Abstract
BAL30072 is a monosulfactam conjugated with an iron-chelating dihydroxypyridone moiety. It is active against Gram-negative bacteria, including multidrug-resistant Pseudomonas aeruginosa. We selected mutants with decreased susceptibilities to BAL30072 in P. aeruginosa PAO1 under a variety of conditions. Under iron-deficient conditions, mutants with overexpression of AmpC β-lactamase predominated. These mutants were cross-resistant to aztreonam and ceftazidime. Similar mutants were obtained after selection at >16× the MIC in iron-sufficient conditions. At 4× to 8× the MIC, mutants with elevated MIC for BAL30072 but unchanged MICs for aztreonam or ciprofloxacin were selected. The expression of ampC and the major efflux pump genes were also unchanged. These BAL30072-specific mutants were characterized by transcriptome analysis, which revealed upregulation of the Fe-dicitrate operon, FecIRA. Whole-genome sequencing showed that this resulted from a single nucleotide change in the Fur-box of the fecI promoter. Overexpression of either the FecI ECF sigma factor or the FecA receptor increased BAL30072 MICs 8- to 16-fold. A fecI mutant and a fecA mutant of PAO1 were hypersusceptible to BAL30072 (MICs < 0.06 μg/ml). The most downregulated gene belonged to the pyochelin synthesis operon, although mutants in pyochelin receptor or synthesis genes had unchanged MICs. The piuC gene, coding for a Fe(II)-dependent dioxygenase located next to the piuA iron receptor gene, was also downregulated. The MICs of BAL30072 for piuC and piuA transposon mutants were increased 8- and 16-fold, respectively. We conclude that the upregulation of the Fe-dicitrate system impacts the expression of other TonB-dependent iron transporters and that PiuA and PiuC contribute to the susceptibility of P. aeruginosa PAO1 to BAL30072.
INTRODUCTION
The outer membranes of Gram-negative bacteria present very efficient permeability barriers that limit the access of many antibiotics to their targets. This potentiates enzymatic and efflux-mediated mechanisms of resistance. In the absence of novel antimicrobial classes, recent efforts have focused on optimizing the efficiency of existing and well-characterized classes of antimicrobials. An obvious issue is to increase the local (intracellular) concentration of these molecules at their target site. For instance, β-lactamase inhibitors efficiently reduce hydrolysis of certain β-lactam antibiotics (1), while efflux pump inhibitors block the activity of multidrug efflux pumps, thus increasing the cytoplasmic (or periplasmic) antibiotic concentration (2, 3). Another option is to subvert the function of efficient and specialized transport systems to increase the uptake of antimicrobials. This “Trojan Horse” strategy (4, 5) is exemplified by natural siderophore-antibiotic conjugates such as salmycins, albomycins (6), ferrimycins, and microcins (7) produced by various microorganisms. Inspired by these structures, synthetic siderophore conjugates have been designed in the past mainly as a combination between β-lactams and a catechol substituent to promote uptake through dedicated siderophore receptors (8–11).
A recently developed example of a siderophore/β-lactam conjugate is the monosulfactam antibiotic BAL30072 (5). This monocyclic β-lactam contains an iron-chelating dihydroxypyridone substituent. The molecule has potent in vitro activity against many Gram-negative bacteria (12, 13) and is in clinical development for the treatment of infections caused by Gram-negative bacilli, including multidrug-resistant P. aeruginosa.
Here, we investigated the possibility of resistance emergence in the P. aeruginosa reference strain PAO1. Using a transcriptome approach, we identified the FecIRA Fe3+-dicitrate transporter as a regulatory cascade and the PiuA iron receptor as a putative uptake system affecting susceptibility to BAL30072.
(This study was presented in part at the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, abstr. F1-144, 17 to 20 September 2011.)
MATERIALS AND METHODS
Bacterial strains and growth.
The strains, plasmids, and primers used in the present study are listed in Table 1. Bacterial strains were routinely grown in Luria-Bertani (LB) medium at 37°C with shaking (220 rpm). Experiments with controlled ferric iron concentrations were performed in IsoSensiTest broth (IST), using 2,2′-bipyridyl (BPL) at 16 μg/ml to scavenge free iron if necessary. MICs were determined in Mueller-Hinton (MH) broth according to Clinical and Laboratory Standards Institute guidelines. Determinations of MICs were repeated on at least three different occasions.
Table 1.
Bacterial strains, plasmids, and primers
| Strain, plasmid, or primer | Relevant characteristics or sequence (5′–3′)a | Source or reference |
|---|---|---|
| Strains | ||
| PT5 | PAO1, mexT nonfunctional | Laboratory collection |
| PAO1-FV | PAO1, mexT functional | M. Camara (Nottingham, United Kingdom) |
| LESB58 | Lacks piuA | C. Winstanley (Liverpool, United Kingdom) |
| 39016 | Lacks piuA | C. Winstanley (Liverpool, United Kingdom) |
| MPAO1fecA | fecA (PA3901)::TnphoA, Tetr | 24 |
| MPAO1nirJ | nirJ::TnlacZ, Tetr | 24 |
| MPAO1piuA | piuA (PA4514)::TnlacZ, Tetr | 24 |
| MPAO1piuC | piuC (PA4515)::TnlacZ, Tetr | 24 |
| MPAO1fecI | fecI (PA3899)::TnlacZ, Tetr | 24 |
| MPAO1femA | femA (PA1910)::TnlacZ, Tetr | 24 |
| MPAO1fiuA | fiuA (PA0470)::TnlacZ, Tetr | 24 |
| MPAO1fiuA | pfuA (PA1322)::TnlacZ, Tetr | 24 |
| PAO1-furA4 | Fur H86Y, constitutive siderophore expression | 29 |
| Plasmids | ||
| pIApX2 | Broad-host-range expression vector, Apr | I. Attree (Grenoble, France) |
| pfecI | fecI PCR fragment of PT5 cloned in pIApX2, Apr | This study |
| pfecA | fecA PCR fragment of PT5 cloned in pIApX2, Apr | This study |
| Primers | ||
| FecI-Bam | CCGGGATCCTAATCCTTCTGCTCCGGGAAT | This study |
| FecI-Hind | CCGAAGCTTACGGCCTGTTCAGCCACCTG | This study |
| FecA-Bam | CCGGGATCCGTGCTACCTGTGGAGGTACGG | This study |
| FecA-Hind | CCGAAGCTTGCTGCTGATGAAACAGTTGGAG | This study |
Restriction sites in the primer sequences are underlined. Tetr, tetracycline resistance; Apr, ampicillin resistance.
