Abstract
The importance of macrolide-resistant (MR) Mycoplasma pneumoniae has become much more apparent in the past decade. We investigated differences in the therapeutic efficacies of macrolides, minocycline, and tosufloxacin against MR M. pneumoniae. A total of 188 children with M. pneumoniae pneumonia confirmed by culture and PCR were analyzed. Of these, 150 patients had a strain with an MR gene and 134 had one with an A-to-G mutation at position 2063 of M. pneumoniae 23S rRNA domain V. Azithromycin (n = 27), clarithromycin (n = 23), tosufloxacin (n = 62), or minocycline (n = 38) was used for definitive treatment of patients with MR M. pneumoniae. Defervescence within 48 h after the initiation of antibiotic therapy was observed in 41% of the patients in the azithromycin group, 48% of those in the clarithromycin group, 69% of those in the tosufloxacin group, and 87% of those in the minocycline group. The average number of days of fever after the administration of antibiotic treatment was lower in the minocycline and tosufloxacin groups than in the macrolide groups. The decrease in the M. pneumoniae burden, as estimated by the number of DNA copies, after 48 to 96 h of treatment was more rapid in patients receiving minocycline (P = 0.016) than in those receiving tosufloxacin (P = 0.049), azithromycin (P = 0.273), or clarithromycin (P = 0.107). We found that the clinical and bacteriological efficacies of macrolides against MR M. pneumoniae pneumonia was low. Our results indicated that minocycline rather than tosufloxacin can be considered the first-choice drug for the treatment of M. pneumoniae pneumonia in children aged ≥8 years.
INTRODUCTION
Mycoplasma pneumoniae is a common causative pathogen of respiratory infections in children and young adults. For the treatment of M. pneumoniae, which, unlike most other types of bacteria, has no cell wall, protein synthesis inhibitors such as macrolides or tetracyclines or DNA synthesis inhibitors such as quinolones are used in adults. In general, 14- and 15-membered ring macrolides are chosen for the initial treatment of children. However, macrolide-resistant (MR) M. pneumoniae has been reported in Japan since 2000 and has become widespread in Japan and China (1–7). MR M. pneumoniae is now spreading throughout Europe and North America, especially in children (8–13). The most frequent mechanism of resistance is an A-to-G mutation at position 2063 of M. pneumoniae 23S rRNA domain V (A2063G) (5). M. pneumoniae with the A2063G and A2063C mutations is highly resistant to 14- and 15-membered ring macrolides, but the degree of resistance to 16-membered ring macrolides varies by drug or strain (1, 4, 14). M. pneumoniae with the A2064G mutation is highly resistant to all 14-, 15-, and 16-membered ring macrolides. M. pneumoniae with the C2617G mutation varies from sensitive to neutral for 14- and 15-membered ring macrolides and sensitive to 16-membered ring macrolides.
Because macrolides are less effective against MR M. pneumoniae infection than against macrolide-sensitive (MS) M. pneumoniae infection (14–16), the Japanese guidelines for the management of respiratory infectious diseases in children recommend the use of minocycline or tosufloxacin instead of macrolides when MR M. pneumoniae pneumonia is suspected and a lack of defervescence within 48 h after the initiation of macrolide therapy is observed (17). Tosufloxacin, a fluoroquinolone antimicrobial agent that has been reported to have a broader spectrum and potent activity against Gram-positive and Gram-negative bacteria, including M. pneumoniae, was approved for pediatric use in patients with community-acquired pneumonia (CAP) by the Ministry of Health, Welfare, and Labor in 2010 (18, 19). The MIC of tosufloxacin against M. pneumoniae is as low as that of minocycline (18). However, tosufloxacin has not yet been approved for infections with M. pneumoniae. The purpose of this study was to investigate differences in the clinical and bacterial efficacy of macrolides, minocycline, and tosufloxacin against MR M. pneumoniae. We performed the first multicenter, prospective epidemiological study of MR M. pneumoniae in Japan.
MATERIALS AND METHODS
Study population.
All of the pediatric patients with CAP who visited 62 institutions located in seven areas of Japan (Kyushu, Chugoku, Shikoku, Kinki, Tokai, Kanto, and Hokkaido) participating in the Atypical Pathogen Study Group from June 2005 to June 2012 were enrolled in this study. A complete list of participating facilities is located in the Acknowledgments. The diagnosis of pneumonia was based on clinical signs and symptoms (cough, fever, productive sputum, dyspnea, chest pain, or abnormal breath sounds) and radiographic pulmonary abnormalities that were at least segmental and were caused by pre-existing or other known causes. Informed consent was obtained from the parents of all patients, and the study protocol was approved by the Ethics Committee at the Kawasaki Medical School.
Study protocol.
The first visit was set as day 0, the second visit was at days 2 to 4, and the third visit was at days 7 to 14. Nasopharyngeal swab specimens were collected for culture and real-time PCR at each visit. Peripheral white blood cell count determination, a blood test for C-reactive protein, M. pneumoniae antibody titer determination by particle agglutination test (Serodia-Myco II kit; Fujirebio, Tokyo, Japan), and a chest X-ray were performed at the first and third visits. It was possible to determine the presence or absence of the resistance gene about 3 days later. Clinical information, including symptoms, signs, and physical examination results, was collected from all patients at each visit.
The primary antibiotic selection was made by the attending pediatrician. The dosage of azithromycin was 10 mg/kg once daily, and clarithromycin, minocycline, and tosufloxacin were administered twice daily at doses of 15, 4, and 12 mg/kg, respectively, in accordance with the package insert accompanying each drug. If clinical symptoms and signs had not improved by the second visit, the antibiotic treatment was changed by the attending pediatrician to minocycline if the patient was ≥8 years old or to tosufloxacin when the patient was <8 years old.
