Abstract
Pseudomonas aeruginosa invades epithelial and phagocytic cells, which may play an important role in the persistence of infection. We have developed a 24-h model of THP-1 monocyte infection with P. aeruginosa PAO1 in which bacteria are seen multiplying in vacuoles by electron microscopy. The model has been used to quantitatively assess antibiotic activity against intracellular and extracellular bacteria by using a pharmacodynamic approach (concentration-dependent experiments over a wide range of extracellular concentrations to calculate bacteriostatic concentrations [Cs] and maximal relative efficacies [Emax]; Hill-Langmuir equation). Using 16 antipseudomonal antibiotics (three aminoglycosides, nine β-lactams, three fluoroquinolones, and colistin), dose-response curves were found to be undistinguishable for antibiotics of the same pharmacological class if data were expressed as a function of the corresponding MICs. Extracellularly, all of the antibiotics reached a bacteriostatic effect at their MIC, and their Emax exceeded the limit of detection (−4.5 log10 CFU compared to the initial inoculum). Intracellularly, Cs values remained unchanged for β-lactams, fluoroquinolones, and colistin but were approximately 10 times higher for aminoglycosides, whereas Emax values were markedly reduced (less negative), reaching −3 log10 CFU for fluoroquinolones and only −1 to −1.5 log10 CFU for all other antibiotics. The decrease in intracellular aminoglycoside potency (higher Cs) can be ascribed to the acid pH to which bacteria are exposed in vacuoles. The decrease in the Emax may reflect a reversible alteration of bacterial responsiveness to antibiotics in the intracellular milieu. The model may prove useful for comparison of antipseudomonal antibiotics to reduce the risk of persistence or relapse of pseudomonal infections.
INTRODUCTION
Pseudomonas aeruginosa is among the leading causes of nosocomial infections in humans, with a particular tropism for the respiratory tract (see references 1 and 2 for reviews). Beside causing acute infections, it also chronically colonizes the lungs of cystic fibrosis patients, causing protracted and relapsing infections that are a primary cause of increased morbidity and mortality (3, 4).
P. aeruginosa is difficult to eradicate for at least two non-mutually exclusive reasons. First, P. aeruginosa is not only naturally resistant to many common antibiotics (because of the poor permeability of its outer membrane) but can also express several mechanisms of resistance to most, if not all, of the drugs that have been selected and clinically developed over the years for their antipseudomonal activity (2). Second, P. aeruginosa can adopt specific modes of life that afford protection from host defenses and antibiotic action. The first one consists of the production of biofilm, a physical barrier to the access of antimicrobial agents that contributes to the persistence of infection with P. aeruginosa (5). The second one stems from the capacity of P. aeruginosa to invade and survive within eukaryotic cells. This has been clearly documented for both epithelial (6–14) and phagocytic (15) cells in vitro and also observed in vivo for lung epithelial cells and alveolar macrophages of infected mice (16). Thus, although it is usually considered an extracellular organism, P. aeruginosa may actually behave as an opportunistic intracellular organism. On the whole, one can estimate that approximately half of the clinical, laboratory, and environmental isolates obtained demonstrate measurable internalization (17). This ability to invade depends on multiple factors, among which the level of expression of two secretion systems, H2 type VI and a type III secretion system, may play a critical role, as the former favors and the latter inhibits internalization (8, 18).
Survival of bacteria in eukaryotic cells has been associated with the reduced efficacy of most antibiotics, as demonstrated for Staphylococcus aureus, Listeria monocytogenes, Legionella pneumophila, and Mycobacterium tuberculosis (see references 19 to 21 for typical examples and references 22 and 23 for reviews). However, no data are available regarding the activity of antipseudomonal antibiotics against the intracellular forms of P. aeruginosa. The aim of the present study was therefore to set up a model of intracellular P. aeruginosa infection allowing a comparative evaluation of the activities of antipseudomonal antibiotics. We selected THP-1 human monocytes as host cells because these have proven useful in obtaining reproducible levels of intracellular infection with other bacteria and in quantitatively assessing the activities of antibiotics by a pharmacodynamic approach (19, 20, 24–26). We included in our study most of the currently used antipseudomonal agents (27) and tested them against reference strain PAO1.
MATERIALS AND METHODS
Bacterial strain, susceptibility testing, and time and dose-kill curve studies in extracellular medium.
P. aeruginosa strain ATCC PAO1 was used for all experiments. Bacteria were grown in Mueller-Hinton broth, and CFU counting was performed by plating on tryptic soy agar. MICs were measured by serial 2-fold microdilution according to CLSI guidelines (inoculum of approximately 106 CFU/ml, reading after 20 to 24 h of incubation) in cation-adjusted Mueller-Hinton broth (CA-MHB) (28) or in the medium used for experiments with THP-1 cells (RPMI 1640 medium supplemented with 10% fetal calf serum buffered at pH 7.4 with 10 mM phosphate buffer for MIC testing and with 2 g/liter NaHCO3 for cell cultures incubated in a 5% CO2 atmosphere). Concentration-kill curves were determined as previously described, with a starting inoculum of 106 CFU/ml (19) in the same media.
Cells, cell culture, and intracellular infection.
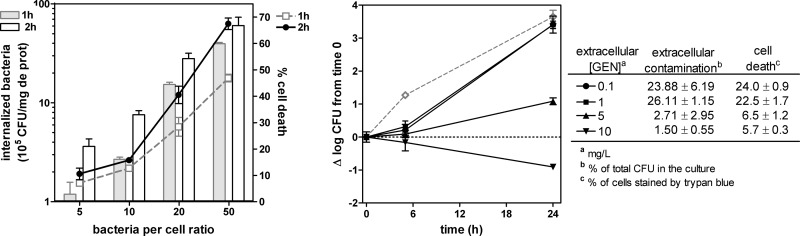
Human THP-1 cells were cultivated in RPMI 1640 medium supplemented with 10% fetal calf serum as described previously (19). Intracellular infection was performed on the basis of the protocol previously developed for S. aureus (19), with specific adaptations. In brief, bacteria were opsonized by 1 h of incubation with 10% human serum in RPMI 1640 medium without fetal calf serum. Phagocytosis was allowed for 2 h at a bacterium/cell ratio of 10:1, after which nonphagocytosed and adherent bacteria were eliminated by incubation for 60 min with gentamicin at 100 mg/liter. After three washes in phosphate-buffered saline (PBS) and resuspension in culture medium, the inoculum was typically 5 × 105 to 7 × 105 CFU/mg of cell protein (defined as time zero). Extracellular contamination at the end of the incubation period was assessed in pilot experiments by CFU counting in the culture fluid pooled with the liquids used for successive washings (see Fig. 1).
Fig 1.