Construction of expression plasmids.
The coding regions of fecI and fecA were amplified by PCR from cell lysates of strain PT5 (PAO1) using the primers listed in Table 1. The PCR conditions were as follows: denaturation at 95°C for 2 min, followed by 27 cycles of 95°C for 20 s, 57°C for 30 s, and 72°C for 2 min and a final extension at 72°C for 4 min. The fragments were digested with BamHI and HindIII (fecI, fecA) and ligated to plasmid pIApX2 cleaved with the appropriate restriction enzymes, yielding the plasmids pfecI and pfecA, respectively. Plasmids were transferred into P. aeruginosa by electroporation.
Microarray analysis and genome sequencing.
Gene expression was determined in exponentially growing cells in LB or MH medium. RNA was extracted from exponentially growing cells (optical density at 600 nm of 2.0) in LB medium using the RNeasy kit (Qiagen), and cDNA was prepared by reverse transcription (RT; ImPromII; Promega) of 500 ng of RNA. For quantitative RT-PCR (qRT-PCR), expression of genes was normalized with respect to the housekeeping gene rpsL and expressed as the fold change compared to expression in strain PAO1. The amount of cDNA copies was determined with standard curves of PAO1 genomic DNA in a RotorGene 3000 real-time PCR machine using the QuantiTect-Sybr mix (Qiagen) and gene-specific primers.
For comparative transcriptome analysis, three separate cultures and three Affymetrix microarrays were used for each strain or condition. Quality of RNA was verified on a Bioanalyzer (Agilent). Preparation of cDNA and hybridization of arrays was performed according to the Affymetrix protocol by the Genomics Platform at the University of Geneva. The data were processed using Affymetrix MAS 5.0 and analyzed with the analysis of variance model in Partek Genomics suites 6.5 (Partek, Inc., St. Louis, MO). Differences in gene expression ratios of >2.0 or of <−2.0 with a P value of <0.05 were considered significant. All microarray data are available through ArrayExpress (http://www.ebi.ac.uk/arrayexpress/, accession no. E-MTAB-1381).
Whole-genome sequencing of mutant BAL6 and the susceptible parental strain PT5 (PAO1) was performed on an Illumina Analyzer at Fasteris SA (Geneva, Switzerland). Assembly of the reads was done on the reference PAO1 genome (NC_002516, www.pseudomonas.com).
β-Lactamase activity assay.
The β-lactamase activity in clarified cell extracts was measured using nitrocefin and normalized with respect to total protein (14).
RESULTS
Mutant selection.
We investigated the possibility of resistance emergence in the P. aeruginosa strain PAO1 under various conditions of iron availability. Colonies growing in the presence of 16, 20, 25, 32, 40, 48, or 64 μg of BAL30072/ml were selected on IST medium at frequencies of 10−8 to 10−7 in the presence of BPL (free ferrous iron at ca. 10 to 12 M) and 10−9 to 10−8 in the presence of 10−6 M ferrous iron. Among 11 colonies from the iron-deficient conditions and 8 colonies from the iron-supplemented condition, all had elevated expression of AmpC β-lactamase and were cross-resistant to aztreonam (strains PAO1-M1, -M3, -M9, and -M18 in Table 2) and ceftazidime (data not shown). In contrast, no mutants with efflux pump overexpression (as indicated by resistance to ciprofloxacin) were identified.
Table 2.
MICs for PAO1 and mutants selected on BAL30072
| Strain | Growth mediuma | Selective agent (μg/ml)b | MIC (μg/ml)c |
Relative nitrocefin hydrolysis | |||
|---|---|---|---|---|---|---|---|
| BAL | ATM | MEM | CIP | ||||
| PAO1 | NA | NA | 1 | 4 | 1 | 0.125 | 1.0 |
| PAO1-M1 | IST + BPL | BAL (20) | 32 | >32 | 1 | 0.125 | 5.1 |
| PAO1-M3 | IST + AFC | BAL (20) | 32 | >32 | 1 | 0.125 | 7.3 |
| PAO1-M9 | IST + BPL | BAL (25) | >32 | >32 | 1 | 0.125 | 16 |
| PAO1-M18 | IST + AFC | BAL (25) | >32 | >32 | 1 | 0.125 | 19 |
| BAL5 | LB | BAL (4) | 16 | 4 | 1 | 0.125 | 0.7 |
| BAL6 | LB | BAL (4) | 16 | 4 | 1 | 0.125 | 0.9 |
| BAL13 | LB | BAL (8) | 16 | 4 | 1 | 0.125 | 0.8 |
| BAL14 | LB | BAL (8) | 16 | 4 | 1 | 0.125 | 1.1 |
| PAO1-FV | NA | NA | 16 | 4 | 2 | 0.25 | 1.2 |
| PAO1-FV16 | IST + BPL | BAL (75) | >64 | 32 | 2 | 0.25 | 8.1 |
| PAO1-FV25 | IST + BPL | ATM (8) | 16 | 32 | 16 | 1 | 1.9 |
Colonies of strain PAO1 able to grow on LB medium in the presence of lower BAL30072 concentrations (4 or 8 μg/ml) were selected at frequencies of 10−8 to 10−7. Two colonies selected on 4 μg/ml (BAL5 and BAL6) and 8 μg/ml (BAL13 and BAL14) BAL30072 were retained for further analysis (Table 2). MIC determinations showed that only BAL30072 susceptibilities were affected in the mutants, whereas the MICs of aztreonam, the parental molecule of BAL30072, and those of ciprofloxacin were unchanged (Table 2). Since the Mex efflux pumps of P. aeruginosa are able to extrude aztreonam (MexAB-OprM) and ciprofloxacin (MexAB-OprM, MexCD-OprJ, MexXY, MexEF-OprN), the unchanged MICs of these molecules in the four mutants tested suggested that efflux pumps are not involved in decreased susceptibility to BAL30072. Indeed, the expressions of the efflux pump genes mexA, mexC, mexE, and mexX were similar (<2-fold change) between PAO1 and mutant BAL6 strains (http://www.ebi.ac.uk/arrayexpress/, accession no. E-MTAB-1381). The expression of ampC, encoding the chromosomal cephalosporinase, was also not affected in mutant BAL6, confirming that ampC overexpression did not contribute to increased BAL30072 MICs in the mutants selected at a low BAL30072 concentration.