Sample preparation and real-time PCR.
Nasopharyngeal swab specimens were collected with a sterile swab (Nippon Menbo, Saitama, Japan). After collection, the swab was placed into 3.0 ml of Universal Vial Transport Medium (Becton, Dickinson, Tokyo, Japan) and transported at room temperature within 2 days to our hospital by a parcel delivery system. Three hundred microliters of each sample was used for PCR, and the remainder was stored at −80°C for culture. The medium used for isolation and MIC determination was pleuropneumonia-like organism broth (Oxoid, Franklin Lakes, NJ) supplemented with 0.5% glucose (Wako Pure Chemicals Inc., Osaka, Japan), 20% Mycoplasma Supplement-G (Oxoid), and 0.0025% phenol red (Sigma-Aldrich Co. LLC, St. Louis, MO) (20). DNA was then extracted with a QIAamp DNA Minikit (Qiagen K. K., Tokyo, Japan) in accordance with the manufacturer's instructions. M. pneumoniae DNA was detected by real-time PCR targeting a conserved part of the gene coding for the P1 adhesin (20). Three replicate assays with each respiratory sample were performed by real-time PCR with the same reaction volume. A positive result was recorded if all three tests were positive. If a positive reaction could not be obtained upon repeated testing, the sample was judged to be negative. M. pneumoniae DNA copy numbers were calculated from the mean value of the three test results.
Detection of point mutations for macrolide resistance in domain V of 23S rRNA.
A search for mutations at sites 2063, 2064, and 2617 in the M. pneumoniae 23S rRNA domain V gene region was performed by direct sequencing of isolates or samples with a positive PCR result as reported previously (2, 16, 21). Specifically, nested PCR was performed with a thermal cycler (PCR Thermal Cycler Dice Gradient; TaKaRa Bio, Inc., Shiga, Japan) with primers (Sigma-Aldrich, Japan), Taq polymerase (TaKaRa Ex Taq Version; TaKaRa Bio, Inc.), and extracted DNA. The PCR products were purified with a QIAquick PCR purification kit (Qiagen). The purified products were electrophoresed in a 3% NuSieve 3:1 agarose gel (Lonza) and, after the single band was confirmed, labeled with a BigDye Terminator V3.1 cycle sequencing kit (Applied Biosystems) and applied to an ABI Prism 3130xl Genetic Analyzer (Applied Biosystems) in accordance with the manufacturer's instructions. The presence or absence of gene mutations at each site was determined with a sequence scanner (Applied Biosystems)
Patients infected with M. pneumoniae showing a point mutation in domain V of the 23S rRNA gene were defined as patients with MR M. pneumoniae (MR patients), and those infected with M. pneumoniae without a mutation were defined as patients with MS M. pneumoniae (MS patients).
Antibiotic susceptibility.
The MICs of five antimicrobial agents for the isolates were determined by the microdilution method (22). Briefly, medium containing 105 to 106 CFU/ml M. pneumoniae was placed in 96-well microplates and incubated at 37°C for 6 to 8 days. The MIC was defined as the lowest concentration of an antimicrobial agent at which the growth of the organism was inhibited, as evidenced by a lack of color change in the medium when the drug-free control first showed a color change. The antimicrobial agents used for MIC determination were as follows: erythromycin, clarithromycin, azithromycin, minocycline, and tosufloxacin.
Statistical analysis.
Statistical analysis of the data was performed with Microsoft Excel 2010 for Statistics (SSRI, Tokyo, Japan). The mean age of the patients and the mean number of febrile days after antibiotic administration were compared with the Wilcoxon rank-sum test. The M. pneumoniae bacterial counts at three points after the administration of each antibiotic were analyzed by a paired t test. P values of <0.05 were considered to indicate statistical significance.
RESULTS
Patient characteristics.
Samples from a total of 2,505 patients with CAP were sent to our hospital. Among these, there were 523 cases positive by culture or real-time PCR for M. pneumoniae, and 291 of these cases fit the study protocol. Finally, 188 cases were analyzed for gene mutations. The patient characteristics are shown in Table 1. Of the patients diagnosed with pneumonia caused by M. pneumoniae, 33% were ≤5 years old. In 2011 and 2012, an epidemic of M. pneumoniae infection especially among children occurred throughout Japan and the incidence was the highest observed during the past decade (23). This surge in M. pneumoniae infections may reflect the high rate in patients ≤5 years old. A total of 150 (80%) of 188 pediatric patients with M. pneumoniae pneumonia were determined to have an MR M. pneumoniae isolate. Of these, 134 patients had an isolate with the A2063G mutation, 8 had an isolate with the A2063T mutation, 2 had an isolate with the A2063C mutation, and 6 had an isolate with the A2064G mutation. A total of 141 isolates of M. pneumoniae were obtained by cultivation of samples from these patients (125 isolates had mutations). Fifteen patients with MR strains were hospitalized, but no patients with MS strains were hospitalized. The statistical significance of differences in categorical variables such as age of onset, gender, and fever disappeared within 48 h after antibiotic administration, as determined by the χ2 test or Fisher's exact test. No significant difference between MR and MS patients in mean age, the number of patients in each age group, gender, or the number of patients hospitalized was observed.
Table 1.
