. Agents Chemother. 45:1058–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jain R, Chen D, White RJ, Patel DV, Yuan Z. 2005. Bacterial peptide deformylase inhibitors: a new class of antibacterial agents. Curr. Med. Chem. 12:1607–1621 [DOI] [PubMed] [Google Scholar]
- 8. Aubart K, Benowitz A, Campobasso N, Dreabit J, Fang Y, Karpinski J, Kelly S, Liao X, Lee J, Mercer D, Lewandowski T, VanAller G, Zonis R, Christensen S, Zalacain M. 2010. Hydrazinopyrimidines as a new class of peptide deformylase inhibitors. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA, 12 to 15 September 2010 http://www.icaac.org/ [Google Scholar]
- 9. Lewandowski T, Demarsh P, Peters T, Kulkarni S. 2010. Potent activity of GSK1322322 a novel peptide deformylase inhibitor after oral dosing in a murine multi-drug resistant Staphylococcus aureus infection. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA, 12 to 15 September 2010 http://www.icaac.org/ [Google Scholar]
- 10. Naderer OJ, Jones LS, Zhu J, Kurtinecz M, Dumont E. 2010. A novel antibacterial peptide deformylase inhibitor (GSK1322322): first time in human safety and pharmacokinetics. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA, 12 to 15 September 2010 http://www.icaac.org/ [Google Scholar]
- 11. Butler D, Chen D, O'Dwyer K, Zalacain M. 2010. Two methodologies confirm the unique potent sub-MIC effect of peptide deformylase inhibitors on the growth of Staphylococcus aureus. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA, 12 to 15 September 2010 http://www.icaac.org/ [Google Scholar]
- 12. Ross JE, Scangarella-Oman NE, Miller LA, Sader HS, Jones RN. 2011. Determination of disk diffusion and MIC quality control ranges for GSK1322322, a novel peptide deformylase inhibitor. J. Clin. Microbiol. 49:3928–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanofi-Aventis US, LLC 2010. Rifater package insert. Sanofi-Aventis US, LLC, Bridgewater, NJ [Google Scholar]
- 14. Vuilleumier N, Rossier MF, Chiappe A, Degoumois F, Dayer P, Mermillod B, Nicod L, Desmeules J, Hochstrasser D. 2006. CYP2E1 genotype and isoniazid-induced hepatotoxicity in patients treated for latent tuberculosis. Eur. J. Clin. Pharmacol. 62:423–429 [DOI] [PubMed] [Google Scholar]
- 15. Singley C, Hoover J, DeMarsh P, Elefante P, Zalacain M. 2010. Efficacy of PDF inhibitor GSK1322322 against abscess infections caused by MRSA using a computer-controlled infusion system to recreate human PK profiles in rats. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA, 12 to 15 September 2010 http://www.icaac.org/ [Google Scholar]