Colonies of the strain PAO1-FV, which has a functional mexT gene and an elevated MIC for BAL30072 (Table 2), growing on IST medium in the presence of BPL (free ferrous iron at ∼10−12 M) in the presence of high BAL30072 concentrations (32 to 100 μg/ml) were selected at a frequency of 10−6. The most resistant colonies, exemplified by strain PAO1-FV16 (Table 2), had 5- to 8-fold increased activities of AmpC β-lactamase and were cross-resistant to aztreonam. The ciprofloxacin MIC was similar to the parental strain, suggesting that the MexEF-OprN efflux pump, although overexpressed, is not active. Parallel selection using aztreonam, under otherwise identical conditions, resulted in mutants with unchanged MICs for BAL30072 but elevated MICs for aztreonam, meropenem, and ciprofloxacin, which is consistent with activation of the MexAB-OprM efflux pump (strain PAO1-FV25 in Table 2). The unchanged MIC for BAL30072 of mutant PAO1-FV25 suggests that the efflux pumps do not contribute to the elevated MIC of BAL30072 and that one of the other systems under the control of MexT (15) is responsible for the intrinsically elevated MIC of PAO1-FV.
Transcriptome analysis.
Transcriptome analysis of the mutant BAL6 to clarify resistance mechanisms was performed. A total of 88 genes were significantly up- or downregulated in mutant BAL6 versus PAO1 (Fig. 1). The most downregulated gene was pchB (−6.5-fold) from the pyochelin siderophore biosynthesis operon (Table 3). qRT-PCR confirmed repression of the pyochelin operon genes ranging from 2-fold (pchR) to 10-fold (fptA) in strains BAL6 and BAL13 compared to PAO1 (data not shown). However, BAL30072 MICs for a pyochelin receptor mutant (fptA) and for a pyochelin regulator mutant (pchR) of PAO1 were unchanged (MIC of 1 μg/ml), suggesting that deficiency in pyochelin uptake or synthesis alone should not be responsible for the decreased BAL30072 susceptibilities. The only other downregulated gene in BAL6, linked directly to iron metabolism, was PA4515 (piuC) belonging to a family of Fe(II)-dependent oxygenases (Prosite PDOC51471) (Fig. 2). Next to piuC, but transcribed in the opposite direction, was the open reading frame PA4514, annotated as piuA and coding for a putative outer membrane iron receptor. The role of PiuA and PiuC in BAL30072 susceptibility is described below.
Fig 1.
Venn diagram established using the Partek Genomics Suite program shows differentially expressed genes between the three conditions. “+” indicates that the strain was grown in the presence of 4 μg of BAL30072/ml.
Table 3.
Genes most induced and repressed in mutant BAL6 versus PAO1
| Gene | Name | Description | Fold change (BAL6 vs PAO1) |
|---|---|---|---|
| Induced | |||
| PA3901 | fecA | Fe(III) dicitrate transport | 26.9 |
| PA0513 | nirG | Transcriptional regulator | 5.9 |
| PA3899 | fecI | ECF sigma factor | 5.7 |
| PA3394 | nosF | ABC transport family | 5.1 |
| PA2171 | Di-iron sulfur repair protein | 5.1 | |
| PA4648 | cupE1 | Pilin subunit E1 | 5.0 |
| PA1914 | Hvn halovibrin | 4.9 | |
| PA0515 | nirD | transcriptional regulator | 4.8 |
| PA1874 | Hypothetical, biofilm resistance antibiotics | 4.7 | |
| PA0510 | nirE | Heme d1 biosynthesis | 4.7 |
| PA0511 | nirJ | Heme d1 biosynthesis | 4.7 |
| PA0514 | nirL | Heme d1 biosynthesis | 4.5 |
| PA2146 | Hypothetical, stress-induced protein motif | 4.5 | |
| PA4306 | Flp pilus assembly | 4.3 | |
| PA0509 | nirN | Probable c-type cytochrome | 4.1 |
| Repressed | |||
| PA4230 | pchB | Salicylate biosynthesis | –6.5 |
| PA5445 | Acetyl-CoA hydrolase | –3.5 | |
| PA2761 | Hypothetical | –2.8 | |
| PA2353 | Hypothetical | –2.8 | |
| PA2624 | Isocitrate dehydrogenase | –2.7 | |
| PA4515 | piuC | Fe(II)-dependent hydroxylase | –2.6 |
| PA1183 | dctA | C4-dicarboxylate transport | –2.5 |
Fig 2.
Alignment of the piu locus in Gram-negative organisms. PiuA is the TonB-dependent siderophore receptor, and PiuC contains the signature for a Fe(II)-dependent dioxygenase. The genes piuA (black arrows), piuC (light gray arrows), and piuB (dark gray arrows) are preceded by Fur-boxes in P. aeruginosa, suggesting their upregulation under iron-depleted conditions. PA4516 homologues (white arrows) are present in Azotobacter vinelandii and Burkholderia spp. PA numbers are shown as reference for the PAO1 genome (www.pseudomonas.com). The gene alignment was obtained with the SEED view program (http://www.theseed.org/wiki/Main_Page).
The most upregulated genes in BAL6 compared to PAO1 were fecA (26.9-fold) and fecI (5.7-fold) coding for the Fe3+-dicitrate outer membrane receptor (16) and a putative extracytoplasmic function (ECF) sigma factor, respectively (Table 3 and Fig. 3). Genes of the heme d1 biosynthesis operon (PA0510-PA0518) required for denitrification (17) were also upregulated (3.8- to 5.9-fold) in mutant BAL6 (Table 3 and see Table S1 in the supplemental material). Another group of upregulated genes are involved in Flp pilus assembly (PA4306, tadD) and the synthesis of cup fimbria genes (cupE1 to cupE5) (18).
Fig 3.
