Abstract
The efficacy of intravenous peramivir against influenza A (H1N1) 2009 virus infection was evaluated in mice in which the immune system was suppressed by cyclophosphamide (CP) treatment. The mortality rate of the vehicle control group was 100%, and the mice lost 20% of their body weight on average by day 13 postinfection (p.i.). Repeated administration of peramivir (40 mg/kg of body weight once a day, given intravenously for 20 days), starting at 1 h p.i., significantly reduced mortality, body weight loss, viral titers, and cytokine production in infected mice compared with results for administration of vehicle (P < 0.01). In addition, repeated administration of peramivir, starting at 24 h, 48 h, or 72 h p.i., also resulted in increases in survival rates and reduction of viral titers in the lungs (P < 0.01). The mean days to death (MDD) of the vehicle group was 14.5 days, while in the groups treated with peramivir starting at 24 h, 48 h, and 72 h p.i., the MDDs were >23.0, 20.9, and 21.8 days, respectively. In comparison, repeated administration of oseltamivir phosphate (5 mg/kg twice a day, given orally for 20 days), starting at 24 h, 48 h, and 72 h p.i., also significantly prevented body weight loss, whereas no significant differences in mortality rates and viral titers in the lungs were observed compared with results for the vehicle group. These data indicated that repeated administration of peramivir was effective in promoting the survival and reducing virus replication in immunosuppressed mice infected with influenza A (H1N1) 2009 virus.
INTRODUCTION
The pandemic 2009 influenza (A/H1N1pdm) virus emerged in April 2009, and in June the World Health Organization (WHO) raised the warning level to phase 6, indicating a global epidemic (pandemic). Although the pathogenicity of the pandemic virus was clearly higher than that of seasonal influenza virus in animal models and humans (1–3) and many cases of A/H1N1pdm virus infection in Japan were reported during the 2009-2010 epidemic (4), the mortality rate of A/H1N1pdm in Japan was similar to that of seasonal influenza. One of the reasons was that the Japanese, especially children, did not develop serious illnesses because of treatment with neuraminidase (NA) inhibitors (5). In vitro and in vivo studies demonstrated that A/H1N1pdm was sensitive to NA inhibitors, e.g., oseltamivir, zanamivir, laninamivir, and peramivir (6–8). Although vaccination plays a critical role in influenza prophylaxis, there is usually insufficient time to prepare and distribute new vaccines before the peak of a pandemic. Therefore, antivirals are important for mitigating the impact of influenza pandemics.
Most people infected with influenza virus develop transient fever and respiratory symptoms but recover within 7 days without developing any complications. However, influenza virus infection occasionally causes serious and fatal outcomes in patients whose immune system is compromised by genetic factors, treatment with anticancer drugs, or use of immunosuppressive drugs after organ transplantation (9, 10). Therefore, it is recommended that high-risk patients be immediately treated with anti-influenza drugs to prevent serious conditions (11, 12), but it is often difficult to administer oral or inhaled drugs, such as oseltamivir or zanamivir, to patients with severe symptoms and those who require respiratory management. Furthermore, it is well recognized that immunosuppression results in prolonged periods of viral replication and provides an environment conducive to the emergence of drug-resistant mutation (13–17). Therefore, anti-influenza virus agents need to be administered intravenously for long periods to immunosuppressed patients.
Peramivir is an anti-influenza drug that selectively inhibits NA of human type A and type B influenza viruses. It was developed as an intravenous preparation and approved in Japan at the beginning of 2010 after clinical trials (18, 19). In randomized, controlled, and double-blinded studies in adults, a single dose of peramivir was demonstrated to significantly reduce the duration of influenza virus infection without safety concerns. On the basis of these results, the U.S. Food and Drug Administration issued an emergency use authorization for intravenous peramivir exclusively for patients hospitalized due to infection associated with A/H1N1pdm virus on 23 October 2009, even though it was still under development in the United States (20). The most important characteristic of peramivir is its rapid bioavailability on intravenous administration. Therefore, peramivir can be used as a first-line therapy, especially for patients at risk for complications or who cannot take oral or inhaled drugs.
Repeated intravenous injections of peramivir for 5 days had beneficial effects in influenza virus-infected patients at high risk for complications, and no major safety issues were identified (21). However, high-risk patients, e.g., immunocompromised patients, might require administration of peramivir for more than 5 days, since virus has been detected for extended periods in these patients (17). Therefore, to examine the effects of peramivir administration for more than 5 days, we used an immunosuppressed-mouse model by administration of cyclophosphamide (CP), a drug used widely in antitumor therapy. Due to reduction of NK cell activity and inhibition of T and B cell proliferative responses (22–24), immunosuppression induced by CP converted harmless infection with influenza virus in immunocompetent mice into a fatal pulmonary illness in immunocompromised mice infected with a low titer of virus. Using CP treatment for immunosuppression, as described for a previous experiment that examined the efficacy of oral administration of peramivir in mice infected with influenza A/NWS/33(H1N1) virus (25), we examined the efficacy of intravenous peramivir against A/H1N1pdm virus infection, since intravenous injection of peramivir rather than oral or inhaled administration might be required in patients with severe symptoms.
In the present study, we found that the virus replicated for longer periods in immunosuppressed mice than in normal mice due to suppression of immune responses. We demonstrated that intravenous administration of peramivir was more effective than oral administration of oseltamivir in immunosuppressed mice infected with A/H1N1pdm virus with respect to mortality, body weight change, and virus titers in lungs. These results suggest that peramivir has the potential to be used to treat patients with A/H1N1pdm virus infection, especially immunosuppressed patients.
MATERIALS AND METHODS
Compounds.
Peramivir was synthesized by BioCryst Pharmaceuticals (Birmingham, AL). Oseltamivir phosphate was purchased from Sequoia Research Products (Oxford, United Kingdom). Oseltamivir carboxylic acid was purchased from Toronto Research Chemicals (Ontario, Canada).
Virus and cells.
A pandemic 2009 influenza virus, A/Osaka/129/2009 (A/H1N1pdm), not mouse adapted, was kindly provided by Osaka Prefectural Institute of Public Health. Madin-Darby canine kidney (MDCK) cells were obtained from the American Type Culture Collection (Manassas, VA) and were grown in minimum essential medium (MEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen) and 100 μg/ml kanamycin sulfate (Invitrogen) in a humidified atmosphere of 5% CO2 at 37°C.
Animals.