Categorical variables age, gender, number of patients hospitalized, and mutation type of 188 pediatric patients with MS and MR M. pneumoniae pneumonia
| Variable | MS patients | MR patients | P value |
|---|---|---|---|
| No. of patients | 38 | 150 | |
| Mean age (range), yr | 8.4 (1–15) | 8.0 (0–15) | 0.670 |
| No. (%) of patients: | |||
| <1 yr old | 2 (5.3) | 7 (4.7) | 0.786 |
| 2–5 yr old | 11 (28.9) | 42 (28.0) | 0.908 |
| 6–7 yr old | 7 (18.4) | 23 (15.3) | 0.643 |
| 8 yr old | 18 (47.4) | 78 (52.0) | 0.610 |
| No. of males/females | 22/16 | 84/66 | 0.833 |
| No. (%) hospitalized | 0 | 15 (10.0) | 0.218 |
| No. (%) with point mutation in domain V of 23S rRNA | |||
| A2063G | 134 (89.4) | ||
| A2063T | 8 (5.3) | ||
| A2063C | 2 (1.3) | ||
| A2064G | 6 (4.0) |
Of the 38 MS patients, 16 received azithromycin and 22 received clarithromycin (Table 2). There was no significant difference in mean age, the number in each age group, or gender between the azithromycin and clarithromycin groups. Of the 150 MR patients, 27 patients received azithromycin, 23 patients received clarithromycin, 62 patients received tosufloxacin, and 38 patients received minocycline. Hospitalization of one patient in the azithromycin group, three patients in the clarithromycin group, five patients in the tosufloxacin group, and six patients in the minocycline group occurred after the initiation of antibiotics (Table 2). The antibiotic was changed to tosufloxacin or minocycline at the second visit for 10 patients in the azithromycin group and 13 in the clarithromycin group. There was no significant difference in the gender of hospitalized patients in the four treatment groups, but the mean age was significantly higher in the minocycline group than in the tosufloxacin group, and the number in each age group and of each gender differed between the azithromycin and clarithromycin groups. The number of patients 2 to 5 years old was significantly lower in the minocycline group than in the tosufloxacin group (P = 0.0401); the number of patients 6 to 7 years old was significantly lower in the minocycline group than in the other treatment groups (P < 0.0001), and the number of patients >8 years old was significantly higher in the minocycline group than in the other treatment groups (P < 0.0001).
Table 2.
Categorical variables age, gender, number of patients hospitalized, and change in antibiotics of 188 pediatric patients with MS and MR M. pneumoniae pneumonia according to treatment group
| Variable | MS patient treatment group: |
MR patient treatment group: |
||||
|---|---|---|---|---|---|---|
| AZMa | CLRb | AZM | CLR | TFXc | MINd | |
| No. of patients | 16 | 22 | 27 | 23 | 62 | 38 |
| Mean age (range), yr | 8.6 (1–13) | 8.0 (2–15) | 8.4 (1–14) | 8.0 (3–15) | 6.5 (0–15) | 9.8 (1–15) |
| No. (%) of patients: | ||||||
| <1 yr old | 1 (6.3) | 1 (4.6) | 1 (3.7) | 0 | 5 (8.1) | 1 (2.6) |
| 2–5 yr old | 5 (31.3) | 6 (27.3) | 5 (18.5) | 8 (34.8) | 23 (37.1) | 6 (15.8) |
| 6–7 yr old | 4 (25) | 3 (13.6) | 7 (25.9) | 3 (13.0) | 13 (21.0) | 0 |
| 8 yr old | 6 (37.5) | 12 (54.5) | 14 (51.9) | 12 (52.2) | 21 (33.9) | 31 (81.6) |
| No. of males/females | 8/8 | 14/8 | 13/14 | 14/9 | 35/27 | 22/16 |
| No. (%) hospitalized | 0 | 0 | 1 (3.7) | 3 (13.0) | 5 (8.1) | 6 (15.8) |
| No. (%) with antibiotic change at second visit | 0 | 0 | 10 (37.0)e | 13 (56.5)f | 0 | 0 |
AZM, azithromycin.
CLR, clarithromycin.
TFX, tosufloxacin.
MIN, minocycline.
Antibiotic changed to TFX for five patients and to MIN for five patients.
Antibiotic changed to TFX for six patients and to MIN for seven patients.
Antibiotic susceptibility.
Table 3 shows the MIC ranges, MIC50s, and MIC90s for the five agents according to the presence or absence of a mutation of the 23S rRNA gene in the 141 M. pneumoniae isolates. Among the 125 isolates of MR M. pneumoniae, the MIC90s of 14- and 15-membered macrolides such as erythromycin, clarithromycin, and azithromycin were >128, >128, and 128 μg/ml, respectively. Tosufloxacin and minocycline showed good antimycoplasmal activity, including against MR isolates, for which the MIC90s were 0.5 and 2 μg/ml, respectively.
Table 3.
In vitro antimycoplasma activity against 141 clinical isolates of M. pneumoniae according to the presence or absence of a mutation in the 23S rRNA gene
| Antimicrobial agent | MIC (μg/ml) for: |
|||||
|---|---|---|---|---|---|---|
| MS M. pneumoniae (n = 16) |
MR M. pneumoniae (n = 125) |
|||||
| Range | 50% | 90% | Range | 50% | 90% | |
| Erythromycin | 0.001–0.0078 | 0.0039 | 0.0078 | 128–>128 | >128 | >128 |
| Clarithromycin | 0.001–0.0156 | 0.002 | 0.0039 | 128–>128 | >128 | >128 |
| Azithromycin | 0.000125–0.001 | 0.00025 | 0.0005 | 16–>128 | 64 | 128 |
| Minocycline | 0.25–2 | 1 | 2 | 0.25–4 | 1 | 2 |
| Tosufloxacin | 0.125–0.5 | 0.25 | 0.5 | 0.125–0.5 | 0.25 | 0.5 |
Clinical efficacy.