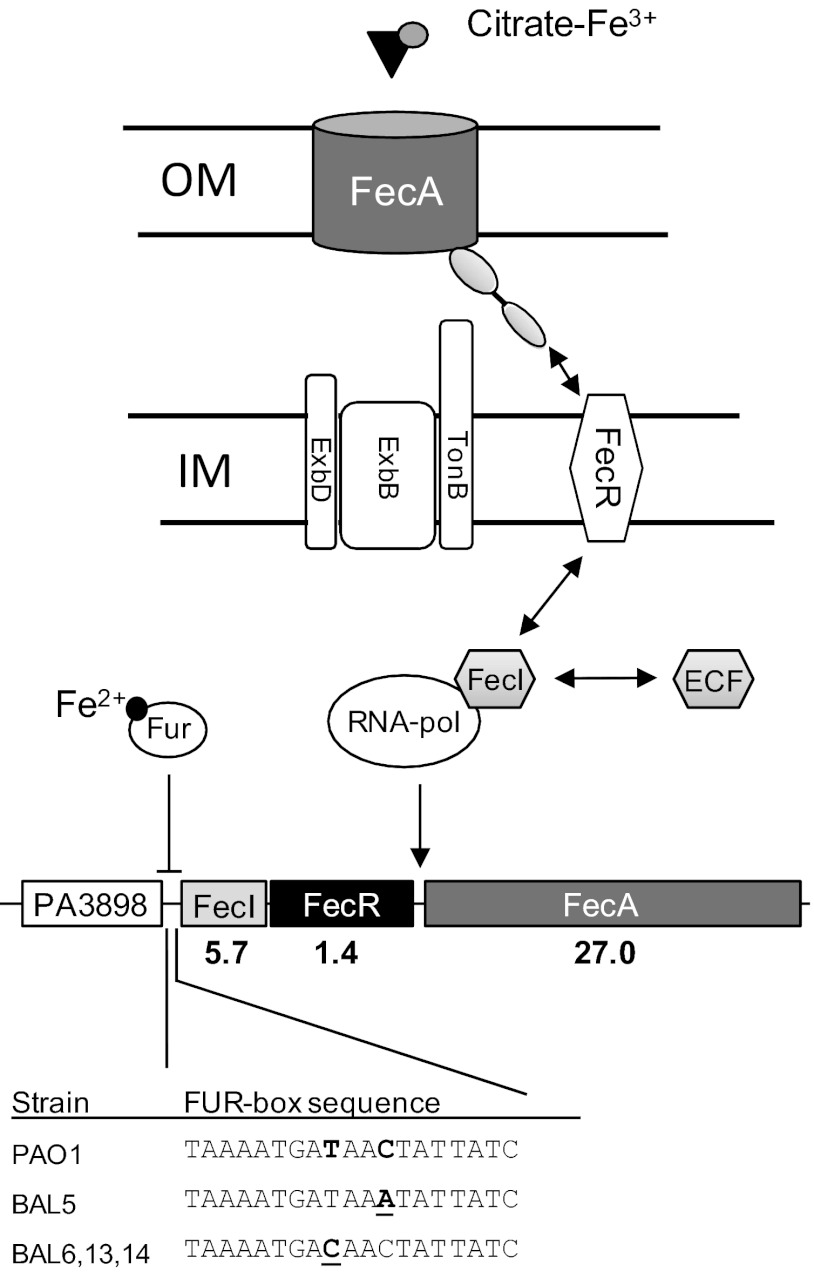
fecIRA operon in P. aeruginosa PAO1. Mutations in the Fur-box motif upstream of fecI identified in the selected mutants are shown below. Numbers indicate changes in gene expression for the fecIRA genes, determined by microarray analysis. OM, outer membrane; IM, inner membrane; ECF, extracytoplasmic function sigma factor.
Genes affected by BAL30072.
The Venn diagram identified a set of 27 genes that were differentially regulated (P < 0.05) in mutant BAL6 when grown in the presence of BAL30072 (BAL6+; 4 μg/ml) (Fig. 1 and Table 4). The most upregulated gene was hcpC, coding for the Hcp1 effector protein secreted by the type VI secretion system of P. aeruginosa (19). A LysR-type transcriptional regulator (PA0191), showing 59% amino acid similarity to the protocatechuate-inducible PcaQ regulator of Pseudomonas sp., and the putative taurine transporter (tauA, PA3938) (20) were also induced in the presence of BAL30072.
Table 4.
Genes affected by BAL30072 in PAO1 and mutant BAL6
| Gene | Name | Description | Fold change |
||
|---|---|---|---|---|---|
| BAL6+ vs BAL6 | BAL6+ vs PAO1 | BAL6 vs PAO1 | |||
| Induced | |||||
| PA0263 | hcpC | Type VI secreted protein Hcp1 | 6.0 | 6.0 | 1.0 |
| PA0201 | Esterase | 5.7 | 5.6 | 1.0 | |
| PA0284 | Hypothetical | 5.5 | 6.1 | 1.1 | |
| PA0191 | LysR-type transcriptional regulator | 4.8 | 4.5 | –1.1 | |
| PA0525 | norD | Denitrification | 4.5 | 3.9 | –1.2 |
| PA3938 | tauA | Periplasmic Bdg protein | 4.0 | 4.2 | 1.1 |
| PA2204 | ABC-type amino acid transporter | 4.0 | 2.5 | –1.6 | |
| PA3450 | Peroxiredoxin (Prx) family | 3.9 | 3.8 | 1.0 | |
| PA4195 | ABC-type amino acid transporter | 3.9 | 4.0 | 1.0 | |
| PA0283 | sbp | ABC-type sulfate transporter | 3.6 | 3.4 | –1.1 |
| Repressed | |||||
| PA0638 | F-pyocin | –6.1 | –4.3 | 1.4 | |
| PA0617 | R-pyocin | –6.0 | –5.1 | 1.2 | |
| PA0613 | R-pyocin | –5.4 | –2.7 | 2.0 | |
| PA0633 | F-pyocin | –4.7 | –3.8 | 1.2 | |
| PA3038 | opdQ | OpdK porin subfamily | –4.5 | –8.1 | –1.8 |
| PA0985 | Pyocin S5 | –4.1 | –3.7 | 1.1 | |
| PA3509 | Hydrolase | –4.0 | –4.2 | 1.0 | |
| PA4501 | opdD | OpdK porin subfamily | –3.7 | –4.3 | –1.2 |
Among the BAL30072-repressed genes (3- to 6-fold) are those belonging to the R and F pyocin operons (PA0613 to PA0648), as well as the pyocin S5 gene (PA0985) (21). We further identified OpdQ and OpdD, both members of the OpdK porin subfamily (22), whose genes were significantly downregulated in the presence of BAL30072 (Table 4). The expression of the genes listed in Table 4 was unchanged between the mutant BAL6 and PAO1, suggesting that differences in expression resulted only from antibiotic exposure. A complete list of the gene expression profile is available in Table S1 in the supplemental material.
Regulation by the fecIRA operon Fe-dicitrate system.