Specific-pathogen-free 6-week-old female BALB/c mice (Charles River Laboratories Japan, Inc.) were used in the challenge experiments. Their body weights and survival rates were monitored daily, and the mice were euthanized when they lost more than 30% of their body weight compared to their weight at preinfection. All mouse studies were conducted under applicable laws and guidelines and with the approval of the Shionogi Animal Care and Use Committee.
NA inhibition assay.
Whole viruses inactivated by Nonidet P-40 were used as the source of NA activity (26). The substrate was 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (MUNANA) (Sigma-Aldrich, St. Louis, MO). The virus, a compound, and MUNANA (final concentration, 10 μM) were mixed in reaction buffer and then incubated at 37°C for 30 min. Virus solution was diluted to obtain a 5,000 to 20,000 fluorescence intensity of NA activity in the reaction mixture. The fluorometric intensity of 4-methylumbelliferon released from MUNANA was measured, the percent inhibition at each drug concentration was determined, and the 50% inhibitory concentration (IC50) was calculated. The results were reported as the averages for three experiments.
Antiviral study with immunosuppressed mice.
BALB/c mice were treated intraperitoneally (i.p.) with 100 mg/kg of body weight of cyclophosphamide (Endoxan; Shionogi and Co., Ltd., Osaka, Japan) at 24 h pre-virus exposure and days +3, +7, +11, +15, and +19 after infection. Infection was achieved by intranasal (i.n.) administration of 100 μl of A/Osaka/129/2009 (1,000 mean 50% tissue culture infective dose [TCID50]) in phosphate-buffered saline (PBS) except where indicated. This dose of virus was approximately 10 times higher than the 90% mouse lethal dose (MLD90) (1.1 × 102 TCID50) as described in Results. Mice were treated intravenously with peramivir at a dose of 40 mg/kg once daily for 1, 5, 10, or 20 days, beginning at 1, 24, 48, or 72 h after virus inoculation. Oseltamivir phosphate at a dose of 5 mg/kg was administered orally twice daily for 20 days with 0.5% methylcellulose solution (MC). Control animals inoculated with virus were treated with 0.5% MC for 20 days. Ten of these animals were observed daily for 20 to 23 days.
To monitor virus replication, inflammatory cytokine/chemokine production, and the emergence of resistant variants in lungs, three mice in each group were euthanized on days 2, 6, 10, 14, and 18 postinfection (p.i.). The collected samples were stored at −80°C until use. For virus quantitation, serial dilutions of lung homogenates were inoculated onto confluent MDCK cells in 96-well plates. After a 1-h incubation, the suspension was removed, and the cells were cultured in MEM, including 0.5% bovine serum albumin (BSA) (Sigma-Aldrich) and 3 μg/ml trypsin. The cells were incubated at 37°C in 5% CO2 for 3 days. The presence of cytopathic effects (CPE) was determined under a microscope, and viral titers were calculated as log10 TCID50/ml. When no CPE was observed using undiluted viral solution, the undetectable level was defined as being less than 1.4 log10 TCID50/ml. To monitor the emergence of resistant variants, dilutions of lung homogenates were inoculated onto confluent MDCK cells in 12-well plates, and MDCK cells were cultured as described above for 2 days. The supernatant was used in the NA inhibition assay.
Levels of inflammatory cytokines and chemokines (interleukin 6 [IL-6], IL-10, IL-12p70, tumor necrosis factor alpha [TNF-α], monocyte chemotactic protein 1 [MCP-1], and gamma interferon [IFN-γ]) in lung homogenates were assessed using a cytometric bead assay (Becton, Dickinson and Company, Franklin Lakes, NJ) according to the manufacturer's instructions. The results of the assays were analyzed using the FCAP Array software program (Becton, Dickinson and Company).
HI assay.
Sera from mice were treated with receptor-destroying enzyme (RDEII; Denka Seiken, Tokyo, Japan). Serially diluted sera were mixed with 4 hemagglutinin (HA) units of virus antigen for 1 h at room temperature. The mixture was then incubated with 0.5% chicken red blood cells for 30 min at room temperature. The hemagglutination inhibition (HI) titers were expressed as the reciprocal of the highest dilution of serum samples that completely inhibited hemagglutination.
Sequence analysis of NA genes.
Viral RNA was isolated from lung homogenates of infected mice by using the RNeasy Mini kit (Qiagen, Duesseldorf, Germany). The neuraminidase region of influenza virus was amplified by PCR using the OneStep reverse transcriptase PCR (RT-PCR) kit (Qiagen) and specific primers: 5′-TATTGGTCTCAGGGAGCAAAAGCAGGAGT-3′ and 5′-ATATGGTCTCGTATTAGTAGAAACAAGGAGTTTTTT-3′. The amplified DNA was sequenced in an Applied Biosystems 3730xl DNA analyzer by the TaKaRa sequencing service. The sequences of the NA region derived from isolated viruses were compared with those of the inoculated virus, and amino acid substitutions were analyzed.
Pathological examination.
After autopsy, lung tissue samples fixed with 10% formalin were processed for hematoxylin and eosin (H&E) staining.
Statistical analysis.
Differences in survival rates on day 20 or 23 after virus inoculation between the control and antiviral agent-treated groups were analyzed by Fisher's exact test. Virus titers, body weights, and cytokine levels on each day were compared with those of the control group and analyzed by using Dunnett's multiple comparison method. The efficacy of peramivir for body weight loss was compared with that of oseltamivir by Student's t test. Statistical analysis was performed using the statistical analysis software program SAS version 9.2 for Windows (SAS Institute, Cary, NC). P values below 0.05 were considered statistically significant.
RESULTS
In vitro NA inhibition activity of peramivir against A/H1N1pdm virus.
Initially, we examined the sensitivity of NA of A/H1N1pdm virus to peramivir and oseltamivir carboxylate in vitro. The IC50s of peramivir and oseltamivir carboxylate against NA activity of A/Osaka/129/2009 were 0.67 ± 0.04 nM and 0.80 ± 0.03 nM (means and standard deviations), respectively. These values were comparable to previous IC50s for peramivir against NA activities of other strains of A/H1N1pdm virus of 0.05 to 0.75 nM and to IC50s of oseltamivir carboxylate of 0.10 to 2.27 nM (6, 8, 27).
Virulence and virus replication of pandemic virus in mice treated with cyclophosphamide.
