Abstract
Nevirapine is one of the most extensively prescribed antiretrovirals worldwide. The present analyses used data and specimens from two prior studies to characterize and compare plasma nevirapine phase I metabolite profiles following a single 200-mg oral dose of nevirapine in 10 HIV-negative African Americans and a steady-state 200-mg twice-daily dose in 10 HIV-infected Cambodians. Nevirapine was assayed by high-performance liquid chromatography (HPLC). The 2-, 3-, 8- and 12-hydroxy and 4-carboxy metabolites of nevirapine were assayed by liquid chromatography-tandem mass spectrometry (LC/MS/MS). Pharmacokinetic parameters were calculated by noncompartmental analysis. The metabolic index for each metabolite was defined as the ratio of the metabolite area under the concentration-time curve (AUC) to the nevirapine AUC. Every metabolite concentration was much less than the corresponding nevirapine concentration. The predominant metabolite after single dose and at steady state was 12-hydroxynevirapine. From single dose to steady state, the metabolic index increased for 3-hydroxynevirapine (P < 0.01) but decreased for 2-hydroxynevirapine (P < 0.001). The 3-hydroxynevirapine metabolic index was correlated with nevirapine apparent clearance (P < 0.001). These findings are consistent with induction of CYP2B6 (3-hydroxy metabolite) and a possible inhibition of CYP3A (2-hydroxy metabolite), although these are preliminary data. There were no such changes in metabolic indexes for 12-hydroxynevirapine or 4-carboxynevirapine. Two subjects with the CYP2B6 *6*6 genetic polymorphism had metabolic indexes in the same range as other subjects. These results suggest that nevirapine metabolite profiles change over time under the influence of enzyme induction, enzyme inhibition, and host genetics. Further work is warranted to elucidate nevirapine biotransformation pathways and implications for drug efficacy and toxicity.
INTRODUCTION
In resource-limited settings, the nonnucleoside HIV-1 reverse transcriptase inhibitor (NNRTI) nevirapine (NVP) is among WHO-recommended components of first-line antiretroviral therapy. At the time of this study, nevirapine in combination with two nucleoside reverse transcriptase inhibitors, such as stavudine or zidovudine, and together with lamivudine was the preferred regimen for treatment-naïve patients, in part because of the availability of a WHO-prequalified, low-cost, generic, fixed-dose combination (1, 2). In addition, single-dose nevirapine administered to pregnant, HIV-infected women at delivery has been widely prescribed to prevent mother-to-child transmission (3–6). Despite its major therapeutic benefits, treatment with nevirapine may cause severe hepatotoxicity and/or skin rash in some patients. Molecular mechanisms of nevirapine toxicity are incompletely understood, but a causal role of metabolites has been suggested (7, 8).
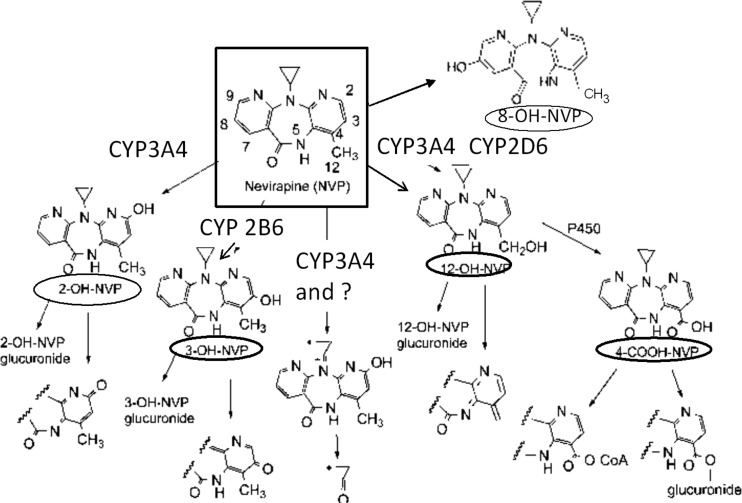
Despite its widespread use, there remain gaps in understanding of nevirapine metabolism and disposition. Its pharmacokinetic characteristics include a long plasma half-life after single-dose administration which decreases with repeated doses due to autoinduction of its biotransformation (6, 9–11). Nevirapine is 60% bound to plasma proteins, and elimination occurs mainly through oxidative metabolism. Five metabolites, including hydroxyl metabolites at positions 2, 3, 8, and 12 (Fig. 1) and 4-carboxynevirapine derived from the 12-hydroxy metabolite, have been identified. In vitro microsome data suggest that CYP3A is involved in 2-hydroxynevirapine formation and CYP2B6 is involved in 3-hydroxynevirapine formation (12). Several CYPs are involved in the other pathways (12, 13). These metabolites are eliminated in the urine as conjugates, mainly glucuronides (14). Relatively little is known regarding nevirapine biotransformation and metabolite disposition following a single dose and at steady state, in part due to the lack of a direct and sensitive assay (13, 15).