The fecI and fecA genes were the most upregulated genes in mutant BAL6. They form the fecIRA operon, which is under the control of the Fur repressor protein in P. aeruginosa (23) (Fig. 3). We suspected that increased expression of fecI and fecA could result from mutation(s) in the structural genes or in the promoter regions. The entire 5-kbp region in mutant BAL6 was sequenced. The only nucleotide difference was a T-to-C transition upstream of the fecI gene. The mutation is located within the putative Fur box (Fig. 3). Sequencing of the fecI promoter region revealed the same T-to-C transition in mutants BAL13 and BAL14 (selected on 8 μg/ml), whereas a C-to-A transversion was found in the Fur-box of strain BAL5 (Fig. 3). In parallel to the microarray analysis, we initiated the sequencing of the genomes of mutant BAL6 and of the parental PAO1 strain. The only confirmed difference between the two strains was the T-to-C transition in the Fur-box motif. Our data suggest that the mutations identified in the Fur-box of the selected mutants prevent Fur binding, leading to constitutive and Fe-independent expression of the fecIRA operon.
To further analyze the role of FecI and FecA, we cloned separately the corresponding genes in an expression vector, yielding plasmids pfecI and pfecA, respectively. When transferred into the susceptible strain PAO1, both plasmids increased the MICs of BAL30072 (Table 5); however, fecI expression yielded the same BAL30072 MICs as for BAL6 (32 μg/ml), while pfecA overexpression increased MICs by 8-fold. MICs of aztreonam were not affected, suggesting that the FecIRA system affects specifically the activity of the siderophore-drug conjugate. We also introduced pfecI in P. aeruginosa strain PA14. MICs were increased to the same level as in PAO1 (data not shown), suggesting that the fecI-mediated BAL30072 resistance was not specific to PAO1. Since the mutation in the fecI Fur-box was the only mutational event in BAL6, we hypothesized that the majority of the genes whose expression was affected in BAL6 were controlled by the FecIRA system, some of which could be directly regulated by the FecI ECF sigma factor.
Table 5.
MICs for receptor mutants and complemented PAO1 strains
| Strain | MIC (μg/ml)a |
||
|---|---|---|---|
| BAL | ATM | BAL + 0.25 mM BPLb | |
| PT5 (PAO1) | 1 | 4 | 0.5 |
| BAL6 | 32 | 4 | 16 |
| PAO1(pIApX2) | 1 | 4 | 0.5 |
| PAO1(pfecI) | 32 | 2 | 16 |
| PAO1(pfecA) | 8 | 2 | 1 |
| MPAO1fecA(pIApX2) | <0.06 | 2 | ND |
| MPAO1fecA (pfecI) | <0.06 | 4 | ND |
| MPAO1fecA (pfecA) | 8 | 4 | ND |
| MPAO1fecI | <0.06 | 4 | ND |
| MPAO1piuA | 8–16 | 8 | 8 |
| MPAO1piuC | 16 | 4 | 8 |
| MPAO1femA | 1 | 4 | 0.5 |
| MPAO1pfuA | 1 | 4 | 0.5 |
| MPAO1fiuA | 1 | 4 | 0.5 |
| PAO1furA4 | <0.06 | 2 | ND |
| PAO1ΔfptA | 1 | 4 | 1 |
| PAO1ΔpchR | 1 | 4 | 1 |
The MICs for strain MPAO1 were identical to those for PAO1 strain PT5.
BPL, 2,2′-bipyridyl. ND, not determined.
Mutants in the FecIRA system are hypersusceptible to BAL30072.
To further explore the role of the FecIRA system, we tested the susceptibilities of a fecA and a fecI mutant against BAL30072. Both mutants were hypersusceptible to the sulfactam (MICs < 0.06 μg/ml), whereas aztreonam MICs were not affected (Table 5). The Fur mutant FurA4, which expresses constitutively iron-repressed genes, showed the same hypersusceptibility to BAL30072. This suggests that under iron depletion, overexpression of one or several of the Fur-controlled iron receptors could lead to increased BAL30072 uptake. Determination of BAL30072 MICs in the presence of the iron chelator 2,2′-bipyridyl, however, decreased MICs only 2-fold with the notable exception of the pfecA overexpressing wild-type strain showing 8-fold reduced MICs (Table 5).
PiuA and PiuC involved in BAL30072 susceptibility.
Based on the reduced expression of piuC in mutant BAL6 identified in the microarray analysis (Table 3), we hypothesized that PiuA and PiuC could be involved in BAL30072 uptake or metabolism. We therefore tested the activity of BAL30072 in a piuA and a piuC mutant from the PAO1 Tn5 mutant library (24). We further tested mutants in other TonB-dependent iron-uptake systems of P. aeruginosa, including FemA (PA1910), PfuA (PA1322), FiuA (PA0470), and CirA (PA1922), and the latter two were shown to play a role in siderophore-β-lactam uptake into Escherichia coli (25). Interestingly, MICs of BAL30072 were increased 8- to 16-fold for piuA and 16-fold for the piuC mutant, while the other iron-receptor mutants had unchanged susceptibilities (Table 5). Aztreonam activities were not affected in the mutants with the exception of piuA, which showed a weak but reproducible 2-fold increase compared to PAO1 (8 μg/ml versus 4 μg/ml). PiuA is a putative TonB-dependent siderophore receptor which bears significant amino acid sequence identity (35% identity, 50% similarity) with the Fiu hydroxamate receptor of E. coli. Although the piuC gene is present in all eight completely sequenced P. aeruginosa strains, the piuA gene is present in only 50% (4/8) of them (PAO1, PA14, PACS2, and 2192). The susceptibility of two of the strains lacking piuA (LESB58 and 39016) toward BAL30072 was independent of the induction state of the iron transport systems (Table 6), whereas the susceptibility of strains with piuA shows some degree of dependency on iron concentration (Tables 5 and 6).
Table 6.
BAL30072 MICs for P. aeruginosa strains with or without the PiuA protein in different states of induction of the iron uptake systems
| Strain | Presence (+) or absence (–) of PiuA | MIC (μg/ml) |
|
|---|---|---|---|
| Repressed (20 μM Fe3+) | Induced (1 mM BPL) | ||
| PAO1 | + | 8 | 0.5 |
| PA14 | + | 4 | 0.25 |
| LESB58 | − | 2 | 2 |
| 39016 | − | 1 | 1 |
In the intergenic region between piuC and piuA of PAO1 are two Fur-box motifs, one in the direction of piuA (GCCAATGATATTGATTTGC) and the other one upstream of piuC (GCAAATCAATATCATTGGC), suggesting that both genes are under the control of the Fur repressor protein (26). The piuA and piuC genes are also linked in other Gram-negative organisms, suggesting that both proteins work in concert. In P. aeruginosa, as well as in Azotobacter vinelandii and Burkholderia species, a third gene with unknown function (an orthologue of PA4516) is found adjacent to piuC forming an operon (Fig. 2). In P. aeruginosa and A. vinelandii a gene annotated as piuB and preceded by a Fur-box motif (TTTATCGCAACTGATTATC) is located downstream of piuA but probably transcribed independently. PiuB contains a sulfite reductase domain and is predicted to be localized in the cytoplasmic membrane. Our data suggest that the thus-far-uncharacterized PiuA/PiuC siderophore transport system of P. aeruginosa is involved in the specific uptake of BAL30032 and perhaps other siderophore-drug conjugates (28).