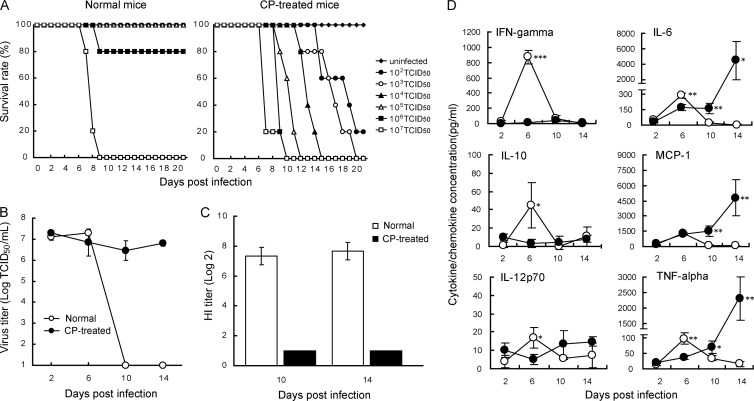
To evaluate the effects of treatment with CP on the virulence and replication of influenza virus in mice, we infected both normal and immunosuppressed BALB/c mice with 102 to 107 TCID50 of H1N1 pandemic virus A/Osaka/129/2009. This virus showed mild pathogenicity in mice without immunosuppression(MLD50 and MLD90 [mouse lethal dose: a dose required to kill 50% or 90% of the mice] = 1.1 × 106 TCID50 and 1.3 × 106 TCID50, respectively), whereas the same virus exhibited high virulence in CP-treated mice (MLD50 < 102 TCID50 and MLD90 = 1.1 × 102 TCID50) (Fig. 1A). These data indicated that immunosuppression made the hosts more susceptible to the virus than immunocompetent hosts.
Fig 1.
Effects of cyclophosphamide on survival, replication of influenza virus in lungs, and production of anti-HA antibodies in mice infected with pandemic influenza virus A/Osaka/129/2009 (A/H1N1pdm). BALB/c mice were treated intraperitoneally with 100 mg/kg of cyclophosphamide on days −1, +3, +7, +11, +15, and +19 after virus infection. (A) The numbers of live and dead mice were monitored for 21 days. (B) Viral titers in lung samples collected on indicated days after virus inoculation are indicated as means ± SD for three animals. (C) Sera were collected on indicated days after infection. Diluted sera were incubated with 4 HA units of virus antigen for HI tests. HI titers were expressed as reciprocals of the highest dilution that completely inhibited hemagglutination and are indicated as means ± SD for three animals. (D) Levels of cytokine and chemokine production in lungs collected on indicated days were measured by the bead array assay and are indicated as means ± SD for three animals. Levels of IFN-γ, IL-10, IL-12p70, IL-6, and TNF-α secretion were significantly increased on day 6 p.i. in normal mice (open circles) compared with those in CP-treated mice (filled circles). Levels of IL-6, MCP-1, and TNF-α secretion on days 10 and 14 p.i. in CP-treated mice were significantly higher than those in normal mice (*, P < 0.05: **, P < 0.01: ***, P < 0.001).
We infected BALB/c mice with 104 TCID50 of A/Osaka/129/2009 and determined the viral titers in their lungs on days 2, 6, 10, and 14 p.i. (Fig. 1B). After intranasal inoculation with virus, viral titers in normal mice reached a maximum level on day 6 p.i. and then decreased rapidly to an undetectable level. In contrast, in CP-treated mice, the viral titers remained from day 6 to day 14 p.i. We also examined the effects of CP on the production of anti-HA antibodies by the HA inhibition (HI) test. Increases in HI titers in sera of normal mice were observed on days 10 and 14 p.i., whereas no HI activity was detected in sera of CP-treated mice (Fig. 1C). These results indicate that the virus replicated for a longer period in CP-treated mice than in normal mice due to the lack of anti-HA antibodies caused by the CP treatment.
We measured the production of inflammatory cytokines and chemokines in the lungs after infection (Fig. 1D). Levels of IFN-γ, IL-10, IL-12p70, IL-6, and TNF-α secretion were significantly increased on day 6 p.i. in normal mice over those in CP-treated mice. Significant increases of IL-6, MCP-1, and TNF-α production were observed on days 10 and 14 p.i. in CP-treated mice compared to those in normal mice. These results suggested that the cytokines and chemokines detected on day 6 p.i. were produced by cells affected by CP but those detected on days 10 to 14 p.i. were produced by another cell population.
Effects of duration of treatment with peramivir in CP-treated mice.
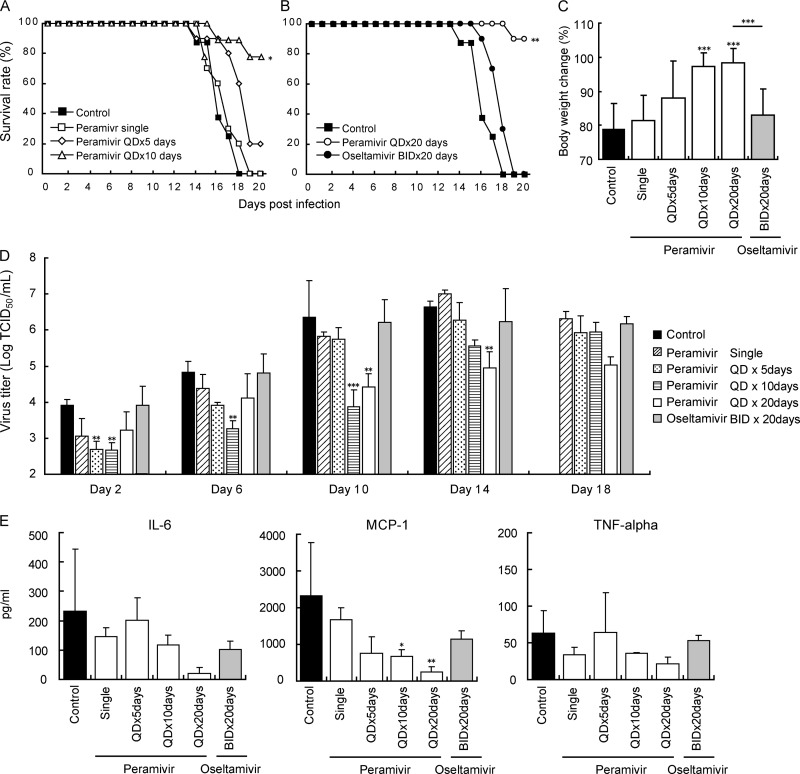
We evaluated the efficacy of peramivir and oseltamivir on the basis of weight changes, survival rates measured for 20 days p.i., and viral titers in lungs. BALB/c mice treated with CP and infected with A/Osaka/129/2009 (1 × 103 TCID50) were administered 40 mg/kg of peramivir intravenously once daily for 1, 5, 10, or 20 days, starting at 1 h post-virus inoculation (Fig. 2). The 40-mg/kg dose of peramivir used in this study was approximately equivalent to the area under the concentration-time curve (AUC) for the injection of 600 mg of peramivir in humans (unpublished results). Since 10 mg/kg/day of oseltamivir in mice was equivalent to the oral administration of 75 mg of oseltamivir in humans (28), we administered 5 mg of oseltamivir orally twice a day for 20 days as a comparison. All untreated mice inoculated with A/Osaka/129/2009 died by day 18 p.i., giving a mean days to death (MDD) of 16.4 ± 1.3 days (Fig. 2A). Survival rates on day 20 of mice treated with peramivir for 5, 10, and 20 days were 20%, 78% (P < 0.01), and 90% (P < 0.001), and their MDD were 18.2 ± 2.1, 19.4 ± 1.3, and 19.9 ± 0.3 days, respectively. On the other hand, mice treated with oseltamivir phosphate died by day 19, and their MDDs were 17.9 ± 1.0 days (Fig. 2B).