The clinical efficacy of anti-M. pneumoniae antibiotics against MS and MR M. pneumoniae strains is shown in Tables 4 and 5, respectively. Among the patients with MS strains, azithromycin and clarithromycin showed good clinical efficacy, with defervescence within 48 h after the initiation of antibiotics observed in 88 and 100% of the patients, respectively (P = 0.333) (Table 4). The average number of days of fever after the initiation of macrolide therapy was 1.62 days in the azithromycin group and 1.04 days in the clarithromycin group (P = 0.058).
Table 4.
Clinical efficacies of macrolides against MS M. pneumoniae pneumonia
| Treatment group (no. of patients) or parameter | No. (%) of patients whose fever disappeared within 48 h after antibiotic administration | Avg no. of days of fever after antibiotic administration |
|---|---|---|
| AZMa (16) | 14 (88) | 1.62 |
| CLRb (22) | 22 (100) | 1.04 |
| P value | 0.333 | 0.058 |
AZM, azithromycin.
CLR, clarithromycin.
Table 5.
Clinical efficacies of macrolides, minocycline, and tosufloxacin against MR M. pneumoniae pneumonia
| Treatment group (no. of patients) or parametera | No. (%) of patients whose fever disappeared within 48 h after antibiotic administration | Avg no. of days of fever after antibiotic administration |
|---|---|---|
| AZM (27) | 11 (41) | 3.06b |
| CLR (23) | 11 (48) | 3.15b |
| TFX (62) | 43 (69) | 2.31 |
| MIN (38) | 33 (87) | 1.83 |
| P value for: | ||
| AZM vs CLR | 0.698 | 0.869 |
| AZM vs TFX | 0.017 | 0.062 |
| AZM vs MIN | 0.0002 | 0.002 |
| CLR vs TFX | 0.067 | 0.081 |
| CLR vs MIN | 0.001 | 0.008 |
| TFX vs MIN | 0.047 | 0.152 |
AZM, azithromycin; CLR, clarithromycin; MIN, minocycline, TFX, tosufloxacin.
Antibiotic changed to TFX or MIN at the second visit for 10 patients in the AZM group and 13 in the CLR group.
Among the MR patients, defervescence within 48 h after the initiation of antibiotics was observed in 41% of the azithromycin group, 48% of the clarithromycin group, 69% of the tosufloxacin group, and 87% of the minocycline group (Table 5). Treatment was more effective in the minocycline and tosufloxacin groups than in the macrolide groups (statistically significantly different, although the difference between the clarithromycin and tosufloxacin groups was not significant) and significantly more effective in the minocycline group than in the tosufloxacin group (P = 0.047). The average number of days of fever after antibiotic administration was significantly shorter in the minocycline group than in the macrolide groups (versus azithromycin, P = 0.002; versus clarithromycin, P = 0.008). No significant differences were observed between the tosufloxacin and minocycline groups (P = 0.152) or between the tosufloxacin and macrolide groups (versus azithromycin, P = 0.062; versus clarithromycin, P = 0.081).
Antibacterial efficacy.
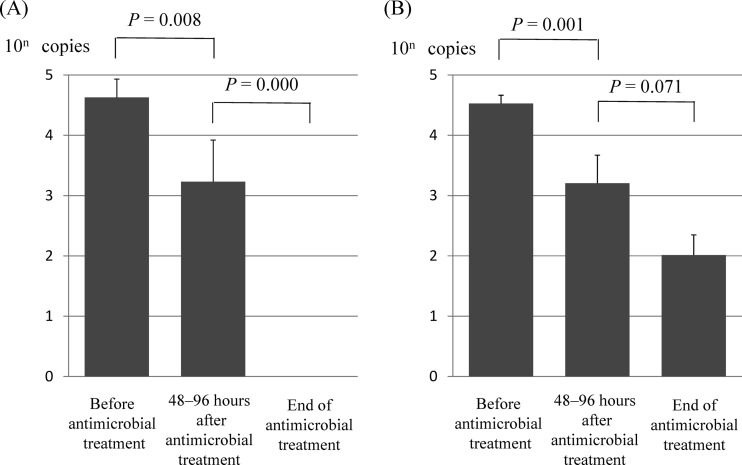
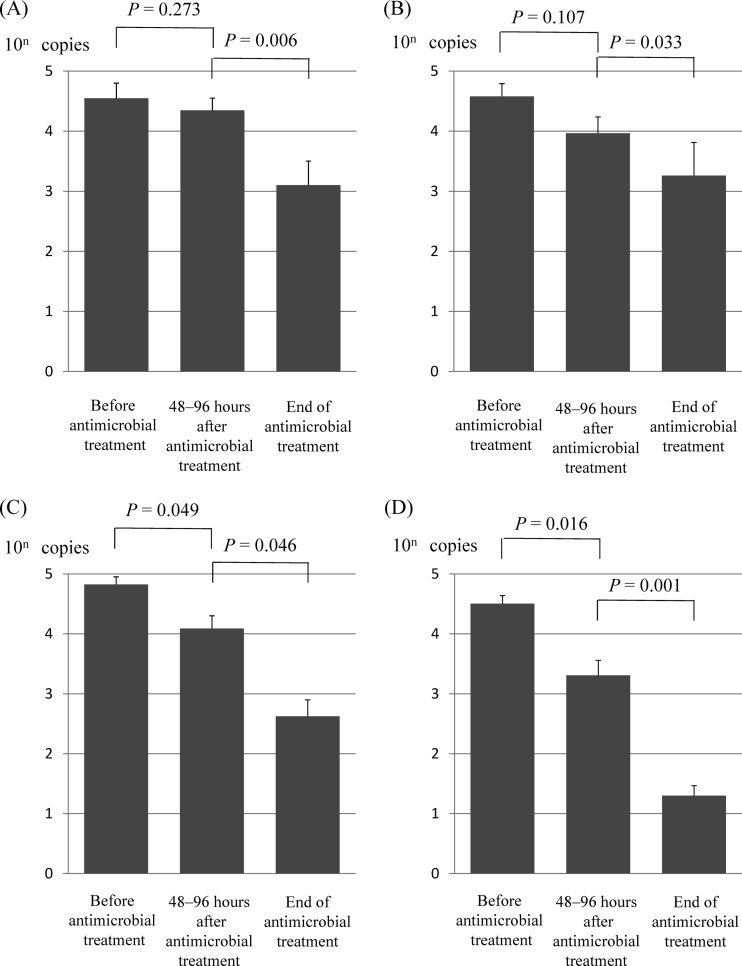
M. pneumoniae DNA copy numbers estimated from real-time PCR results at three time points before and after antibiotic treatment in MS patients and MR patients are shown in Fig. 1 and 2, respectively. DNA copy numbers indicate the mean values (standard deviations) of all patients in each treatment group. The M. pneumoniae DNA copy numbers after 48 to 96 h of macrolide treatment in the control group decreased promptly from 4.2 × 104 (3.3 × 103; range, 2.7 × 103 to 2.1 × 105) to 1.7 × 103 (1.4 × 103; range, 0 to 5.9 × 103) in patients receiving azithromycin (P = 0.008) and from 3.3 × 104 (5.0 × 103; range, 1.6 × 103 to 1.4 × 105) to 1.6 × 103 (1.2 × 103; range, 0 to 1.1 × 104) in patients receiving clarithromycin (P = 0.001) (Fig. 1). However, the M. pneumoniae DNA copy numbers after 48 to 96 h of macrolide treatment in MR patients decreased slowly from 3.5 × 104 (3.8 × 103; range, 1.9 × 103 to 3.3 × 105) to 2.2 × 104 (1.4 × 103; range, 1.5 × 102 to 1.5 × 105) in patients receiving azithromycin (P = 0.273) and from 3.8 × 104 (3.6 × 103; range, 8.5 × 103 to 9.0 × 105) to 9.2 × 103 (7.0 × 102; range, 5.8 × 102 to 1.8 × 104) in patients receiving clarithromycin (P = 0.107) (Fig. 2A and B). The M. pneumoniae DNA copy numbers in MR patients decreased promptly after the administration of tosufloxacin (from 6.6 × 104 [2.7 × 103; range, 1.6 × 103 to 1.3 × 106] to 1.2 × 104 [5.4 × 10; range, 0 to 3.9 × 105]) and minocycline (from 3.1 × 104 [3.2 × 103; range, 1.1 × 103 to 4.2 × 105] to 2.0 × 103 [4.2 × 103; range, 0 to 1.2 × 105]), but the decrease in M. pneumoniae was more rapid in patients receiving minocycline (P = 0.016) than in those receiving tosufloxacin (P = 0.049) (Fig. 2C and D).