Setting up the intracellular model. (Left panel) Internalization of P. aeruginosa PAO1 after 1 (gray bars) or 2 (open bars with black border) h of phagocytosis of PAO1 at increasing bacterium-to-cell ratios (left axis) and percent mortality of THP-1 cells as assessed at the end of the phagocytosis period (right axis). All data are means ± standard deviations (n = 3). (Right panel) Apparent intracellular growth of P. aeruginosa PAO1 in THP-1 cells incubated for 24 h with increasing extracellular concentrations of gentamicin compared to the extracellular growth (dotted gray line) measured in RPMI culture medium supplemented with 10% fetal calf serum in the absence of antibiotic. The graph shows the change in the number of CFU (Δlog CFU), from the initial, postphagocytosis inoculum achieved after 2 h of phagocytosis for an initial inoculum of 10 CFU/cell (approximately 7 × 105 CFU/mg of protein). The table shows the percentages of contamination of the culture medium by P. aeruginosa and of cell death at the end of the experiment. GEN, gentamicin. All data are means ± standard deviations (n = 3).
Intracellular antibiotic activity.
Antibiotics were added to infected cells at extracellular concentrations ranging from 0.01 to 200 mg/liter to obtain a complete description of the concentration-response curve, as described in detail in a previous publication (19). After appropriate incubation times (up to 24 h), cells were collected by centrifugation, resuspended in PBS, centrifuged again to eliminate extracellular bacteria, and collected in distilled water. Complete cell lysis was achieved by sonication (10 s), and lysates were used for determination of CFU counts (after plating on agar of appropriate dilutions) and of protein content by Lowry's assay with a commercially available detection kit (Bio-Rad DC Protein Assay, Bio-Rad laboratories, Hercules, CA). The activity was expressed as the change from the initial inoculum after 24 h of incubation.
Assessment of cell viability.
The percentage of dead cells was assessed by trypan blue exclusion test, with unstained and stained cells counted by optical microscopy after 5 min of incubation with the dye at a final concentration of 0.5% in PBS.
Morphological studies.
Infected cells were harvested, washed with PBS, fixed with 2% glutaraldehyde (30 min, 4°C) in 0.1 M sodium cacodylate buffer (pH 7.4), washed, postfixed for 1 h with 1% osmium tetroxide in cacodylate buffer in the dark, and then stained en bloc with uranyl acetate (29). Ultrathin sections (75 nm) were counterstained with uranyl acetate and lead citrate and observed in a Philips CM12 microscope.
Antibiotics.
Amikacin (disulfate salt; potency, 73.6%), ticarcillin (disulfate salt; potency, 84.65%), piperacillin (sodium salt; potency, 94.2%), and colistin (sulfate salt; potency, 67.50%) were purchased from Sigma-Aldrich (St. Louis, MO). The following antibiotics were obtained as microbiological standards from their manufacturers: ciprofloxacin (chlorhydrate; potency, 85%) and moxifloxacin (chlorhydrate; potency, 91%) from Bayer AG, Wuppertal, Germany; tobramycin (potency, 100%) from SMB-GalePhar, Marche-en-Famenne, Belgium; and imipenem (potency, 100%) from Merck, Darmstadt, Germany. The following antibiotics were procured as commercial products registered in Belgium or France for parenteral use from their marketing authorization holders or resellers: gentamicin (Gentalline; potency, 100%) from MSD-France (Courbevoie, France), levofloxacin (Tavanic; potency, 95%) from Aventis (Romainville, France), piperacillin-tazobactam (Tazocin; potency, 97%) from Wyeth Pharmaceuticals (Ottignies-Louvain-la-Neuve, Belgium), cefepime (Maxipime; potency, 84.5%) and aztreonam (Azactam; potency, 95.4%) from Bristol-Myers Squibb Co. (Brussels, Belgium), ceftazidime (Glazidim; potency, 88.2%) from Glaxo-SmithKline (Genval, Belgium), meropenem (Meronem; potency, 74.0%) from Astra Zeneca (Brussels, Belgium), and doripenem (Doribax; potency, 95.7%) from Janssen-Cilag (Beerse, Belgium).
Other reagents.
Unless stated otherwise, all other reagents were of analytical grade and were purchased from Sigma-Aldrich-Fluka. Cell culture or microbiology media were from Invitrogen (Paisley, Scotland) and Difco (Sparks, MD).
Curve fittings and statistical analyses.
Curve fittings were performed with GraphPad Prism (version 4.02) software for Windows (GraphPad Prism Software, San Diego, CA), and statistical analyses were performed with GraphPad InStat 3 version 3.10 (GraphPad Prism Software).
RESULTS
Susceptibility testing.
Table 1 shows the MICs of a series of antibiotics currently used for the treatment of infections with P. aeruginosa, together with their maximal concentrations in serum (Cmax) of humans (total drug) as determined in patients receiving conventional dosages. MICs were measured in CA-MHB at pHs 7.4 and 5.5 to mimic the extracellular and phagolysosomal environments, respectively, and in RPMI 1640 supplemented with 10% fetal calf serum (i.e., the medium used in experiments with THP-1 cells). Acid pH decreased the potency of aminoglycosides (MICs increased by at least 2 log2 dilutions) but not that of the other antibiotics (maximum, 1-dilution increase). When measured in RPMI 1640, MICs were similar to or only 1 dilution higher than those observed in CA-MHB.
Table 1.
MICs of antibiotics in CA-MHB at pHs 7.4 and 5.5 and in RPMI 1640 against P. aeruginosa PAO1 compared to the Cmax
| Antibiotic | Abbreviation | MIC (mg/liter) |
Dosagea | Human Cmax (mg/liter)b | Reference(s) | ||
|---|---|---|---|---|---|---|---|
| CA-MHB |
RPMI 1640 (pH 7.4) | ||||||
| pH 7.4 | pH 5.5 | ||||||
| Aminoglycosides | |||||||
| Gentamicin | GEN | 2 | 8 | 4 | 3–5 mg/kg/day i.v. | 18 | 37 |
| Amikacin | AMK | 4 | 64 | 4 | 7.5 mg/kg/day i.v. | 38 | 37 |
| Tobramycin | TOB | 1 | 8 | 1 | 3–5 mg/kg/day i.v. | 6 | 37 |
| β-Lactams | |||||||
| Ticarcillin | TIC | 32 | 64 | 32 | 2 g/3–4 days i.v. | 200 | 37 |
| Piperacillin | PIP | 16 | 16 | 16 | 4 g/3–4 days i.v. | 389 | 37 |
| Piperacillin-tazobactam | TZP | 16 | 16 | 16 | 4 g/3–4 days i.v. | 224 | 37 |
| Cefepime | CEP | 4 | 8 | 4 | 2 g/2–3 days i.v. | 160 | 37 |
| Ceftazidime | CAZ | 2 | 4 | 2 | 2 g/2–3 days i.v. | 160 | 37 |
| Aztreonam | ATM | 8 | 16 | 8 | 2 g/3–4 days i.v. | 210 | 37 |
| Meropenem | MEM | 1 | 1 | 2 | 1 g/2–3 days i.v. | 57 | 37, 38 |
| Imipenem | IMI | 1 | 1 | 1 | 1 g/3–4 days i.v. | 91 | 37, 38 |
| Doripenem | DOR | 0.5 | 1 | 1 | 1 g/3–4 days i.v. | 73 | 37, 38 |
| Fluoroquinolones | |||||||
| Moxifloxacin | MXF | 1 | 2 | 1 | 0.4 g/day p.o.-i.v. | 4.3 | 37 |
| Levofloxacin | LVX | 1 | 2 | 1 | 0.5 g/day p.o.-i.v. | 6.2 | 37 |
| Ciprofloxacin | CIP | 0.125 | 0.25 | 0.125 | 0.4 g/day i.v. | 4.6 | 37 |
| Colistin (polymyxin) | CST | 1 | 2 | 2 | 2.5–5 mg/kg/day i.v. | 5 | 39 |
Conventional doses for the treatment of P. aeruginosa infections. p.o., oral; i.v., intravenous.