DISCUSSION
In this study, the evolution of resistance to the siderophore monosulfactam BAL30072 was investigated. Under iron limitation and at high antibiotic concentrations, mutants with high-level resistance due to overexpression of the chromosomal β-lactamase was observed, as expected from previous studies (14). Moya et al. reported ceftazidime-resistant mutants of PAO1 obtained with similar frequency and phenotype that were due to inactivation of PBP4 (27). There was no evidence for the selection of mutants overexpressing any of the Mex efflux systems, although parallel experiments with efflux substrates such as aztreonam rendered such mutants. Although PAO1 derivatives with efflux pump deletions were previously shown to become hypersusceptible to BAL30072 (14), it might be that this is due to side effects of the pump deletion on regulation and not to the decreased activity of the pump itself.
Since iron-limiting conditions might be expected to disfavor the selection of mutants defective in iron siderophore uptake, we also selected for mutants in defined medium supplemented with ferrous iron present and in rich medium (LB broth). The mutation frequency was similar under the different conditions, and high-level resistance was still predominantly due to mutants with increased levels of β-lactamase. At lower antibiotic concentrations (4 and 8 μg/ml), mutants that were specifically resistant to BAL30072, without overexpression of β-lactamase, were observed on LB medium. Transcriptome analysis of one of these mutants (BAL6) identified the FecIRA Fe3+-dicitrate transporter and the putative TonB-dependent Fe receptor PiuA as key elements in this context. Classical resistance mechanisms such as efflux pumps, as well as AmpC overexpression, could be excluded.
The FecIRA system of P. aeruginosa (16) is similar to the FecIRA Fe3+-dicitrate uptake system in E. coli, which is citrate inducible and transports Fe3+ dicitrate into the periplasm (28). In. E. coli, the FecI ECF sigma factor is bound to the regulator FecR under noninducing conditions. Upon citrate induction, the N-terminal extension of FecA interacts with FecR, leading to signaling and the release of FecI ECF sigma factor, which subsequently binds RNA-polymerase and increases fecA expression. FecI expression is repressed under iron-replete conditions by the Fur repressor (Fig. 3). In BAL mutants, the repression of fecI by FUR is relieved due to mutations in the Fur-box, causing constitutive activation of the FecIRA signaling cascade.
How does this activation cause resistance to the siderophore-drug conjugate? We hypothesize that FecI has to compete with other iron receptor-regulating ECFs for binding to RNA-polymerase (Fig. 3). P. aeruginosa possesses 21 TonB-dependent iron receptors of which 11 have adjacent ECF sigma factor genes (26). When FecI is overexpressed from a plasmid, it probably outcompetes other ECF sigma factors and prevents induction of the cognate iron-receptors which could promote uptake of BAL30072 and other drug-siderophore conjugates. The surprising observation that both a fecA and a fecI transposon mutant were hypersusceptible to this molecule favors this hypothesis. Both mutants were as susceptible as the Fur mutant, which constitutively expresses iron receptors (29). This finding is reminiscent of a fecA mutant of E. coli, which was shown to be more susceptible than the wild type to the synthetic dihydroxypyridone-conjugated cephem KP-736 (MICs of 0.006 versus 0.024 for the wild-type strain) (8, 30) and to the catechol-conjugated monobactam BMS-180680 (MICs of 0.0005 versus 0.015 for the wild type) (10). Interestingly, a fecA mutant of E. coli was shown in a separate study to overexpress several siderophore receptors (Cir, FhuA, FhuE, FepA, and four other unidentified iron receptors) (31). Since KP-736 and BMS-180680 are taken up via Cir and Fiu receptors in E. coli (10, 30), overexpression of these proteins in a fecA mutant can explain its hypersusceptibility to these conjugates. These data are in agreement with our model on ECF sigma factor competition for binding to RNA-polymerase. Alternatively, overexpression of FecA could titrate the TonB system, which would decrease the amount of TonB available to energize other iron transport systems, including PiuA.
Homologues of Cir and Fiu are annotated on the genome of P. aeruginosa as CirA (FeuA, PA1922) and FiuA (PA0470), respectively, and are present in all eight sequenced strains. However, susceptibilities to BAL30072 for the cirA (PA1922) and fiuA (PA0470) mutants were not affected. This could result from the moderate overall amino acid identity between the E. coli and P. aeruginosa Cir and Fiu homologues of 35 and 37%, respectively. In comparison, the FecA receptor proteins of the two organisms share 63% amino acid identity, suggesting that the Fe-dicitrate system has maintained an important regulatory role in evolution. Our transcriptome analysis identified the uncharacterized PiuA receptor and the adjacent piuC gene, coding for a putative Fe(II) 2-ketoglutarate dioxygenase (Prosite PDOC51471), as the most likely uptake system for BAL30072 in P. aeruginosa. The closest homologue of PiuA in E. coli is Fiu with 35% amino acid identity, whereas PiuC shows 49% identity with the uncharacterized oxygenase YbiX. Interestingly, this family of dioxygenases also contains enzymes that participate in the biosynthesis of penicillins and cephalosporins in Streptomycetes (32). Whether PiuC is able to modify or inactivate β-lactams remains to be determined. MICs for the piuA and piuC mutants were close to those of the BAL6 mutant. Interestingly, the PAO1-FV variant with a functional MexT that has similarly elevated BAL30072 MIC, should have derepressed expression of the MexS and XenB oxidoreductases (33).