Fig 2.
Therapeutic efficacy of single or repeated administration of peramivir and repeated administration of oseltamivir against A/Osaka/129/2009 (A/H1N1pdm) in immunosuppressed mice. Eight to ten mice per group were treated with CP as described in the legend for Fig. 1, intranasally infected with 1,000 TCID50 (>10 MLD50), and then administered peramivir intravenously (40 mg/kg/day), oseltamivir orally (10 mg/kg/day), or 0.5% methylcellulose (control) orally on indicated days. The administration started at 1 h p.i. (A) Comparison of survival rates among peramivir treatments. The survival rate in groups that received peramivir for 10 days was significantly higher than those in other groups (*, P < 0.01). (B) Comparison of survival rates between peramivir and oseltamivir treatments. The survival rate in the group that received peramivir for 20 days was significantly higher than that in the group treated with oseltamivir or methylcellulose for 20 days (**, P < 0.001). (C) Body weight changes in infected mice on day 13 p.i. The values are means ± SD for 8 to 10 mice. The groups treated with peramivir for 10 days and 13 days showed significantly less body weight loss than did the control group (***, P < 0.001). (D) Viral titers in lungs collected on indicated days after virus inoculation are given as means ± SD for three animals. Significant differences were observed in groups treated with peramivir for 5, 10, and 20 days in comparison with the control group on day 2, days 2 to 10, and days 10 to 14 p.i., respectively. The group administered with oral oseltamivir showed a reduction in viral titers on day 14 in comparison with the control, but the difference was not statistically significant. (E) Levels of cytokines/chemokines in lungs from immunosuppressed mice 14 days p.i. were measured by the bead array assay and given as means ± SD for three animals. The levels of MCP-1 production in the groups treated with peramivir for 10 and 14 days (QD×20days) were significantly lower than that in the control group (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
We compared body weight loss on day 13 among the various groups, since the earliest mortality was observed in the control group on day 14. The mean weight with standard deviation of the control group on day 13 was 79% ± 8% of that before infection (Fig. 2C). The groups treated with peramivir once daily (QD) for 10 days (QD×10 days) and 13 days (mice in group QD×20 days) showed significantly less reduction of body weight than the control group (10 days, 97% ± 4%; 13 days, 98% ± 4%) (P < 0.0001), while the group treated with oseltamivir phosphate (twice daily [BID] for 20 days [BID×20 days]; 83% ± 7%) showed decreases in body weight comparable to those for the control group.
On day 10 p.i., although viral titers in lungs of the groups treated with peramivir for 10 days were significantly lower than those in the control group (P < 0.01) (Fig. 2D), viral titers in mice injected with peramivir for 5 days increased after discontinuation of treatment, and the viral titers were comparable to the viral titers in the control group. On day 14 p.i., a significant reduction of viral titers was observed in the group treated with peramivir for 14 days (three mice in group QD×20 days), but no significant difference was observed in the group treated with peramivir for 10 days (three mice in group QD×10 days). On day 18 p.i., the reduction of viral titers was maintained in the group treated with peramivir for 18 days (three mice in group QD×20 days), although statistical analysis was not conducted due to a lack of mice in the control group. We did not observe any obvious signs of drug-related toxicity in groups treated repeatedly with 40 mg/kg of peramivir. No significant reduction in virus titers was observed in the group treated with oseltamivir compared to findings for the control group.
The production of inflammatory cytokines and chemokines in lung homogenates was examined on day 14 p.i. (Fig. 2E). Levels of MCP-1 production in the groups treated with peramivir for 10 days (QD×10 days) and 14 days (three mice in group QD×20 days) were significantly lower than those of the control group. The level of IL-6 production in the group treated with peramivir for 14 days (group QD×20 days) was lower than that in the control group, although the difference was not statistically significant. On the other hand, there was no significant effect of drugs on TNF-α production.
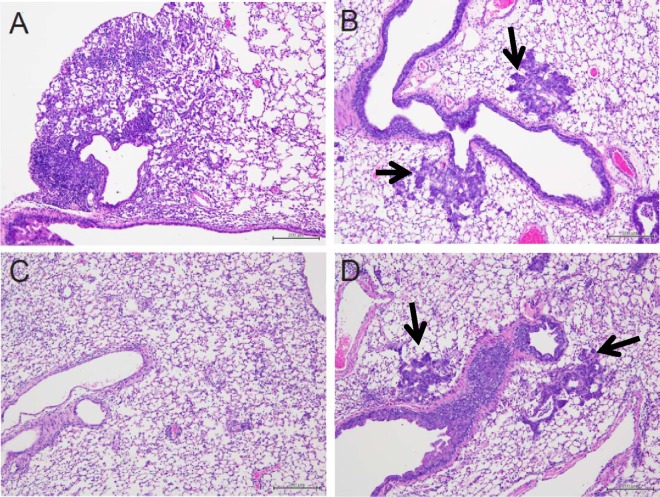
We histologically examined inflammation in the lungs of mice treated with peramivir. Lung tissues collected on day 15 p.i. from mice infected with the pandemic virus without CP and antiviral treatment revealed severe focal infiltration of lymphocytes and neutrophils in the alveoli and thickened alveolar walls (Fig. 3A), though no virus was detected (Fig. 1B). In immunosuppressed mice (Fig. 3B to D), lymphocytes were sparse in the alveoli but lymphoid and neutrophilic infiltration was not apparent in the alveolar walls. In mice treated with methylcellulose or oseltamivir (Fig. 3B and D), bronchial metaplasia of alveolar epithelial cells was observed. However, the mice treated with peramivir showed no bronchial metaplasia of alveolar epithelial cells in the observed lung sections (Fig. 3C). Since we found significant effects on viral titers, body weight, cytokine production, and lung inflammation in the groups treated with peramivir for more than 10 days consecutively, the daily regimen was chosen for subsequent assessments.