Cmax, commonly observed maximal concentration (total drug) in serum after intravenous or oral administration of these doses.
Validation of the intracellular model.
In a first series of experiments, we examined in parallel (i) the level of internalization of PAO1 by THP-1 cells exposed for 1 or 2 h to increasing inocula and (ii) the extent of cell death induced by bacteria under these conditions (Fig. 1, left panel). Internalization of bacteria clearly increased with the inoculum and the time of incubation but was accompanied by a commensurate toxicity for the host cells. On the basis of these data, we selected a phagocytosis time of 2 h and an initial bacterium-to-cell ratio of 10, which allowed us to obtain reproducibly an intracellular inoculum of about 7 × 105 CFU/mg cell protein with only 15% cell death. We then monitored, under these conditions, the apparent intracellular growth of bacteria for 24 h in the presence of low concentrations of gentamicin. This antibiotic was selected on the basis of the model developed previously for intracellular infection of THP-1 cells with S. aureus because it was shown to prevent extracellular contamination while at the same time only minimally impairing its intracellular growth (19). As shown in Fig. 1 (right panel), the increase in the number of cell-associated CFU at 24 h was almost as large as for bacteria grown in CA-MHB as long as the gentamicin concentrations were lower than the MIC measured in RPMI 1640. However, extracellular contamination was very important (leading to an overestimation of the cell-associated counts) and associated with a marked (>20%) loss of cell viability. When the extracellular gentamicin concentration was brought to 5 mg/liter (i.e., close to its MIC in RPMI 1640 [4 mg/liter]), extracellular contamination became negligible but bacteria still grew in cells (with a gain of about 1 log10 CFU over the 24-h incubation period) and with no apparent increase in cell toxicity (trypan blue assay). Any further increase in the extracellular gentamicin concentration caused complete impairment of intracellular growth, denoting the development of significant activity in cells beyond what was obtained against extracellular bacteria.
Morphological studies.
Electron microscopy was used to examine the morphology of infected cells, as well as the subcellular localization of P. aeruginosa (Fig. 2). At the end of the phagocytosis period (2 h), we observed bacteria located in vacuoles limited by a double membrane (suggesting a phagosomal nature) and close to the cell surface, where they started multiplying. After 24 h of incubation (in the presence of 5 mg/liter gentamicin in the extracellular medium), we noticed the presence of larger vacuoles hosting several bacteria.
Fig 2.
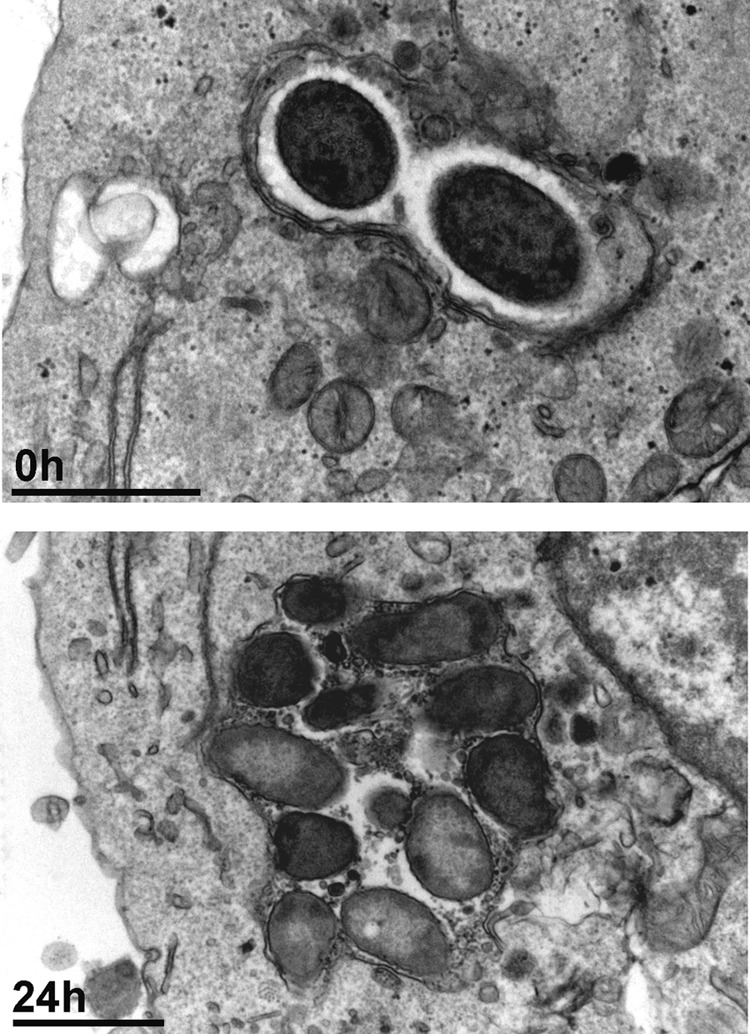
Electron microscopic appearance of THP-1 cells infected with P. aeruginosa PAO1. Infected cells were observed after 2 h of phagocytosis (corresponding to the postphagocytosis conditions) and after 24 h of incubation in the presence of gentamicin at an extracellular concentration of 5 mg/liter. Bars, 1 μm.
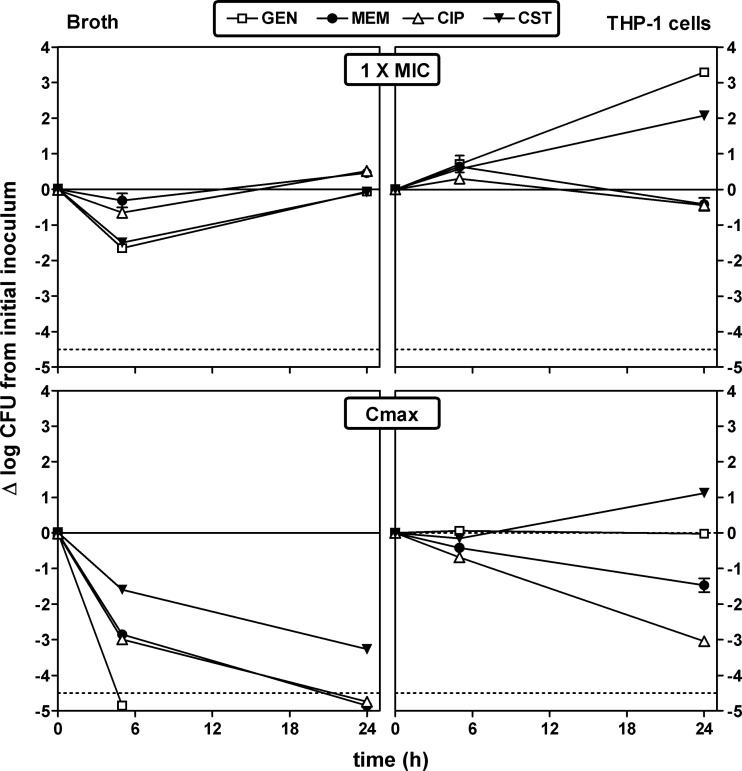
Kinetics and influence of concentration on antibacterial effects in the MIC-to-Cmax range.