Fig 1.
Structures of nevirapine (parent drug) and its 5 metabolites quantitated in this study (8).
A sensitive liquid chromatography-tandem mass spectrometry (LC/MS/MS) assay was recently developed to quantitate the phase I metabolites of nevirapine (16). The present analyses applied this assay to specimens from two prior studies to characterize plasma nevirapine phase I metabolite profiles in two different situations, one involving a single 200-mg oral dose of nevirapine given to 10 HIV-negative African Americans and the other at steady state with a 200-mg oral dose twice daily in 10 HIV-infected Cambodians. We also compare metabolite profiles between these situations.
MATERIALS AND METHODS
Patients and study design.
Data and specimens for these pharmacokinetic analyses were from individuals who had participated in two previously published studies (17, 18). This analysis was approved by the National Ethics Committee of Cambodia and by the Vanderbilt University Institutional Review Board. Plasma samples were from 10 healthy, HIV-negative African Americans (group A) who had received a single 200-mg oral dose of nevirapine (17) and from 10 HIV-infected Cambodians (group B) from the ESTHER (Ensemble pour une Solidarité Thérapeutique en Réseau) at the Calmette Hospital (Phnom Penh, Cambodia) (18). In both groups, plasma was kept frozen until analysis, and genotype data for CYP2B6 *1*6 were available from the previous studies (17, 18).
Group B patients were on chronic, steady-state antiretroviral therapy with 200 mg nevirapine twice daily plus two nucleoside analogs (lamivudine with either stavudine or zidovudine) for about 3 years and agreed to participate in this extensive pharmacokinetic study as part of the ANRS12154 study. In that study, plasma HIV-1 RNA was measured in addition to standard laboratory tests.
Plasma samples from group A subjects were obtained at 0.5, 1, 2, 4, 6, 8, 12, and 24 h and on days 2, 3, 5, 7, 9, and 13 after a single dose of nevirapine. Plasma samples from group B subjects were collected at predose and at 1, 2, 4, and 8 h after the morning nevirapine dose.
Assay of nevirapine and metabolites in plasma.
Plasma nevirapine assays for group A were performed in the United States (17), and those for group B were performed in Cambodia (18), both by liquid chromatography according to previously validated assays. The lower limit of quantification was 50 ng/ml. Standard curves were linear up to 5,000 ng/ml (17) or 10,000 ng/ml (18).
Nevirapine metabolites were assayed using a validated LC/MS/MS assay recently published by the Biomedical Mass Spectrometry Laboratory at the Ohio State University (16). The lower limit of quantification was 1 ng/ml for each hydroxy metabolite and 5 ng/ml for 4-carboxynevirapine. All concentrations were converted in molar equivalents based on molecular weights as follows: nevirapine, 266; hydroxy nevirapine, 282; and 4-carboxynevirapine, 292.
Pharmacokinetic analysis.