Fig 3.
Histological analysis of lung tissues. Three mice per group were intranasally infected with 1,000 TCID50 (>10 MLD50) of A/Osaka/129/2009 (A/H1N1pdm). The mice were treated from 1 h p.i. for 15 days and were autopsied on day 15 p.i. to collect lung tissues. Representative pictures with H&E staining of each group are shown. (A) Normal infected mice; (B) immunosuppressed and infected mice treated with 0.5% methylcellulose (control); (C) immunosuppressed and infected mice treated with peramivir (40 mg/kg/day); (D) immunosuppressed and infected mice treated with oral oseltamivir (10 mg/kg/day). Arrows indicate metaplasia.
Effects of 24-, 48-, and 72-h-p.i. delayed treatment with peramivir in CP-treated mice.
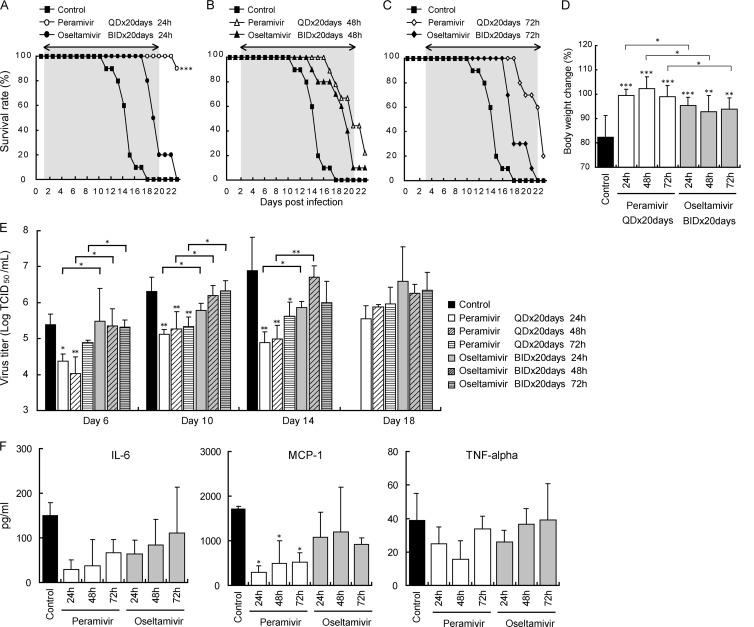
We previously showed that early treatment with a single injection with peramivir reduced virus titers in immunocompetent animals (32). Therefore, we examined the effects of late administration of peramivir in CP-treated mice to estimate the efficacy of peramivir after disease onset in immunocompromised patients. We started injection of peramivir at 24 h to 72 h p.i. and continued the treatment for up to 20 days with observation until day 23. All mice inoculated with A/Osaka/129/2009 (1 × 103 TCID50) without treatment died by day 18, giving an MDD of 14.6 ± 1.8 days. One of the 10 mice for which treatment with peramivir was initiated at 24 h p.i. died on day 23 (90% survival; P < 0.0001 versus results with vehicle) (Fig. 4A). When peramivir treatment was delayed until 48 and 72 h p.i., survival rates were 22% and 20%, yielding MDDs of 20.9 ± 2.4 and 21.8 ± 1.9 days, respectively (Fig. 4B and C). Therefore, early treatment with peramivir after infection resulted in a higher survival rate than late treatment in immunocompromised mice. In contrast, when oseltamivir treatment was delayed until 24, 48, or 72 h p.i., the groups treated with oseltamivir survived for a longer period than the control group (MDD, 24 h, 19.6 ± 1.5 days; 48 h, 19.2 ± 2.8 days; 72 h, 18.7 ± 1.9 days) (Fig. 4A to C). However, no significant difference in survival curves was observed among groups treated with oseltamivir.
Fig 4.
Therapeutic efficacy of repeated administration of peramivir and oseltamivir against A/Osaka/129/2009 (A/H1N1pdm) in immunosuppressed mice. Nine to ten mice per group were intranasally infected with 1,000 TCID50 (>10 MLD50) and then treated intravenously with peramivir (40 mg/kg/day), orally with oseltamivir (10 mg/kg/day), or orally with 0.5% methylcellulose (control) for 20 days from 24 h p.i. (A), 48 h p.i. (B), or 72 h p.i. (C). Survival was monitored daily for 23 days. Treatment periods were indicated with arrows and gray zones. The survival rate of the group that received peramivir for 20 days starting at 24 h was significantly higher than those of the groups treated with oseltamivir or methylcellulose (***, P < 0.0001). (D) Body weight changes in infected mice on day 10 p.i. The values are means ± SD of 9 to 10 mice. The groups treated with peramivir and oseltamivir from 24, 48, or 72 h p.i. showed significantly less body weight loss than did the control group (*, P < 0.01; **, P < 0.001; ***, P < 0.0001). (E) Viral titers for lungs collected on indicated days after virus inoculation are given as means ± SD for three animals. Significant differences were observed in groups treated with peramivir from 24, 48, and 72 h p.i. in comparison with the control group (*, P < 0.05; **, P < 0.01; ***, P < 0.001). The group administered oral oseltamivir showed no significant reduction in viral titers in comparison with the control. (F) Levels of cytokines/chemokines in lungs from immunosuppressed mice 14 days after infection were measured by the bead array assay and are given as means ± SD for three animals. The levels of MCP-1 production in the groups treated with peramivir were significantly lower than that in the control group (*, P < 0.05).
The mean relative weight on day 10, when all mice were alive, was 82% ± 9% in the vehicle-treated group (Fig. 4D). All three groups treated with peramivir showed significantly less body weight loss than the vehicle group (24 h, 99% ± 3%; 48 h, 102% ± 5%; 72 h, 99% ± 5%) (P < 0.0001). Significant decreases in body weight loss were also observed in the groups treated with oseltamivir compared with results for the control (24 h, 95% ± 3%; 48 h, 93% ± 7%; 72 h, 94% ± 5%,) (P < 0.001 or 0.0001). Comparing the efficacy of peramivir and oseltamivir for body weight loss for the same starting points of treatment, the groups given peramivir showed less decrease of body weight than those treated with oseltamivir (P < 0.01).