Fig 1.
Mean MS M. pneumoniae DNA copy numbers estimated from real-time PCR results at three time points before and after antibiotic treatment. Data for 16 patients receiving azithromycin (A) and 22 patients receiving clarithromycin (B) are shown. Bars indicate standard errors.
Fig 2.
Mean MR M. pneumoniae DNA copy numbers estimated from real-time PCR results at three time points before and after antibiotic treatment. Data for 27 patients receiving azithromycin (A), 23 patients receiving clarithromycin (B), 62 patients receiving tosufloxacin (C), and 38 patients receiving minocycline (D) are shown. Bars indicate standard errors.
Among MS patients, eradication of M. pneumoniae from the nasopharynx at the end of treatment was observed in 100% of the patients in the azithromycin group and 73% of the patients in the clarithromycin group (P = 0.0679). Among the MR patients, the rate of M. pneumoniae eradication in the nasopharynx at the end of treatment was 34% of the patients in the azithromycin group, 35% of those in the clarithromycin group, 59% of those in the tosufloxacin group, and 77% of those in the minocycline group. The eradication rate was significantly better in the minocycline group than in the macrolide groups (versus azithromycin, P = 0.0008; versus clarithromycin, P = 0.0025), but no significant differences were observed between the clarithromycin and tosufloxacin groups (P = 0.0864) or between the minocycline and tosufloxacin groups (P = 0.0843).
DISCUSSION
The increase in MR M. pneumoniae has become a problem in Japan. In cases with genetic resistance, macrolides are less effective than they are against MS M. pneumoniae (14–16). Defervescence and disappearance of coughing could not be achieved in a number of MR patients who initially received macrolides. In the present MR patients, defervescence within 48 h after the initiation of antibiotics was observed in 41% of the azithromycin group, 48% of the clarithromycin group, 87% of the minocycline group, and 69% of the tosufloxacin group. The decreasing numbers of M. pneumoniae DNA copies after the initiation of antibiotics were well correlated with clinical improvement in all of the antibiotics used.
Similar results for therapeutic efficacy in pediatric MR patients were reported in a recent study (24). Okada et al. reported that defervescence within 48 h after the initiation of antibiotic therapy was observed in 46% of the patients who received macrolides (five received azithromycin, and eight received clarithromycin), 90% of the patients who received minocycline and 69% of the patients who received tosufloxacin (24). They also evaluated bacterial disappearance by real-time PCR method after the initiation of the antibiotics and found that the decrease in the MR M. pneumoniae bacterial count was significantly more rapid in patients receiving minocycline than in those receiving tosufloxacin. Although the tosufloxacin-treated patient group was small (n = 6), our study with a larger sample size supported the results of Okada et al., which indicate that minocycline was more effective than macrolides or tosufloxacin against MR M. pneumoniae pneumonia in terms of shortening of the clinical course and decreasing the M. pneumoniae burden. However, it needs to be considered in clinical practice that tetracyclines can cause the side effect of tooth discoloration in children <8 years old. In order to prevent the spread of MR M. pneumoniae infections, studies of new drugs that are safe for use by children <8 years old are needed.