We cannot exclude that other iron receptors, not tested here, or a simultaneous knockout of the tested receptors would also affect susceptibilities, as observed for a cir-fiu double mutant in E. coli (30). However, the fact that in the presence of 2,2′-bipyridyl the BAL30072 MICs decreased only marginally suggest that the PiuA-PiuC system is the major uptake pathway for BAL30072 in the iron-sufficient conditions used here. The PiuA receptor was recently reported to contribute to resistance of PAO1 toward another siderophore-conjugated β-lactam, MC-1 (34). In strains lacking the PiuA receptor, the MIC of MC-1 was raised 32-fold in iron-sufficient medium, compared to the 8- to 16-fold increase observed for BAL30072 in a similar specific deletion. A BLAST analysis within the pseudomonas.com website reveals that the piuA gene is present in only four (PA14, PAO1, PACS2, and 2192) of the eight completely sequenced P. aeruginosa genomes, suggesting that different P. aeruginosa isolates may display different sensitivity toward induction of iron uptake systems or resistance selection frequencies to BAL30072.
The transcriptome analysis further allowed us to observe the effect of BAL30072 on gene transcription. Genes induced by the monosulfactam include a sulfate (sbp) and the taurine ABC-type transport systems. The TauABC transporters in E. coli catalyze the uptake of periplasmic taurine (2-aminoethanesulfonic acid), which can be used by bacteria as a sulfur source (20). A sulfonic acid group is present on BAL30072; however, whether it promotes uptake of BAL30072 from the periplasm into the cytoplasm or contributes to the resistance phenotype was not investigated. Induction could also result from a general overlap between iron depletion and sulfate starvation (26). Among the genes repressed by BAL30072 are those of the S, R, and F pyocins. Interestingly, these genes were also downregulated when PAO1 was exposed to ceftazidime, a cephalosporin targeting PBP3, like BAL30072 (35). The reason for this is unclear. The pyocin genes are induced upon exposure to inhibitors of DNA replication (mitomycin C and ciprofloxacin) (36, 37) and under oxidative stress conditions (hydrogen peroxide) (38). Of note is the reduced expression of the two porins OpdQ and OpdD, which belong to the OpdK subfamily (22). It is possible that these porins could promote a nonspecific uptake mechanism for BAL30072, which could further increase the FecIRA-mediated resistance in P. aeruginosa, when exposed to this molecule. A similar observation was made in E. coli, where deletion of OmpC and OmpF further increased resistance to KP-736 in a fiu-cir double mutant (30).
In summary, the uncharacterized PiuA iron transporter is the most likely uptake system for the siderophore monosulfactam BAL30072. Susceptibility to this antibiotic is influenced by the expression level of this transporter, as well as by overexpression of the AmpC β-lactamase. Our study also highlights the potential of siderophore-drug conjugates as an efficient strategy for increasing the uptake of antimicrobial molecules. The “Trojan Horse” strategy is illustrated by the exquisite hypersusceptibility of the Fur mutant, which to our knowledge has not been tested in this or any other organisms in any previous study relating to siderophore antimicrobials. Fur mutants constitutively express iron receptor genes and thereby mimic iron deficiency conditions encountered by bacterial pathogens in vivo. Our results should encourage further research in this field, including also members of other antimicrobial classes.
Supplementary Material
ACKNOWLEDGMENTS
We thank P. Descombes and M. Docquier and the genomic platform of the University of Geneva for performing the transcriptomic experiments and O. Schaad for help with the microarray data analysis.
This work was supported by an unrestricted grant from Basilea Pharmaceutica International, Ltd., Basel, Switzerland.
Footnotes
Published ahead of print 19 February 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02474-12.
REFERENCES
- 1. Bush K, Macielag MJ. 2010. New beta-lactam antibiotics and beta-lactamase inhibitors. Expert. Opin. Ther. Pat. 20:1277–1293 [DOI] [PubMed] [Google Scholar]
- 2. Viveiros M, Martins M, Couto I, Rodrigues L, Spengler G, Martins A, Kristiansen JE, Molnar J, Amaral L. 2008. New methods for the identification of efflux-mediated MDR bacteria, genetic assessment of regulators and efflux pump constituents, characterization of efflux systems and screening for inhibitors of efflux pumps. Curr. Drug Targets 9:760–778 [DOI] [PubMed] [Google Scholar]
- 3. Pages JM, Masi M, Barbe J. 2005. Inhibitors of efflux pumps in Gram-negative bacteria. Trends Mol. Med. 11:382–389 [DOI] [PubMed] [Google Scholar]
- 4. Möllmann U, Heinisch L, Bauernfeind A, Köhler T, Ankel-Fuchs D. 2009. Siderophores as drug delivery agents: application of the “Trojan Horse” strategy. Biometals 22:615–624 [DOI] [PubMed] [Google Scholar]
- 5. Page MG, Heim J. 2009. New molecules from old classes: revisiting the development of beta-lactams. IDrugs 12:561–565 [PubMed] [Google Scholar]
- 6. Braun V, Gunthner K, Hantke K, Zimmermann L. 1983. Intracellular activation of albomycin in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 156:308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duquesne S, Destoumieux-Garzon D, Peduzzi J, Rebuffat S. 2007. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat. Prod. Rep. 24:708–734 [DOI] [PubMed] [Google Scholar]
- 8. Maejima T, Inoue M, Mitsuhashi S. 1991. In vitro antibacterial activity of KP-736, a new cephem antibiotic. Antimicrob. Agents Chemother. 35:104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsuji K, Tsubouchi H, Yasumura K, Matsumoto M, Ishikawa H. 1996. Synthesis and structure-activity relationships of cephalosporins, 2-isocephems, and 2-oxaisocephems with C-3′ or C-7 catechol or related aromatics. Bioorg. Med. Chem. 4:2135–2149 [DOI] [PubMed] [Google Scholar]
- 10. Fung-Tomc J, Bush K, Minassian B, Kolek B, Flamm R, Gradelski E, Bonner D. 1997. Antibacterial activity of BMS-180680, a new catechol-containing monobactam. Antimicrob. Agents Chemother. 41:1010–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arisawa M, Sekine Y, Shimizu S, Takano H, Angehrn P, Then RL. 1991. In vitro and in vivo evaluation of Ro 09-1428, a new parenteral cephalosporin with high antipseudomonal activity. Antimicrob. Agents Chemother. 35:653–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins PG, Stefanik D, Page MG, Hackel M, Seifert H. 2012. In vitro activity of the siderophore monosulfactam BAL30072 against meropenem-nonsusceptible Acinetobacter baumannii. J. Antimicrob. Chemother. 67:1167–1169 [DOI] [PubMed] [Google Scholar]