Viral titers in the groups treated with peramivir from 24, 48, and 72 h p.i. were significantly lower than those in the control group on days 10 and 14 p.i. (P < 0.01) (Fig. 4E). On the other hand, no significant reduction in viral titers was observed in the groups treated with oseltamivir compared with results for the control. On day 14 p.i., levels of MCP-1 production in all groups treated with peramivir until day 14 were significantly lower than those in the control group (Fig. 4F). On the other hand, there was no significant effect of both drugs on IL-6 and TNF-α production.
To monitor the emergence of resistant variants during or after repeated peramivir and oseltamivir phosphate treatment, we conducted an NA inhibition assay and determined NA gene sequences by using isolated virus obtained from lung homogenates on days 6, 10, 14, and 18 p.i. There were no changes in IC50s of isolates recovered from 0.5% MC-treated mice. Although viruses isolated in the groups treated with oseltamivir phosphate showed a similar IC50 to oseltamivir, the virus isolated on day 18 from one mouse treated with peramivir for 17 days starting at 1 h p.i. showed a reduction in susceptibility to peramivir (change in IC50 from 0.67 nM to 17.91 nM, a 26-fold increase in the IC50 compared to that of the wild-type virus). The isolated virus also showed a reduction in susceptibility to oseltamivir (from 0.80 nM to 105.13 nM IC50, a 131-fold increase in the IC50 compared to that of the wild-type virus) and a mutation resulting in an amino acid change at position 275 (H to Y) of the NA in the N1 numbering by direct sequencing (dominant virus population). The viruses isolated from samples of other mice (98.8%) treated with peramivir were susceptible to peramivir.
DISCUSSION
We used an immunosuppressed-mouse model by treatment with CP to evaluate the efficacy of repeated intravenous injections of peramivir against A/H1N1pdm virus infection. Treatment with CP has been reported to induce a reduction in NK cell activity, inhibition of T and B cell proliferative responses, and decrease in interferon production and also to enhance susceptibility to viral infection (22–24). The immunosuppressed-mouse model mimicked to some extent immunocompromised human patients, in whom prolonged viral replication induced progressive illness (17). In the present study, we found that IFN-γ, IL-10, IL-12p70, IL-6, and TNF-α responses in the lungs of immunosuppressed mice on day 6 p.i. were lower than those in mice without CP treatment. However, the levels of IL-6, MCP-1, and TNF-α production in the lungs of immunosuppressed mice on days 10 and 14 p.i. were higher than those in mice not given CP treatment (Fig. 1). These results suggested that cytokine and chemokine responses after viral infection in immunosuppressed mice might be limited in the early phase of infection because of impaired leukocyte function and the responses caused by nonlymphoid cells in the later phase might not be early enough to regulate viral propagation and cause severe symptoms. Furthermore, we observed metaplasia of alveolar epithelial cells replaced by bronchiolar-type epithelium in lungs of immunosuppressed mice on 15 days p.i. without treatment (Fig. 3). This change was thought to be a regenerative change after severe alveolar injury (29, 30). In the present study, metaplasia was observed in immunosuppressed mice without treatment and with oseltamivir treatment, in which viral titers were higher than those in mice treated with peramivir (Fig. 2). Therefore, we thought that histological changes might be one of the criteria for evaluating the efficacy of antiviral agents.
Our results demonstrated that repeated intravenous injection of peramivir, starting at 1 h p.i., was effective for the reduction in viral titers, prevention of death, inhibition of body weight loss, and amelioration of the mild histological changes in immunosuppressed mice infected with A/H1N1pdm virus (Fig. 2). A single injection or repeated injections for 5 days with peramivir reduced viral replication for only a short period, and viral titers in these groups after discontinuation of treatment reached levels similar to those in the untreated group due to the suppression of immune responses against influenza virus. However, treatment with peramivir for 20 days led to a significant reduction in viral titers during the treatment period and improved survival and body weight loss. These results suggested that suppression of viral replication not only in the early phase but also in the late phase during infection was crucial for ameliorating symptoms in the immunosuppressed-mouse model.
When the treatment was initiated at 24 h p.i., treatment with peramivir for 20 days was more effective for reduction of viral titers, prevention of death, and inhibition of body weight loss than treatment with peramivir beginning at 48 h and 72 h p.i. (Fig. 4), though early oral treatment with peramivir did not improve survival rates and MDDs of immunocompromised mice compared with results with delayed initiation of treatments (25). When treatment with peramivir was initiated at 48 h or 72 h p.i., the MDDs were longer than that of the vehicle control group but the differences were not statistically significant for protection from mortality, despite significant reductions in viral titers and prevention of body weight loss (Fig. 4). These results agreed with those of a previous study, in which early initiation of treatment with peramivir intramuscularly was strongly associated with a reduction of mortality in mice infected with a lethal dose of A/H1N1pdm virus (6). When repeated treatment with peramivir was initiated at 24 h p.i., viral titers on days 14 and 18 p.i. were substantially lower than those of mice treated with peramivir with starting at 48 h and 72 h p.i. This result suggested that continued viral replication in the late phase of infection contributed to the severity of symptoms, including lethality, and that suppression of viral replication in the late phase of infection was crucial to ameliorating symptoms in the immunosuppressed-mouse model.
Intravenous administration of medicines can reliably provide stable pharmacokinetics in compared to oral administration or inhalation. After oral administration of a 10-mg/kg dose of oseltamivir phosphate, the AUC and maximum concentration (Cmax) of oseltamivir carboxylate in plasma of mice were 3.9 μg · h/ml and 1.2 μg/ml, respectively (31). In contrast, intravenous administration of a 10-mg/kg dose of peramivir achieved a 16.0-μg · h/ml AUC and 44.3 μg/ml C0 (extrapolated plasma concentration at time 0), which were 4 and 40 times higher than those of 10 mg/kg of oseltamivir phosphate (data not shown). The high concentration of peramivir in plasma after injection was thought to be effective to reduce virus replication in the early phase of infection. Furthermore, the low AUC and Cmax of oseltamivir carboxylate in immunosuppressed mice were thought to result in low efficacy in reduction of A/H1N1pdm virus titers.
After repeated intravenous injection of peramivir at a dose of 40 mg/kg/day for 20 days, we did not observe any obvious signs of drug-related toxicity in mice. In addition, when peramivir was administered continuously by intravenous infusion to macaques at a dose of 720 mg/kg/day for 30 days, no toxic findings were evident in the clinical observations (32). In a clinical study of peramivir in high-risk patients, no clear increases in the incidence of any adverse events were identified as a result of repeated peramivir dosing for 5 days (21).