Tosufloxacin is a newly approved fluoroquinolone antimicrobial agent for pediatric use (18, 19). Use was confined strictly to patients with CAP or acute otitis media caused by penicillin-resistant Streptococcus pneumoniae or non-β-lactamase-producing, ampicillin-resistant Haemophilus influenzae and to patients whose infections did not respond to any agent apart from tosufloxacin. Tosufloxacin has not yet been approved for infections with M. pneumoniae. In this study, we evaluated the effectiveness of tosufloxacin in pediatric MR patients. Although our study showed that tosufloxacin was more effective than macrolides in MR patients, it was less effective than minocycline. We may need a more active agent than tosufloxacin for younger age groups in the future.
In Japan, few reports on MR M. pneumoniae in adults have appeared (5). Because MR M. pneumoniae can spread in a family, resistant M. pneumoniae is likely to increase in adults also. However, there is a possibility that MR M. pneumoniae does not spread because of the frequent use by adults of fluoroquinolones such as garenoxacin, moxifloxacin, and sitafloxacin, which are active against MR M. pneumoniae with MICs of <0.0625 μg/ml (4). If tosufloxacin is used appropriately for M. pneumoniae infections in pediatric patients, the negative cycle of resistance can be broken and MR M. pneumoniae may, it is hoped, decrease in children as in adults. Our data indicate that fluoroquinolones such as tosufloxacin are the treatment of choice for CAP due to MR M. pneumoniae in children <8 years old.
Our study had several limitations. First, we assessed the clinical outcome by using fever. Previous studies also assessed only febrile days to evaluate the clinical outcome in MR patients, but a higher frequency of prescription changes was observed in MR patients than in MS patients (14–16). Previous reports demonstrated that the mean numbers of febrile days of MS patients during macrolide administration were 1.4 and 1.5, respectively (14, 15). They also reported that the mean numbers of febrile days of MR patients during macrolide administration were 4.3 and 4.0, respectively. Thus, the Japanese guidelines for the management of respiratory infectious diseases in children recommend the use of minocycline or tosufloxacin instead of macrolides when MR M. pneumoniae pneumonia is suspected and a lack of defervescence within 48 h after the initiation of macrolide treatment is observed (17). Second, we performed a nonrandomized trial to compare the efficacies of several antibiotics against MR strains. Randomized therapeutic trials are necessary to establish clinical guidelines with regard to the most appropriate antimicrobial agents to use against these MR strains. Third, in our study, 15 MR patients (10%) and no MS patients were hospitalized. The decision to hospitalize was made by the attending physician and not according to pneumonia severity as judged by guidelines (17). Therefore, further study is needed to investigate the differences in severity between pneumonia cases due to MR and MS M. pneumoniae strains.
In conclusion, the numbers of M. pneumoniae bacteria in the nasopharynxes of MR patients decreased promptly after minocycline and tosufloxacin treatment and were closely related to the clinical outcomes. In contrast, we found that the clinical and bacteriological efficacy of macrolides for treating cases of MR M. pneumoniae was low. Our results indicated that minocycline rather than tosufloxacin can be considered the first-choice drug for the treatment of M. pneumoniae pneumonia in children ≥8 years old.
ACKNOWLEDGMENTS
We thank Keiko Fujioka, Department of Pediatrics, Kawasaki Medical School, for her technical assistance.
This study was supported in part by MEXT KAKENHI (19591190 and 21591304) and Project Research Grants from the Kawasaki Medical School (13-401, 14-402, 15-405A, 16-405 M, 17-402 M, 18-401, 19-402 M, 20-4030).