The activity of antibiotics used for the treatment of P. aeruginosa infections was first examined over time and at fixed concentrations (1× the MIC in broth and at the human Cmax) against both extracellular and intracellular bacteria with a panel of antibiotics covering the aminoglycoside, fluoroquinolone, and β-lactam classes and colistin. These data are illustrated for one representative antibiotic in each of these pharmacological classes (gentamicin, ciprofloxacin, meropenem) and for colistin in Fig. 3. With respect to extracellular activity, gentamicin and colistin caused a transient decrease in the inoculum at 5 h when used at an extracellular concentration corresponding to the MIC, and all antibiotics were eventually bacteriostatic at 24 h, as anticipated. When used at its human Cmax (see Table 1 for values), gentamicin was rapidly bactericidal toward extracellular bacteria, yielding a decrease in the CFU count corresponding to the limit of detection at 5 h. Ciprofloxacin and meropenem were also bactericidal against extracellular bacteria at 5 h (3 log10 CFU decrease), but colistin required 24 h of incubation to reach this threshold. With respect to intracellular activity, gentamicin and colistin did not prevent bacterial growth when used at extracellular concentrations corresponding to their MICs, while meropenem and ciprofloxacin reached a bacteriostatic effect after 24 h of incubation. At the human Cmax, colistin still did not completely impair the intracellular growth of P. aeruginosa, gentamicin was bacteriostatic, and meropenem and ciprofloxacin caused 1.5- and 3-log10 CFU decreases at 24 h, respectively. Full data for the other antibiotics tested (amikacin, tobramycin, moxifloxacin, levofloxacin, piperacillin, piperacillin-tazobactam, ticarcillin, ceftazidime, cefepime, imipenem, doripenem, and aztreonam) are shown in Fig. S1 in the supplemental material together with those of the four antibiotics described above. Globally, all of the antibiotics were bacteriostatic at their MICs at 24 h against extracellular bacteria and bactericidal at their Cmax, with the exception of cefepime. Together with gentamicin, amikacin was the only antibiotic capable of sterilizing the culture in 5 h. Against intracellular bacteria and at 24 h, aminoglycosides did not prevent intracellular growth when added to the culture medium at concentrations corresponding to their MICs in RPMI 1640 medium, whereas all β-lactams were bacteriostatic and fluoroquinolones showed variable effects. At extracellular concentrations corresponding to their Cmax, aminoglycosides were almost bacteriostatic at 24 h, β-lactams reduced the inoculum by approximately 1 log10 CFU, but fluoroquinolones reduced the intracellular inoculum by close or equal to 3 log10 CFU compared to the postphagocytosis value.
Fig 3.
Influence of time on the rate and extent of activity of antibiotics against extracellular (broth; left panels) and intracellular (THP-1cells; right panels) P. aeruginosa PAO1 upon incubation at fixed extracellular concentrations corresponding to their MICs in broth (top) and to concentrations (total drug) corresponding to their Cmax observed in humans after the administration of conventional doses (bottom). The ordinate shows the change in the number of CFU (log scale) per milliliter for extracellular bacteria or per milligram of cell protein for intracellular bacteria. The solid horizontal line corresponds to a bacteriostatic effect (no change from the initial inoculum), and the dotted horizontal line shows the limit of detection (−4.5-log CFU decrease). Values are means ± standard deviations (n = 3); when not visible, error bars are smaller than the symbols. GEN, gentamicin; MEM, meropenem; CIP, ciprofloxacin; CST, colistin.
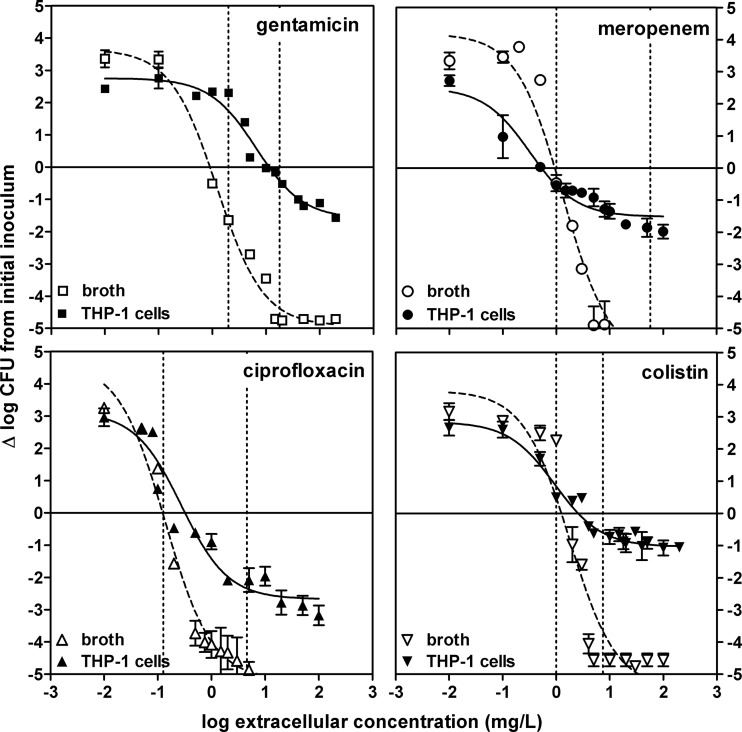
Concentration-effect relationships (pharmacological comparisons).