Prolonged replication of influenza virus in recipients of antiviral agents might be a risk factor in the emergence of drug-resistant variants (14, 16). In the present study, H275Y NA mutant virus with low susceptibility to peramivir was detected in a mouse treated with repeated administration of peramivir for 20 days from 1 h p.i., whereas the mutant virus was not detected in mice treated for 1, 5, or 10 days with peramivir or for 20 days with oseltamivir. In addition, no mutant virus was recovered from mice treated with repeated peramivir for 20 days from 24, 48, and 72 h p.i. These results might be dependent on the strength and period of selective pressure with antiviral agents, since selective pressure by host immune responses was minimal in the immunocompromised model and peramivir showed tighter binding to and a slower off-rate from the NA enzymatic catalytic site in vitro than did oseltamivir carboxylate (33). Indeed, repeated intravenous injection of peramivir for 20 days starting at 1 h p.i. was more effective for reducing viral titers and preventing death than other treatment regimens in the immunosuppressed-mouse model. On the other hand, no significant reduction in viral titers and no prevention of death was observed in the mice treated with oseltamivir for 20 days compared with results for the control, indicating that the selective pressure of repeated oseltamivir treatment was not sufficient to suppress viral replication and select mutant virus.
Although the H275Y NA mutant virus detected in a mouse treated with repeated administration of peramivir showed reduced susceptibility to peramivir, the viral titer of H275Y NA mutant virus was lower than those of other viruses without H275Y mutation obtained from mice treated with the same regimen of peramivir (data not shown). In addition, peramivir has been shown to be effective against an influenza A/WSN/33(H1N1) virus containing the H275Y NA mutation in a lethal mouse model when treatment was initiated at 24 h or 48 h p.i., since peak plasma concentrations of peramivir were higher than the in vitro IC50 of peramivir against H275Y NA mutants (34). Moreover, a single intravenous injection of 600 mg of peramivir resulted in a peak plasma concentration of 34,100 ng/ml in healthy volunteers, and repeated intravenous peramivir was thought to be effective in patients infected with influenza virus, including H275Y NA mutants that prevailed worldwide in the 2008-2009 season at high risk for complications (21, 35). Therefore, repeated administration of peramivir might regulate propagation of H275Y mutants even when they might be detected during the treatment. In the present study, we demonstrated that repeated intravenous injection of peramivir had beneficial effects on viral titers and symptoms in immunosuppressed mice infected with a lethal dose of A/H1N1pdm virus. Therefore, repeated intravenous injection of peramivir could be an alternative to oseltamivir for treating immunosuppressed patients with severe influenza virus infection.
ACKNOWLEDGMENTS
We thank Tetsuo Kase for providing A/Osaka/129/2009, Kaoru Baba, Takahiro Noda, and Yoshinori Ando for animal care, and Tomoyuki Homma and Takeshi Noshi for valuable discussions.
All work described here was financially supported by Shionogi & Co., Ltd.
Footnotes
Published ahead of print 11 March 2013
REFERENCES
- 1. Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, Muramoto Y, Tamura D, Sakai-Tagawa Y, Noda T, Sakabe S, Imai M, Hatta Y, Watanabe S, Li C, Yamada S, Fujii K, Murakami S, Imai H, Kakugawa S, Ito M, Takano R, Iwatsuki-Horimoto K, Shimojima M, Horimoto T, Goto H, Takahashi K, Makino A, Ishigaki H, Nakayama M, Okamatsu M, Takahashi K, Warshauer D, Shult PA, Saito R, Suzuki H, Furuta Y, Yamashita M, Mitamura K, Nakano K, Nakamura M, Brockman-Schneider R, Mitamura H, Yamazaki M, Sugaya N, Suresh M, Ozawa M, Neumann G, Gern J, Kida H, Ogasawara K, Kawaoka Y. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jain S, Benoit SR, Skarbinski J, Bramley AM, Finelli L. 2012. Influenza-associated pneumonia among hospitalized patients with 2009 pandemic influenza A (H1N1) virus—United States, 2009. Clin. Infect. Dis. 54:1221–1229 [DOI] [PubMed] [Google Scholar]
- 3. Maines TR, Jayaraman A, Belser JA, Wadford DA, Pappas C, Zeng H, Gustin KM, Pearce MB, Viswanathan K, Shriver ZH, Raman R, Cox NJ, Sasisekharan R, Katz JM, Tumpey TM. 2009. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science 325:484–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ministry of Health, Welfare and Labour Japan 2010. Infectious Diseases Weekly Report Japan (IDWR) 12:10–5 Ministry of Health Welfare and Labour Japan, Tokyo, Japan: http://idsc.nih.go.jp/idwr/kanja/idwr/idwr2010/idwr2010-10 [Google Scholar]
- 5. Sugaya N, Shinjoh M, Mitamura K, Takahashi T. 2011. Very low pandemic influenza A (H1N1) 2009 mortality associated with early neuraminidase inhibitor treatment in Japan: analysis of 1000 hospitalized children. J. Infect. 63:288–294 [DOI] [PubMed] [Google Scholar]
- 6. Bantia S, Kellogg D, Parker C, Upshaw R, Ilyushina NA, Babu YS. 2011. A single intramuscular injection of neuraminidase inhibitor peramivir demonstrates antiviral activity against novel pandemic A/California/04/2009 (H1N1) influenza virus infection in mice. Antiviral Res. 90:17–21 [DOI] [PubMed] [Google Scholar]
- 7. Kiso M, Shinya K, Shimojima M, Takano R, Takahashi K, Katsura H, Kakugawa S, Le MT, Yamashita M, Furuta Y, Ozawa M, Kawaoka Y. 2009. Characterization of oseltamivir-resistant H1N1 pandemic influenza A viruses. PLoS Pathog. 6:e1001079 doi:10.1371/journal.ppat.1001079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nguyen HT, Sheu TG, Mishin VP, Klimov AI, Gubareva LV. 2010. Assessment of pandemic and seasonal influenza A (H1N1) virus susceptibility to neuraminidase inhibitors in three enzyme activity inhibition assays. Antimicrob. Agents Chemother. 54:3671–3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ljungman P, Andersson J, Aschan J, Barkholt L, Ehrnst A, Johansson M, Weiland O. 1993. Influenza A in immunocompromised patients. Clin. Infect. Dis. 17:244–247 [DOI] [PubMed] [Google Scholar]