The members of the Atypical Pathogen Study Group are Hideki Asaki (Asaki Pediatric Clinic), Kazutoyo Asada (National Mie Hospital), Tomohiro Ichimaru (Saga Prefectural Hospital Koseikan), Toshio Ineda (Inada Clinic), Takuya Inoue (Chayamati Pediatric Clinic), Masakazu Umemoto (Umemoto Pediatric Clinic), Kanetsu Okura (Okura Clinic), Kenji Okada (Fukuoka National Hospital), Takashige Okada (Okada Pediatric Clinic), Teruo Okafuji (Okafuji Pediatric Clinic), Yasuko Okamoto (Okamoto Clinic), Shinichiro Oki (Higashisaga National Hospital), Keiko Oda (Kawasaki Medical School Kawasaki Hospital), Jin Ochiai (Ochiai Pediatric Clinic), Seiko Obuchi (Obuchi Clinic), Yoji Kanehara (Kanehara Pediatric Clinic), You Kanematsu (Kanematsu Pediatric Clinic), Shoji Kouno (Shomonoseki City Central Hospital), Makoto Kuramitsu (Aoba Pediatric Clinic), Katsuji Kuwakado (Kurashiki Central Hospital), Satoshi Kuwano (Kuwano Kids Clinic), Tatuso Koga (Koga Pediatric Clinic), Hayashi Komura (Komura Pediatric Clinic), Hiroshi Sakata (Asahikawa-Kosei General Hospital), Takahisa Sakuma (Sakuma Pediatric Clinic), Kazuhide Shiotsuki (Shiotsuki Internal Medicine Pediatric Clinic), Yasushi Shimada (Shimada Clinic), Makio Sugita (Kurashiki Riverside Hospital), Toru Sugimura (Sugimura Pediatric Clinic), Shumei Takeda (Takeda Pediatric Clinic), Isao Tanaka (Mizushima Central Hospital), Hiroyuki Tanaka (Tanaka Family Clinic), Naohumi Tomita (Tomita Clinic), Kensuke Nagai(Nagai Pediatric Internal Medicine Clinic), Yoshikuni Nagao (Mabi Memorial Hospital), Hidekazu Nakashima (Kojima Central Hospital), Tadashi Nagata (Nagata Pediatric Clinic), Kimiko Nakamura (Enoura Clinic), Kazuyo Nomura (Kama Red Cross Hospital), Kanoko Hashino (Hashino Pediatric Clinic), Yuko Hirata (Hirata Internal Medicine Pediatric Clinic), Kazumi Hiraba (Mokubo Pediatric Clinic), Takuji Fujisawa (Fujisawa Pediatric Clinic), Akiko Maki (Hashima Pediatric Clinic), Toshinobu Matsuura (Yoshino Pediatric Clinic), Nobuyoshi Mimaki (Kurashiki Medical Center), Tatsuhiko Moriguchi (Sakai Hospital Kinki University Faculty of Medicine), Shigeru Mori (Momotaro Clinic), Yoichiro Yamaguchi (Yamaguchi Pediatric Clinic), Syuji Yamada (Yamada Pediatric Clinic), Teruyo Fujimi (Fujimi Clinic), Norio Tominaga (Isahaya Health Insurance General Hospital), Syunji Hasegawa (Yamaguchi University Graduate School of Medicine), Kiyoko Nishimura (Nishimura Pediatric Clinic), Mihoko Mizuno (Daido Clinic), Jiro Iwamoto (Iizuka Hospital), Toshiyuki Iizuka (Hakuai Hospital), Shigeru Yamamoto (Daido Municipal Pediatric Clinic), Tomomichi Kurasaki (Kurosaki Pediatric Clinic), and Tadashi Matsubayashi (Seirei Hamamatsu General Hospital).
Footnotes
Published ahead of print 4 March 2013
REFERENCES
- 1. Okazaki N, Narita M, Yamada S, Izumikawa K, Umetsu M, Kenri T, Sasaki Y, Arakawa Y, Sasaki T. 2001. Characteristics of macrolide-resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro. Microbiol. Immunol. 45:617–620 [DOI] [PubMed] [Google Scholar]
- 2. Matsuoka M, Narita M, Okazaki N, Ohya NH, Yamazaki T, Ouchi K, Suzuki I, Andoh T, Kenri T, Sasaki Y, Horino A, Shintani M, Arakawa Y, Sasaki T. 2004. Characterization and molecular analysis of macrolide-resistant Mycoplasma pneumoniae clinical isolates obtained in Japan. Antimicrob. Agents Chemother. 48:4624–4630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morozumi M, Iwata S, Hasegawa K, Chiba N, Takayanagi R, Matsubara K, Nakayama E, Sunagawa K, Ubukata K, the Acute Respiratory Diseases Study Group 2008. Increased macrolide resistance of Mycoplasma pneumoniae in pediatric patients with community-acquired pneumonia. Antimicrob. Agents Chemother. 52:348–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morozumi M, Takahashi T, Ubukata K. 2010. Macrolide-resistant Mycoplasma pneumoniae: characteristics of isolates and clinical aspects of community-acquired pneumonia. J. Infect. Chemother. 16:78–86 [DOI] [PubMed] [Google Scholar]
- 5. Miyashita N, Kawai Y, Akaike H, Ouchi K, Hayashi T, Kurihara T, Okimoto N, Atypical Pathogen Study Group 2012. Macrolide-resistant Mycoplasma pneumoniae in adolescents with community-acquired pneumonia. BMC Infect. Dis. 12:126 doi:10.1186/1471-2334-12-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Y, Ye X, Zhang H, Xu X, Li W, Zhu D, Wang M. 2009. Antimicrobial susceptibility of Mycoplasma pneumoniae isolates and molecular analysis of macrolide-resistant strains from Shanghai, China. Antimicrob. Agents Chemother. 53:2160–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Qiu S, Yang G, Song L, Su W, Xu Y, Jia Y, Wang L, Hao R, Zhang Liu J, Fu X, He J, Zhang J, Li Z, Song H. 2012. An outbreak of Mycoplasma pneumoniae caused by a macrolide-resistant isolate in a nursery school. Antimicrob. Agents Chemother. 56:3748–3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peuchant O, Menard A, Renaudin H, Morozumi M, Ubukata K, Bebear CM, Pereyre S. 2009. Increased macrolide resistance of Mycoplasma pneumoniae in France directly detected in clinical specimens by real-time PCR and melting curve analysis. J. Antimicrob. Chemother. 64:52–58 [DOI] [PubMed] [Google Scholar]