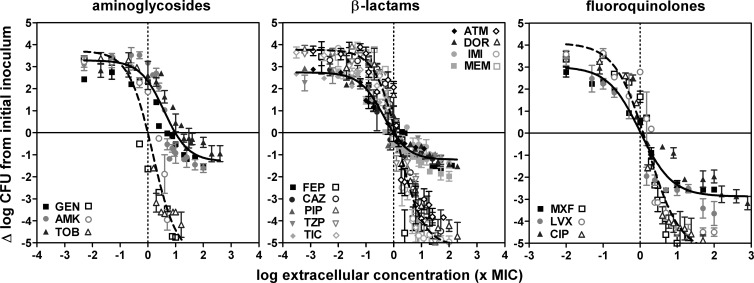
Because only minimal decreases in intracellular CFU counts were observed at 5 h, full pharmacological concentration-response studies (aimed at determining and comparing the apparent bacteriostatic concentrations [Cs] and maximal relative efficacies [Emax] of antibiotics against extracellular and intracellular bacteria) were made only after 24 h of incubation. Typical data are illustrated in Fig. 4 for the same four representative antibiotics. In all cases, monophasic sigmoidal functions (with a slope factor of 1) could be fitted to the experimental values and these were used for interpretation of the data. Against extracellular bacteria, an apparent bacteriostatic effect was observed for each antibiotic at concentrations close to the MIC and the Emax was always below the actual limit of detection (4.5-log10 decrease compared to the original inoculum). Emax values were considerably lower (less negative) against intracellular bacteria than against extracellular bacteria for all four drugs, reaching only a 1- to 1.5-log10 CFU decrease from the original, postphagocytosis inoculum for colistin, gentamicin, and meropenem and a 2.6-log10 CFU decrease for ciprofloxacin. The Cs of colistin, meropenem, and ciprofloxacin were close to the MICs (measured at pH 7.4), while that of gentamicin was about 10-fold higher. These studies were then extended to 12 other antibiotics, and the corresponding data (grouped by drugs of the same pharmacological class) are illustrated in Fig. 5 (for matching pharmacodynamic parameters for each individual antibiotic, see Table S1 in the supplemental material). Quite interestingly, all of the antibiotics within a pharmacological class showed similar activity profiles when the extracellular concentrations were expressed in multiples of the respective MICs (at pH 7.4). A single curve could thus be fitted to the whole set of data obtained with different antibiotics within that class (Table 2 contains the pharmacodynamic parameters calculated from these single-fit curves; see Tables S1 to S3 in supplemental material for the pharmacodynamic parameters of individual curves and detailed statistical analyses); only the aztreonam and amikacin intracellular Emax values were significantly different from those of their respective classes, and only the tobramycin EC50 (concentration yielding a response halfway between the Emin and Emax responses in concentration-dependent experiments) was significantly different from that of the other aminoglycosides. Two major observations could be made from these studies. First, the Cs values were similar for intracellular and extracellular bacteria with β-lactams and fluoroquinolones but were globally 10 times higher (lower potency) for aminoglycosides. Second, the Emax values for intracellular P. aeruginosa were lower (less negative) than those of their extracellular counterparts but to a variable extent. Thus, the Emax still reached about −2.9 log10 CFU for fluoroquinolones but only about −1.2 log10 CFU for all other antibiotics. The MICs for bacteria collected at the end of the experiment were measured and found to be unchanged from those for the infecting strain, indicating that we were not dealing with the mere selection of stable resistant subpopulations (data not shown).
Fig 4.
Concentration-response curves of selected antibiotics against extracellular and intracellular P. aeruginosa. The graphs show the changes in the number of CFU (Δlog CFU from the initial inoculum) per milliliter of broth (extracellular, open symbols, dotted lines) or per milligram of cell protein (intracellular, closed symbols, solid lines) in THP-1 cells after 24 h of incubation at increasing extracellular concentrations (expressed in milligrams per liter [total drug]). The solid horizontal line corresponds to a bacteriostatic effect (no change from the initial inoculum); the vertical dotted lines show the MIC-to-Cmax range limits. Data are means ± standard deviations (n = 3); when not visible, error bars are smaller than the symbols.
Fig 5.
Concentration-response curves of antibiotics against extracellular and intracellular P. aeruginosa. The graphs show changes in the number of CFU (Δlog CFU from the initial inoculum) per milliliter of broth (extracellular; open symbols and dotted line) or per milligram of cell protein (intracellular; closed symbols and solid line) in THP-1 cells after 24 h of incubation at increasing extracellular concentrations (expressed in multiples of the MIC). The solid horizontal line corresponds to a bacteriostatic effect (no change from the initial inoculum); the vertical dotted line shows the MIC. Data are means ± standard deviations (n = 3); when not visible, error bars are smaller than the symbols. For definitions of drug name abbreviations, see Table 1.
Table 2.
Pertinent regression parameters of dose-response curves for extracellular (broth) and intracellular (THP-1 cells) activities of antibiotics against strain PAO1a
| Antibiotic(s) | Mean extracellular activity (95% confidence interval) |
Mean intracellular activity (95% confidence interval) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Eminb | Emaxc | EC50d | Cse | R2 | Eminb | Emaxc | EC50d | Cse | R2 | |
| Aminoglycosides | 3.80 (3.37–4.23)aAf | >−4.5A | 1.77 (1.31–2.37)aA | 1.18 | 0.90 | 3.34 (3.04–3.64)aA | −1.31 (−1.62 to −1.00)aB | 4.22 (3.01–5.93)aA | 10.77 | 0.83 |
| β-Lactams | 3.77 (3.57–3.98)aA | >−4.5A | 1.58 (1.37–1.83)aA | 1.17 | 0.93 | 2.74 (2.60–2.88)bB | −1.23 (−1.34 to −1.11)aB | 0.44 (0.36–0.53)bA | 0.98 | 0.90 |
| Fluoroquinolones | 4.10 (3.57–4.63)aA | >−4.5A | 1.61 (1.29–2.00)aA | 1.21 | 0.94 | 3.02 (2.65–3.39)aB | −2.88 (−3.13 to −2.63)bB | 1.00 (0.73–1.36)bA | 1.04 | 0.91 |
| Colistin | 3.81 (2.75–4.87)aA | >−4.5A | 1.78 (1.08–2.95)aA | 1.25 | 0.92 | 2.84 (2.50–3.18)bB | −1.04 (−1.22 to −0.86)aB | 0.94 (0.63–1.40)bA | 2.52 | 0.92 |
Cells were incubated for 24 h with extracellular drug concentrations ranging from 0.01 to 200 mg/liter. A single curve was fitted to the data obtained for different antipseudomonal antibiotics within a single class. The corresponding data are shown in Fig. 5; for the parameters for the individual fits, see Table S1 in the supplemental material.
CFU count increase (in log10 units) at 24 h from the corresponding initial inoculum as extrapolated for an infinitely low antibiotic concentration.
CFU count decrease (in log10 units) at 24 h from the corresponding initial inoculum as extrapolated from an infinitely high antibiotic concentration.
Extracellular concentration (total drug; in multiples of the MIC) causing a reduction halfway between Emin and Emax, as calculated from the Hill equation of the concentration-response curve (slope factor of 1).
Cs, i.e., the extracellular concentration (total drug; in multiples of the MIC) resulting in no apparent bacterial growth (number of CFU identical to the initial inoculum), as calculated from the Hill equation of the concentration-response curve.
Statistical analysis per column was done by one-way analysis of variance with Tukey's test for multiple comparisons of each parameter for all classes. Values followed by different lowercase letters are significantly different from each other (P < 0.05). Statistical analysis per row was done by unpaired, two-tailed t test between corresponding parameters of extracellular and intracellular activities. Values followed by different uppercase letters are significantly different from each other (P < 0.05).