Pharmacokinetic parameters for nevirapine and its phase I metabolites were assessed by noncompartmental methods (WinNonlin v6.1; Pharsight Corporation, Mountain View, CA). The linear-log trapezoidal method was used to calculate areas under concentration-time curves (AUCs) for group A to the last measurable concentration at time t (AUC0-t) and for group B over a 12-hour dosing interval (AUC12). For group B, we assumed identical predose and 12-hour postdose concentrations. For group A, the AUCs from t to infinity (AUCinf) were calculated by extrapolation whenever determination of the terminal rate constant (λz) was possible. Apparent clearances of nevirapine (CL/F) were calculated by the following standard equations after single (SD) or repeated (SS) doses, respectively: CLSD/F = dose/AUCinf or CLSS/F = dose/AUC12. The metabolic index was defined as the ratio of the metabolite AUC to the nevirapine AUC. For consistency in group A, the AUC0-t to the same last time t of concentration detection was used for metabolites and nevirapine to calculate metabolic indexes due to the inability to accurately determine the terminal rate constants (λz) for metabolites that were detected at very low concentrations. The maximum plasma concentration (Cmax), the observed predose concentration (C0), and the time to Cmax (Tmax) were obtained from visual inspection of concentration-time curves.
Statistical analyses.
This observational pharmacokinetic study assessed plasma concentrations of nevirapine metabolites following single or repeated doses of nevirapine. When this pilot study was designed, there were no data to guide sample size estimates. We chose to study 10 individuals in each group to detect major differences in nevirapine metabolite disposition.
Pharmacokinetic parameters for nevirapine and its metabolites were described by medians and ranges. Parameters were compared between group A and group B using the nonparametric Wilcoxon rank-sum test. When appropriate, associations between pharmacokinetic parameters were examined using a Spearman correlation test. All analyses were performed using Statgraphics 5 plus (Manugistics, Inc., MD).
RESULTS
Study subject characteristics.
Subject characteristics are summarized in Table 1. Group A comprised healthy, HIV-negative African Americans who received a single dose of nevirapine. Group B comprised HIV-infected Asians in Cambodia who were studied at steady state on nevirapine-containing regimens for at least 1 year. All patients had plasma HIV-1 RNA at <400 copies/ml at the time of study, and CD4 T cell counts ranged from 155 to 513 cells/µl (median, 277 cells/µl). Eight patients were receiving concomitant zidovudine and lamivudine, and two received concomitant stavudine and lamivudine. None of the patients were receiving medications that induce or inhibit drug-metabolizing enzymes (e.g., rifampin or fluconazole). In addition to race/ethnicity, the two populations differed by body mass index (BMI) and proportion of female participants. Frequencies of CYP2B6 *1/*6 genotypes were similar between groups, with one subject in each group being homozygous for *6/*6. All but one patient in group B had normal liver function tests (one with alanine aminotransferase [ALAT] of 99 IU/ml), and all had plasma creatinine of <1.1 mg/dl and calculated creatinine clearances of >60 ml/min.
Table 1.
Characteristics of study subjects
| Characteristic | Values for HIV-negative adults in the United States (n = 10) | Values for HIV-infected adults in Cambodia (n = 10) |
|---|---|---|
| Race/ethnicity | African American | Cambodian |
| No. of females | 8 | 5 |
| Age (yrs) (range) | 25 (21–43) | 32 (28–44) |
| Weight (kg) (range) | 68 (59–100) | 52 (42–66) |
| BMI (range) | 23 (18–33) | 21 (19–22) |
| CYP2B6 *1/*6 (n) | ||
| *1/*1 | 5 | 6 |
| *1/*6 | 4 | 3 |
| *6/*6 | 1 | 1 |
Concentrations of nevirapine and its phase I metabolites.