- 9. Dumke R, von Baum H, Luck PC, Jacobs E. 2010. Occurrence of macrolide-resistant Mycoplasma pneumoniae strains in Germany. Clin. Microbiol. Infect. 16:613–616 [DOI] [PubMed] [Google Scholar]
- 10. Chironna M, Sallustio A, Esposito S, Perulli M, Chinellato I, Di Bari C, Quarto M, Cardinale F. 2011. Emergence of macrolide-resistant strains during an outbreak of Mycoplasma pneumoniae infections in children. J. Antimicrob. Chemother. 66:734–737 [DOI] [PubMed] [Google Scholar]
- 11. Uldum SA, Bangsborg JM, Gahrn-Hansen B, Ljung R, Mølvadgaard M, Føns Petersen R, Wiid Svarrer C. 2012. Epidemic of Mycoplasma pneumoniae infection in Denmark, 2010 and 2011. Euro Surveill. 17:20073 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20073 [DOI] [PubMed] [Google Scholar]
- 12. Yamada M, Buller R, Bledose S, Storch GA. 2012. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr. Infect. Dis. J. 31:409–411 [DOI] [PubMed] [Google Scholar]
- 13. Averbuch D, Hidalgo-Grass C, Moses AE, Engelhard D, Nir-Paz R. 2011. Macrolide resistance in Mycoplasma pneumoniae, Israel, 2010. Emerg. Infect. Dis. 17:1079–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsubara K, Morozumi M, Okada T, Matsushima T, Komiyama O, Shoji M, Ebihara T, Ubukata K, Sato Y, Akita H, Sunagawa K, Iwata S. 2009. A comparative clinical study of macrolide-sensitive and macrolide-resistant Mycoplasma pneumoniae infections in pediatric patients. J. Infect. Chemother. 15:380–383 [DOI] [PubMed] [Google Scholar]
- 15. Suzuki S, Yamazaki T, Narita M, Okazaki N, Suzuki I, Andoh T, Matsuoka M, Kenri T, Arakawa Y, Sasaki T. 2006. Clinical evaluation of macrolide-resistant Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 50:709–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawai Y, Miyashita N, Yamaguchi T, Saitoh A, Kondoh E, Fujimoto H, Teranishi H, Inoue M, Wakabayashi T, Akaike H, Ogita S, Kawasaki K, Terada K, Kishi F, Ouchi K. 2012. Clinical efficacy of macrolide antibiotics against genetically determined macrolide-resistant Mycoplasma pneumoniae pneumonia in pediatric patients. Respirology 17:354–362 [DOI] [PubMed] [Google Scholar]
- 17. Committee for the Guidelines in Management of Respiratory Infectious Diseases in Children 2011. Guidelines for the management of respiratory infectious diseases in children in Japan. In Ouchi K, Kurosaki T, Okada K. (ed), Japanese Society of Pediatric Pulmonology/Japanese Society for Pediatric Infectious Diseases, Tokyo, Japan: (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 18. Ishida K, Kaku M, Irifune K, Mizukane R, Takemura H, Yoshida R, Tanaka H, Usui T, Tomono K, Suyama N. 1994. In-vitro and in-vivo activity of a new quinolone AM-1155 against Mycoplasma pneumoniae. J. Antimicrob. Chemother. 34:875–883 [DOI] [PubMed] [Google Scholar]
- 19. Niki Y, Hanaki H, Matsumoto T, Yagisawa M, Kohno S, Aoki N, Watanabe A, Sato J, Hattori R, Koashi N, Terada M, Kozuki T, Maruo A, Morita K, Ogasawara K, Takahashi Y, Matsuda K, Nakanishi K, Sunagawa K, Takeuchi K, Fujimura S, Takeda H, Ikeda H, Sato N, Niitsuma K, Saito M, Koshiba S, Kaneko M, Miki M, Nakanowatari S, Takahashi H, Utagawa M, Nishiya H, Kawakami S, Aoki Y, Chonabayashi N, Sugiura H, Ichioka M, Goto H, Kurai D, Saraya T, Okazaki M, Yoshida K, Yoshida T, Tsukada H, Imai Y, Honma Y, Yamamoto T, Kawai A, Mikamo H, Takesue Y, Wada Y, Miyara T, Toda H, Mitsuno N, Fujikawa Y, Nakajima H, Kubo S, Ohta Y, Mikasa K, Kasahara K, Koizumi A, Sano R, Yagi S, Takaya M, Kurokawa Y, Kusano N, Mihara E, Nose M, Kuwabara M, Fujiue Y, Ishimaru T, Matsubara N, Kawasaki Y, Tokuyasu H, Masui K, Kido M, Ota T, Honda J, Kadota J, Hiramatsu K, Aoki Y, Nagasawa Z, Yanagihara K, Fujita J, Tateyama M, Totsuka K. 2011. Nationwide surveillance of bacterial respiratory pathogens conducted by the Japanese Society of Chemotherapy in 2008: general view of the pathogens' antibacterial susceptibility. J. Infect. Chemother. 17:510–523 [DOI] [PubMed] [Google Scholar]
- 20. Miyashita N, Kawai Y, Yamaguchi T, Ouchi K, Oka M, Atypical Pathogen Study Group 2011. Clinical potential of diagnostic methods for the rapid diagnosis of Mycoplasma pneumoniae pneumonia in adults. Eur. J. Clin. Microbiol. Infect. Dis. 30:439–446 [DOI] [PubMed] [Google Scholar]
- 21. Lucier TS, Heitzman K, Liu SK, Hu PC. 1995. Transition mutations in the 23S rRNA of erythromycin-resistant isolates of Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 39:2770–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Waites KB, Crabb DM, Bing X, Duffy LA. 2003. In vitro susceptibilities to and bactericidal activities of garenoxacin (BMS-284756) and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob. Agents Chemother. 47:161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Institute of Health Infectious Disease Surveillance Center 2004. Mycoplasma pneumonia cases reported per sentinel weekly. National Institute of Health Infectious Disease Surveillance Center, Tokyo, Japan: http://idsc.nih.go.jp/idwr/kanja/weeklygraph/18myco.html [Google Scholar]
- 24. Okada T, Morozumi M, Tajima T, Hasegawa M, Sakata H, Ohnari S, Chiba N, Iwata S, Ubukata K. 2012. Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin. Infect. Dis. 55:1642–1649 [DOI] [PubMed] [Google Scholar]