DISCUSSION
The present study has allowed the development of a well-defined 24-h model of intracellular infection of phagocytic cells with P. aeruginosa that could be used to document the response of intracellular P. aeruginosa to antibiotics on a pharmacodynamic basis.
Considering the model, optimization of the bacterium-to-cell ratio and the time of phagocytosis was critical in obtaining a reproducible infection while at the same time avoiding extensive killing of the host cells. This is important for P. aeruginosa because it produces a variety of virulence factors that can affect host cell viability. In strain PAO1, pili, flagella, a type III secretion system, exotoxin A, and lipopolysaccharide are all known to contribute to virulence (30, 31). Because the expression of these factors is variable between strains and critically depends on their growth status, preliminary investigations such as those performed here will probably be necessary when expanding the model to other laboratory or clinical strains. Selection of the appropriate extracellular concentration of gentamicin to prevent extracellular contamination without impairing the intracellular growth of P. aeruginosa also proved to be critical. Our data suggest that a concentration close to the MIC for the strain (as determined in cell culture medium at pH 7.4) is the most appropriate, as previously established in a model of S. aureus-infected THP-1 cells (19), but we also see that both lower and higher concentrations may give rise to spurious results. Lastly, we show that P. aeruginosa is able to grow slowly within THP-1 monocytes, with a net population increase of about 1 log10 CFU over 24 h. However, it must be emphasized that intracellular growth seems to start only after a 5-h period following phagocytosis. In this context, few studies have monitored the long-term intracellular fate of P. aeruginosa, which makes comparisons of other models with ours quite difficult. However, prolonged intracellular persistence of P. aeruginosa has been demonstrated in airway epithelial cells (14) or in HeLa cervical cancer cells (8) and transient growth has been demonstrated in corneal epithelial cells (9). A switch from apparent intracellular persistence to a net increase in the number of intracellular bacteria is probably related to an equilibrium among intrinsic bacterial growth properties, expression of virulence factors, and the level of host defenses. The latter are notoriously poor in THP-1 cells (32). Electron microscopy suggests that intracellular P. aeruginosa remains confined in vacuoles in THP-1 cells for at least 24 h and can actively replicate therein. A vacuolar localization of P. aeruginosa has also been generally observed in epithelial cells (7, 13, 33), but the nature of the infected vacuoles has not yet been determined (31).
Moving now to the use of the model for the study of antibiotics, we show here that their general pharmacodynamic profile is globally comparable to that observed in the same host cells for other bacteria such as S. aureus, L. monocytogenes, and L. pneumophila (19, 20, 24–26). Thus, (i) all antibiotics clearly showed concentration-dependent effects following the general model described for drugs in interaction with their targets and described by the Hill-Langmuir sigmoid function, (ii) the relative intracellular Emax is always considerably lower than that observed in broth, and (iii) the apparent Cs for intracellular bacteria is similar to the value for bacteria in broth, except that of aminoglycosides (which is markedly increased, denoting a corresponding loss of potency). Remarkably, all of the drugs within a given pharmacological class behave similarly when the bacterial response is expressed as a function of multiples of the respective MICs. This suggests that the intracellular activity of antibiotics depends on the intrinsic activity shown in broth (MIC), which will determine the Cs on the one hand, and on their pharmacological class, which will determine their relative Emax on the other hand. The latter parameter reflects the maximal killing capacity of a given antibiotic in a specific environment. It therefore takes into account not only the way the drug can express its activity in the infected compartment but also the bacterial responsiveness to that antibiotic and the cooperation with cell defense mechanisms to achieve killing (22). The marked reduction in maximal relative intracellular efficacy (compared to what is seen in broth) must therefore be interpreted as clear evidence that a significant part of the intracellular inoculum apparently becomes insensitive to the action of the antibiotic. This could be explained by the slow growth of bacteria when in the intracellular milieu or the ability of intracellular P. aeruginosa to switch to a less susceptible phenotype. While slow growth has been documented here, we know that P. aeruginosa may adopt a biofilm-like phenotype inside cells that makes it poorly responsive to antibiotics (14). Both changes, however, must be reversible because no difference in the MIC was noticed when testing bacteria collected from cells (after phagocytosis and exposure of these cells to antibiotics for 24 h) in comparison with bacteria maintained in broth.
Our previous studies using the THP-1 monocyte model allow comparison of the pharmacodynamic properties of antibiotics against bacteria located in distinct subcellular compartments of the same host. Against an organism that thrives in vacuoles, such as S. aureus, the Emax values of aminoglycosides and β-lactams are of the same order of magnitude as those seen here (about −1 to −1.5 log10 CFU). The values of fluoroquinolones seem species specific, varying from −2 log10 CFU for S. aureus (25, 34, 35) to −2 to −3 log10 CFU for L. pneumophila (25) and −3 log10 CFU for P. aeruginosa. Against an organism multiplying in the cytosol, such as L. monocytogenes, the Emax values of both β-lactams (−2 log10 CFU) and fluoroquinolones (−3 to −4 log10 CFU) are higher (more negative) (24–26) than those we observed here and aminoglycosides are totally ineffective (36). Available data (reviewed in references 22 and 23) show that β-lactams and fluoroquinolones, although they are recovered mainly in the cytosol after cell fractionation studies, are probably capable of moving freely throughout all cell compartments. Conversely, aminoglycosides are always found associated with, and probably restricted to, the endocytic and lysosomal compartment of the cells. Thus, while β-lactams, fluoroquinolones, and aminoglycosides can be effective against intravacuolar P. aeruginosa and S. aureus if their extracellular concentration is high enough (which is what the Emax will document), only β-lactams and fluoroquinolones might be able to reach and act on cytosolic L. monocytogenes. Access to target only, however, does not explain why fluoroquinolones are more effective against intracellular P. aeruginosa than against intracellular S. aureus (when comparing these two bacteria in the same cell host) and more effective than β-lactams against P. aeruginosa in the model studied here. This suggests a specific role of bacterial responsiveness to fluoroquinolones that needs to be investigated further.