- 13. Mima T, Kvitko BH, Rholl DA, Page MG, Desarbre E, Schweizer HP. 2011. In vitro activity of BAL30072 against Burkholderia pseudomallei. Int. J. Antimicrob. Agents 38:157–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Page MG, Dantier C, Desarbre E. 2010. In vitro properties of BAL30072, a novel siderophore sulfactam with activity against multiresistant gram-negative bacilli. Antimicrob. Agents Chemother. 54:2291–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tian ZX, Mac AM, O'Connor HF, Fargier E, Mooij MJ, Adams C, Wang YP, O'Gara F. 2009. MexT modulates virulence determinants in Pseudomonas aeruginosa independent of the MexEF-OprN efflux pump. Microb. Pathog. 47:237–241 [DOI] [PubMed] [Google Scholar]
- 16. Marshall B, Stintzi A, Gilmour C, Meyer JM, Poole K. 2009. Citrate-mediated iron uptake in Pseudomonas aeruginosa: involvement of the citrate-inducible FecA receptor and the FeoB ferrous iron transporter. Microbiology 155:305–315 [DOI] [PubMed] [Google Scholar]
- 17. Kawasaki S, Arai H, Kodama T, Igarashi Y. 1997. Gene cluster for dissimilatory nitrite reductase (nir) from Pseudomonas aeruginosa: sequencing and identification of a locus for heme d1 biosynthesis. J. Bacteriol. 179:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giraud C, Bernard CS, Calderon V, Yang L, Filloux A, Molin S, Fichant G, Bordi C, de Bentzmann S. 2011. The PprA-PprB two-component system activates CupE, the first non-archetypal Pseudomonas aeruginosa chaperone-usher pathway system assembling fimbriae. Environ. Microbiol. 13:666–683 [DOI] [PubMed] [Google Scholar]
- 19. Hood RD, Singh P, Hsu F, Guvener T, Carl MA, Trinidad RR, Silverman JM, Ohlson BB, Hicks KG, Plemel RL, Li M, Schwarz S, Wang WY, Merz AJ, Goodlett DR, Mougous JD. 2010. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7:25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Ploeg, Weiss MA, Saller E, Nashimoto H, Saito N, Kertesz MA, Leisinger T. 1996. Identification of sulfate starvation-regulated genes in Escherichia coli: a gene cluster involved in the utilization of taurine as a sulfur source. J. Bacteriol. 178:5438–5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Michel-Briand Y, Baysse C. 2002. The pyocins of Pseudomonas aeruginosa. Biochimie 84:499–510 [DOI] [PubMed] [Google Scholar]
- 22. Tamber S, Ochs MM, Hancock RE. 2006. Role of the novel OprD family of porins in nutrient uptake in Pseudomonas aeruginosa. J. Bacteriol. 188:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prince RW, Storey DG, Vasil AI, Vasil ML. 1991. Regulation of toxA and regA by the Escherichia coli fur gene and identification of a Fur homologue in Pseudomonas aeruginosa PA103 and PA01. Mol. Microbiol. 5:2823–2831 [DOI] [PubMed] [Google Scholar]
- 24. Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, Will O, Kaul R, Raymond C, Levy R, Chun-Rong L, Guenthner D, Bovee D, Olson MV, Manoil C. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 100:14339–14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nikaido H, Rosenberg EY. 1990. Cir and Fiu proteins in the outer membrane of Escherichia coli catalyze transport of monomeric catechols: study with beta-lactam antibiotics containing catechol and analogous groups. J. Bacteriol. 172:1361–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cornelis P, Matthijs S, Van Oeffelen L. 2009. Iron uptake regulation in Pseudomonas aeruginosa. Biometals 22:15–22 [DOI] [PubMed] [Google Scholar]
- 27. Moya B, Dötsch A, Juan C, Blazquez J, Zamorano L, Haussler S, Oliver A. 2009. Beta-lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. PLoS Pathog. 5:e1000353 doi:10.1371/journal.ppat.1000353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wagegg W, Braun V. 1981. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein FecA. J. Bacteriol. 145:156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barton HA, Johnson Z, Cox CD, Vasil AI, Vasil ML. 1996. Ferric uptake regulator mutants of Pseudomonas aeruginosa with distinct alterations in the iron-dependent repression of exotoxin A and siderophores in aerobic and microaerobic environments. Mol. Microbiol. 21:1001–1017 [DOI] [PubMed] [Google Scholar]
- 30. Tatsumi Y, Maejima T, Mitsuhashi S. 1995. Mechanism of tonB-dependent transport of KP-736, a 1,5-dihydroxy-4-pyridone-substituted cephalosporin, into Escherichia coli K-12 cells. Antimicrob. Agents Chemother. 39:613–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zimmermann L, Hantke K, Braun V. 1984. Exogenous induction of the iron dicitrate transport system of Escherichia coli K-12. J. Bacteriol. 159:271–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kovacevic S, Weigel BJ, Tobin MB, Ingolia TD, Miller JR. 1989. Cloning, characterization, and expression in Escherichia coli of the Streptomyces clavuligerus gene encoding deacetoxycephalosporin C synthetase. J. Bacteriol. 171:754–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fetar H, Gilmour C, Klinoski R, Daigle DM, Dean CR, Poole K. 2011. MexEF-oprN multidrug efflux operon of Pseudomonas aeruginosa: regulation by the MexT activator in response to nitrosative stress and chloramphenicol. Antimicrob. Agents Chemother. 55:508–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McPherson CJ, Aschenbrenner LM, Lacey BM, Fahnoe KC, Lemmon MM, Finegan SM, Tadakamalla B, O'Donnell JP, Mueller JP, Tomaras AP. 2012. Clinically relevant gram-negative resistance mechanisms have no effect on the efficacy of MC-1, a novel siderophore-conjugated monocarbam. Antimicrob. Agents Chemother. 56:6334–6342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blazquez J, Gomez-Gomez JM, Oliver A, Juan C, Kapur V, Martin S. 2006. PBP3 inhibition elicits adaptive responses in Pseudomonas aeruginosa. Mol. Microbiol. 62:84–99 [DOI] [PubMed] [Google Scholar]
- 36. Cirz RT, O'Neill BM, Hammond JA, Head SR, Romesberg FE. 2006. Defining the Pseudomonas aeruginosa SOS response and its role in the global response to the antibiotic ciprofloxacin. J. Bacteriol. 188:7101–7110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brazas MD, Hancock RE. 2005. Ciprofloxacin induction of a susceptibility determinant in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:3222–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chang W, Small DA, Toghrol F, Bentley WE. 2005. Microarray analysis of Pseudomonas aeruginosa reveals induction of pyocin genes in response to hydrogen peroxide. BMC Genomics 6:115 doi:10.1186/1471-2164-6-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.