- 10. Rocha E, Cox NJ, Black RA, Harmon MW, Harrison CJ, Kendal AP. 1991. Antigenic and genetic variation in influenza A (H1N1) virus isolates recovered from a persistently infected immunodeficient child. J. Virol. 65:2340–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harper SA, Bradley JS, Englund JA, File TM, Gravenstein S, Hayden FG, McGeer AJ, Neuzil KM, Pavia AT, Tapper ML, Uyeki TM, Zimmerman RK. 2009. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1003–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaiser L, Keene ON, Hammond JM, Elliott M, Hayden FG. 2000. Impact of zanamivir on antibiotic use for respiratory events following acute influenza in adolescents and adults. Arch. Intern. Med. 160:3234–3240 [DOI] [PubMed] [Google Scholar]
- 13. Baz M, Abed Y, McDonald J, Boivin G. 2006. Characterization of multidrug-resistant influenza A/H3N2 viruses shed during 1 year by an immunocompromised child. Clin. Infect. Dis. 43:1555–1561 [DOI] [PubMed] [Google Scholar]
- 14. Gubareva LV, Matrosovich MN, Brenner MK, Bethell RC, Webster RG. 1998. Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus. J. Infect. Dis. 178:1257–1262 [DOI] [PubMed] [Google Scholar]
- 15. Ison MG, Gubareva LV, Atmar RL, Treanor J, Hayden FG. 2006. Recovery of drug-resistant influenza virus from immunocompromised patients: a case series. J. Infect. Dis. 193:760–764 [DOI] [PubMed] [Google Scholar]
- 16. Memoli MJ, Hrabal RJ, Hassantoufighi A, Eichelberger MC, Taubenberger JK. 2010. Rapid selection of oseltamivir- and peramivir-resistant pandemic H1N1 virus during therapy in 2 immunocompromised hosts. Clin. Infect. Dis. 50:1252–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van der Vries E, Stelma FF, Boucher CA. 2010. Emergence of a multidrug-resistant pandemic influenza A (H1N1) virus. N. Engl. J. Med. 363:1381–1382 [DOI] [PubMed] [Google Scholar]
- 18. Kohno S, Kida H, Mizuguchi M, Shimada J. 2010. Efficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infection. Antimicrob. Agents Chemother. 54:4568–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kohno S, Yen MY, Cheong HJ, Hirotsu N, Ishida T, Kadota J, Mizuguchi M, Kida H, Shimada J. 2011. Phase III randomized, double-blind study comparing single-dose intravenous peramivir with oral oseltamivir in patients with seasonal influenza virus infection. Antimicrob. Agents Chemother. 55:5267–5276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Food and Drug Administration 2009. News and events: FDA authorizes emergency use of intravenous antiviral peramivir for 2009 H1N1 influenza for certain patients, settings. Food and Drug Administration, Washington, DC: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm187813.htm [Google Scholar]
- 21. Kohno S, Kida H, Mizuguchi M, Hirotsu N, Ishida T, Kadota J, Shimada J. 2011. Intravenous peramivir for treatment of influenza A and B virus infection in high-risk patients. Antimicrob. Agents Chemother. 55:2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mastino A, Grelli S, Premrov MG, Favalli C. 1991. Susceptibility to influenza A virus infection in mice immunosuppressed with cyclophosphamide. J. Chemother. 3:156–161 [DOI] [PubMed] [Google Scholar]
- 23. Hurd J, Heath RB. 1975. Effect of cyclophosphamide on infections in mice caused by virulent and avirulent strains of influenza virus. Infect. Immun. 11:886–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singer SH, Noguchi P, Kirschstein RL. 1972. Respiratory diseases in cyclophosphamide-treated mice. II. Decreased virulence of PR8 influenza virus. Infect. Immun. 5:957–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sidwell RW, Bailey KW, Morrey JD, Wong MH, Baldwin TJ, Smee DF. 2003. Inhibition of influenza virus infections in immunosuppressed mice with orally administered peramivir (BCX-1812). Antiviral Res. 60:17–25 [DOI] [PubMed] [Google Scholar]
- 26. Hurt AC, Holien JK, Barr IG. 2009. In vitro generation of neuraminidase inhibitor resistance in A(H5N1) influenza viruses. Antimicrob. Agents Chemother. 53:4433–4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okomo-Adhiambo M, Sleeman K, Ballenger K, Nguyen HT, Mishin VP, Sheu TG, Smagala J, Li Y, Klimov AI, Gubareva LV. 2010. Neuraminidase inhibitor susceptibility testing in human influenza viruses: a laboratory surveillance perspective. Viruses 2:2269–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ward P, Small I, Smith J, Suter P, Dutkowski R. 2005. Oseltamivir (Tamiflu) and its potential for use in the event of an influenza pandemic. J. Antimicrob. Chemother. 55(Suppl 1):i5–i21 [DOI] [PubMed] [Google Scholar]
- 29. Baskerville A, Thomas G, Wood M, Harris WJ. 1974. Histology and ultrastructure of metaplasia of alveolar epithelium following infection of mice and hamsters with influenza virus. Br. J. Exp. Pathol. 55:130–137 [PMC free article] [PubMed] [Google Scholar]
- 30. Betsuyaku T, Fukuda Y, Parks WC, Shipley JM, Senior RM. 2000. Gelatinase B is required for alveolar bronchiolization after intratracheal bleomycin. Am. J. Pathol. 157:525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li W, Escarpe PA, Eisenberg EJ, Cundy KC, Sweet C, Jakeman KJ, Merson J, Lew W, Williams M, Zhang L, Kim CU, Bischofberger N, Chen MS, Mendel DB. 1998. Identification of GS 4104 as an orally bioavailable prodrug of the influenza virus neuraminidase inhibitor GS 4071. Antimicrob. Agents Chemother. 42:647–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kitano M, Itoh Y, Kodama M, Ishigaki H, Nakayama M, Ishida H, Baba K, Noda T, Sato K, Nihashi Y, Kanazu T, Yoshida R, Torii R, Sato A, Ogasawara K. 2011. Efficacy of single intravenous injection of peramivir against influenza B virus infection in ferrets and cynomolgus macaques. Antimicrob. Agents Chemother. 55:4961–4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bantia S, Parker CD, Ananth SL, Horn LL, Andries K, Chand P, Kotian PL, Dehghani A, El-Kattan Y, Lin T, Hutchison TL, Montgomery JA, Kellog DL, Babu YS. 2001. Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob. Agents Chemother. 45:1162–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abed Y, Pizzorno A, Boivin G. 2012. Therapeutic activity of intramuscular peramivir in mice infected with a recombinant influenza A/WSN/33 (H1N1) virus containing the H275Y neuraminidase mutation. Antimicrob. Agents Chemother. 56:4375–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shetty AK, Peek LA. 2012. Peramivir for the treatment of influenza. Expert Rev. Anti Infect. Ther. 10:123–143 [DOI] [PubMed] [Google Scholar]