Previous studies with other bacteria showed that the relative potencies of drugs rely on their MICs on the one hand (intrinsic potency) and on their cellular concentrations on the other (for a review, see reference 23). We have selected the Cs as a more useful parameter than the EC50 commonly used to describe relative antibiotic potencies in pharmacological studies. This is because EC50 are critically dependent on the amplitude of the concentration-response curve, which in our case is entirely related to the Emax (the Emin, which describes bacterial growth in the absence of an antibiotic, is essentially similar for all of the groups studied here). Thus, a given EC50 may actually correspond to very different bacterial response levels (growth, an apparent bacteriostatic effect, or a decrease in the CFU count), depending on how much lower the Emax value is than that of the initial inoculum, which makes it poorly informative in terms of an antibacterial effect. Conversely, the Cs for intracellular bacteria tends to be constant when comparing antibiotics (even if the Emax values are different) and actually close to the MIC measured in broth or in RPMI 1640 medium for all antibiotics except aminoglycosides. While β-lactams reach intracellular concentrations close to the extracellular ones, fluoroquinolones accumulate to much higher concentrations in cells (22). The most plausible explanation for the fact that fluoroquinolones are not more potent than β-lactams against intracellular bacteria is that only a fraction of an accumulated fluoroquinolone expresses its activity at the site of action, as already concluded from studies assessing the susceptibility of intracellular S. aureus and L. monocytogenes to fluoroquinolones with distinct levels of accumulation (see reference 26 and the references cited therein). Colistin accumulation and subcellular distribution are still unknown, making any discussion premature in this context. For aminoglycosides, the marked difference in Cs for intracellular bacteria from the MIC measured in broth at neutral pH can be understood if considering that both the drug and the target could be located in an acidic environment (lysosomes and phagolysosomes on the basis of the data from our electron microscopic studies). The Cs of aminoglycosides for intracellular bacteria are actually close to their MICs if measured at pH 5.5. Data in the literature, however, suggest that the vacuoles in which P. aeruginosa survives and multiplies may be distinct from lysosomes (and therefore not as acidic), at least in epithelial cells (33). Further studies aimed at identifying the real nature of the vacuoles identified here and at determining their actual pH would be useful in this context.
The present study used only one reference strain of P. aeruginosa (ATCC PAO1), which may be considered a major limitation to the application of our conclusions to clinical situations. The choice of a unique, well-characterized strain was actually essential for the development and validation of the model, as well as for the correct comparative assessment of the intrinsic intracellular activity of each antibiotic class. Many resistance mechanisms and virulence factors vary in their level of expression in clinical strains, depending on the environment. This would have created much uncertainty in what is primarily a pharmacological study. Focusing on this aspect, our data show a clear superiority of fluoroquinolones over β-lactams and aminoglycosides when dealing with intracellular forms of P. aeruginosa. They also highlight the poor efficacy of colistin against these forms. Within the limitations inherent to all in vitro experiments, including those that are specific to the model used and that are discussed in details in our previous publications (19, 20, 24), our studies may represent a first step in the rationalization of current antibiotic choices and in the better assessment of new molecules. Further studies examining key clinical strains and other host cells but based on a similar pharmacodynamic approach may eventually further contribute to limitation of the risk of therapeutic failures related to persistence and/or recurrence of pseudomonal infections.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to M. C. Cambier, C. Misson, V. Mohymont, and K. Santos for expert technical assistance. We thank all of the manufacturers for the kind gifts of their antibiotics.
J.M.B. was a postdoctoral fellow of the BioWin program (Région Wallonne, Belgium) and F.V.B. is Maître de Recherche of the Belgian Fonds National de la Recherche Scientifique. This work was supported by the BioWin program, the Fonds de la Recherche Scientifique Médicale (FRSM grants 3.4639.09 and 3.4530.12), and grants from the Fonds Alphonse et Jean Forton and the French Association Mucoviscidose ABCF.
Footnotes
Published ahead of print 11 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02609-12.
REFERENCES
- 1. Fujitani S, Sun HY, Yu VL, Weingarten JA. 2011. Pneumonia due to Pseudomonas aeruginosa: part I: epidemiology, clinical diagnosis, and source. Chest 139:909–919 [DOI] [PubMed] [Google Scholar]
- 2. Mesaros N, Nordmann P, Plesiat P, Roussel-Delvallez M, Van Eldere J, Glupczynski Y, Van Laethem Y, Jacobs F, Lebecque P, Malfroot A, Tulkens PM, Van Bambeke F. 2007. Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin. Microbiol. Infect. 13:560–578 [DOI] [PubMed] [Google Scholar]
- 3. Kerem E, Corey M, Gold R, Levison H. 1990. Pulmonary function and clinical course in patients with cystic fibrosis after pulmonary colonization with Pseudomonas aeruginosa. J. Pediatr. 116:714–719 [DOI] [PubMed] [Google Scholar]
- 4. Li Z, Kosorok MR, Farrell PM, Laxova A, West SE, Green CG, Collins J, Rock MJ, Splaingard ML. 2005. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 293:581–588 [DOI] [PubMed] [Google Scholar]
- 5. Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. 2010. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 35:322–332 [DOI] [PubMed] [Google Scholar]
- 6. Fleiszig SM, Zaidi TS, Fletcher EL, Preston MJ, Pier GB. 1994. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect. Immun. 62:3485–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chi E, Mehl T, Nunn D, Lory S. 1991. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect. Immun. 59:822–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ha U, Jin S. 2001. Growth phase-dependent invasion of Pseudomonas aeruginosa and its survival within HeLa cells. Infect. Immun. 69:4398–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fleiszig SM, Zaidi TS, Pier GB. 1995. Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect. Immun. 63:4072–4077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fleiszig SM, Evans DJ, Do N, Vallas V, Shin S, Mostov KE. 1997. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect. Immun. 65:2861–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Evans D, Kuo T, Kwong M, Van R, Fleiszig S. 2002. Pseudomonas aeruginosa strains with lipopolysaccharide defects exhibit reduced intracellular viability after invasion of corneal epithelial cells. Exp. Eye Res. 75:635–643 [DOI] [PubMed] [Google Scholar]
- 12. Hirakata Y, Yano H, Arai K, Endo S, Kanamori H, Aoyagi T, Hirotani A, Kitagawa M, Hatta M, Yamamoto N, Kunishima H, Kawakami K, Kaku M. 2010. Monolayer culture systems with respiratory epithelial cells for evaluation of bacterial invasiveness. Tohoku J. Exp. Med. 220:15–19 [DOI] [PubMed] [Google Scholar]
- 13. Emam A, Carter WG, Lingwood C. 2010. Glycolipid-dependent, protease sensitive internalization of Pseudomonas aeruginosa into cultured human respiratory epithelial cells. Open Microbiol. J. 4:106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcia-Medina R, Dunne WM, Singh PK, Brody SL. 2005. Pseudomonas aeruginosa acquires biofilm-like properties within airway epithelial cells. Infect. Immun. 73:8298–8305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Del Porto P, Cifani N, Guarnieri S, Di Domenico EG, Mariggio MA, Spadaro F, Guglietta S, Anile M, Venuta F, Quattrucci S, Ascenzioni F. 2011. Dysfunctional CFTR alters the bactericidal activity of human macrophages against Pseudomonas aeruginosa. PLoS One 6:e19970 doi:10.1371/journal.pone.0019970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmiedl A, Kerber-Momot T, Munder A, Pabst R, Tschernig T. 2010. Bacterial distribution in lung parenchyma early after pulmonary infection with Pseudomonas aeruginosa. Cell Tissue Res. 342:67–73 [DOI] [PubMed] [Google Scholar]
- 17. Kierbel A, Gassama-Diagne A, Mostov K, Engel JN. 2005. The phosphoinositol-3-kinase-protein kinase B/Akt pathway is critical for Pseudomonas aeruginosa strain PAK internalization. Mol. Biol. Cell 16:2577–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sana TG, Hachani A, Bucior I, Soscia C, Garvis S, Termine E, Engel J, Filloux A, Bleves S. 2012. The second type VI secretion system of Pseudomonas aeruginosa strain PAO1 is regulated by quorum sensing and Fur and modulates internalization in epithelial cells. J. Biol. Chem. 287:27095–27105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barcia-Macay M, Seral C, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. 2006. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob. Agents Chemother. 50:841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lemaire S, Kosowska-Shick K, Appelbaum PC, Verween G, Tulkens PM, Van Bambeke F. 2010. Cellular pharmacodynamics of the novel biaryloxazolidinone radezolid: studies with infected phagocytic and nonphagocytic cells, using Staphylococcus aureus, Staphylococcus epidermidis, Listeria monocytogenes, and Legionella pneumophila. Antimicrob. Agents Chemother. 54:2549–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hartkoorn RC, Chandler B, Owen A, Ward SA, Bertel Squire S, Back DJ, Khoo SH. 2007. Differential drug susceptibility of intracellular and extracellular tuberculosis, and the impact of P-glycoprotein. Tuberculosis (Edinb) 87:248–255 [DOI] [PubMed] [Google Scholar]
- 22. Carryn S, Chanteux H, Seral C, Mingeot-Leclercq MP, Van Bambeke F, Tulkens PM. 2003. Intracellular pharmacodynamics of antibiotics. Infect. Dis. Clin. North Am. 17:615–634 [DOI] [PubMed] [Google Scholar]
- 23. Van Bambeke F, Barcia-Macay M, Lemaire S, Tulkens PM. 2006. Cellular pharmacodynamics and pharmacokinetics of antibiotics: current views and perspectives. Curr. Opin. Drug Discov. Devel. 9:218–230 [PubMed] [Google Scholar]
- 24. Lemaire S, Van Bambeke F, Mingeot-Leclercq MP, Tulkens PM. 2005. Activity of three {beta}-lactams (ertapenem, meropenem and ampicillin) against intraphagocytic Listeria monocytogenes and Staphylococcus aureus. J. Antimicrob. Chemother. 55:897–904 [DOI] [PubMed] [Google Scholar]
- 25. Lemaire S, Van Bambeke F, Tulkens PM. 2011. Activity of finafloxacin, a novel fluoroquinolone with increased activity at acid pH, towards extracellular and intracellular Staphylococcus aureus, Listeria monocytogenes and Legionella pneumophila. Int. J. Antimicrob. Agents 38:52–59 [DOI] [PubMed] [Google Scholar]
- 26. Vallet CM, Marquez B, Ngabirano E, Lemaire S, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. 2011. Cellular accumulation of fluoroquinolones is not predictive of their intracellular activity: studies with gemifloxacin, moxifloxacin and ciprofloxacin in a pharmacokinetic/pharmacodynamic model of uninfected and infected macrophages. Int. J. Antimicrob. Agents 38:249–256 [DOI] [PubMed] [Google Scholar]
- 27. Sun HY, Fujitani S, Quintiliani R, Yu VL. 2011. Pneumonia due to Pseudomonas aeruginosa: part II: antimicrobial resistance, pharmacodynamic concepts, and antibiotic therapy. Chest 139:1172–1185 [DOI] [PubMed] [Google Scholar]
- 28. Clinical and Laboratory Standards Institute 2012. Performance standards for antimicrobial susceptibility testing. 22th informational supplement (MS100-S22). Clinical and Laboratory Standard Institute, Wayne, PA [Google Scholar]
- 29. Ouadrhiri Y, Scorneaux B, Sibille Y, Tulkens PM. 1999. Mechanism of the intracellular killing and modulation of antibiotic susceptibility of Listeria monocytogenes in THP-1 macrophages activated by gamma interferon. Antimicrob. Agents Chemother. 43:1242–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rajan S, Cacalano G, Bryan R, Ratner AJ, Sontich CU, van Heerckeren A, Davis P, Prince A. 2000. Pseudomonas aeruginosa induction of apoptosis in respiratory epithelial cells: analysis of the effects of cystic fibrosis transmembrane conductance regulator dysfunction and bacterial virulence factors. Am. J. Respir. Cell Mol. Biol. 23:304–312 [DOI] [PubMed] [Google Scholar]
- 31. Hritonenko V, Evans DJ, Fleiszig SM. 2012. Translocon-independent intracellular replication by Pseudomonas aeruginosa requires the ADP-ribosylation domain of ExoS. Microbes Infect. 14:1366–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garcia LG, Lemaire S, Kahl BC, Becker K, Proctor RA, Tulkens PM, Van Bambeke F. 2012. Influence of the protein kinase C activator phorbol myristate acetate on the intracellular activity of antibiotics against hemin- and menadione-auxotrophic small-colony variant mutants of Staphylococcus aureus and their wild-type parental strain in human THP-1 cells. Antimicrob. Agents Chemother. 56:6166–6174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Angus AA, Lee AA, Augustin DK, Lee EJ, Evans DJ, Fleiszig SM. 2008. Pseudomonas aeruginosa induces membrane blebs in epithelial cells, which are utilized as a niche for intracellular replication and motility. Infect. Immun. 76:1992–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lemaire S, Kosowska-Shick K, Appelbaum PC, Glupczynski Y, Van Bambeke F, Tulkens PM. 2011. Activity of moxifloxacin against intracellular community-acquired methicillin-resistant Staphylococcus aureus: comparison with clindamycin, linezolid and co-trimoxazole and attempt at defining an intracellular susceptibility breakpoint. J. Antimicrob. Chemother. 66:596–607 [DOI] [PubMed] [Google Scholar]
- 35. Lemaire S, Tulkens PM, Van Bambeke F. 2011. Contrasting effects of acidic pH on the extracellular and intracellular activities of the anti-gram-positive fluoroquinolones moxifloxacin and delafloxacin against Staphylococcus aureus. Antimicrob. Agents Chemother. 55:649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carryn S, Van Bambeke F, Mingeot-Leclercq MP, Tulkens PM. 2002. Comparative intracellular (THP-1 macrophage) and extracellular activities of beta-lactams, azithromycin, gentamicin, and fluoroquinolones against Listeria monocytogenes at clinically relevant concentrations. Antimicrob. Agents Chemother. 46:2095–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Amsden GW. 2009. Tables of antimicrobial agent pharmacology, p 705–764 In Mandell GL, Bennett JE, Dolin R. (ed), Principles and practice of infectious diseases. Churchill Livingstone, Elsevier, Philadelphia, PA [Google Scholar]
- 38. Bretonnière C, Jacqueline C, Caillon J, Guitton C, Le MV, Miegeville AF, Villers D, Potel G, Boutoille D. 2010. Efficacy of doripenem in the treatment of Pseudomonas aeruginosa experimental pneumonia versus imipenem and meropenem. J. Antimicrob. Chemother. 65:2423–2427 [DOI] [PubMed] [Google Scholar]
- 39. Michalopoulos AS, Falagas ME. 2011. Colistin: recent data on pharmacodynamics properties and clinical efficacy in critically ill patients. Ann. Intensive Care 1:30 doi:10.1186/2110-5820-1-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.