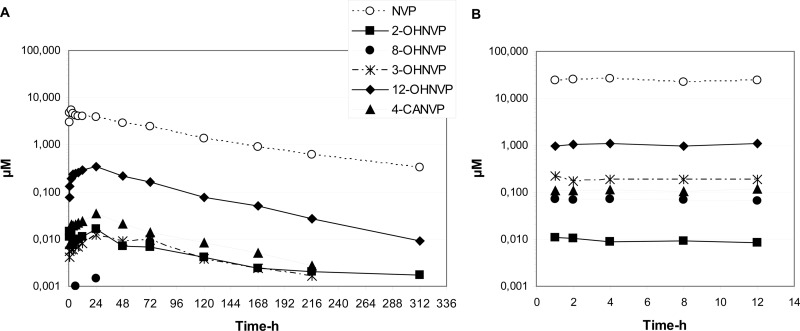
The within-day and day-to-day precisions of nevirapine quality control samples included in each analytical run were below 12%. Day-to-day precision of quality control of nevirapine metabolites was below 14%, and accuracy for between-run validation was within 93% and 116%. In groups A and B, plasma concentrations of each metabolite were well below concentrations of nevirapine at every time point (Fig. 2). Of the metabolites assayed, 12-hydroxynevirapine was the most abundant in both groups. Concentrations of 12-hydroxynevirapine were, on average, 5 times greater in group B than in group A, a result which parallels nevirapine concentrations. Concentrations of 3-hydroxynevirapine and 4-carboxynevirapine increased 5- to 20-fold in group B compared to those in group A. Interestingly, the 2-hydroxynevirapine concentration decreased from group A to group B. Plasma levels of 8-hydroxynevirapine were undetectable following a single dose of nevirapine. In contrast, measurable concentrations of 8-hydroxynevirapine were detected in all samples in group B, with a median Cmax of 0.075 μM. Temporal declines in metabolite concentrations tended to parallel declines in nevirapine concentrations, indicating that the rate of metabolite formation is the rate-limiting step in their disposition and is driven by the slow elimination rate for nevirapine.
Fig 2.
Median plasma concentrations of nevirapine and its 5 metabolites after single-dose administration of 200 mg of nevirapine (A) and at steady state during a 12-h dosing interval after administration of 200 mg of nevirapine twice daily (B).
Pharmacokinetics of nevirapine metabolites.
Pharmacokinetic parameters of nevirapine and its phase I metabolites are summarized in Table 2. As expected, the half-life of nevirapine was long in group A, with considerable interindividual variability (median, 99 h; range, 53 to 217 h). Although median half-lives of some metabolites tended to be shorter than that of nevirapine, these differences were not statistically significant. In group B, we were not able to calculate half-lives for nevirapine or its metabolites, as the dosing interval was too short.
Table 2.
Pharmacokinetic parameters of nevirapine and its metabolites after a single dose (group A) and at steady state (group B)
| Drug | Pharmacokinetic parameters fora: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group A after single dose |
Group B at steady state |
|||||||||
| Cmax (μM) | Tmax (h) | t1/2 (h) | AUC12 (μM · h) | AUCt (μM · h) | AUCinf (μM · h) | Ctrough (μM) | Cmax (μM) | Tmax (h) | AUC12 (μM · h) | |
| Nevirapine | 5.7 (3.8–12.1) | 2 (0.5–8) | 99 (53–217) | 59.2 (38.0–66.9) | 513.0 (380.0–592.0) | 560 (430–684) | 21.8 (13.5–36.5) | 26.7 (19.1–50.2) | 2 (0–4) | 291.8* (161.9–459.8) |
| 2-Hydroxynevirapine | 0.02 (0.01–0.08) | 1 (0.5–24) | 99 (48–882) | 0.10 (0.05–0.32) | 0.97 (0.59–3.59) | 1.55 (0.85–4.36) | 0.01 (0.00–0.01) | 0.01 (0.01–0.02) | 2 (1–8) | 0.11* (0.06–0.17) |
| 8-Hydroxynevirapine | Not detected | Not detected | Not detected | Not detected | Not detected | Not detected | 0.04 (0.03–0.08) | 0.07 (0.05–0.14) | 2 (1–14) | 0.76 (0.46–1.29) |
| 3-Hydroxynevirapine | 0.01 (0.01–0.03) | 24 (6–72) | 59 (35–217) | 0.09 (0.04–0.12) | 1.14 (0.47–2.12) | 1.37 (0.73–2.40) | 0.11 (0.05–0.23) | 0.18 (0.08–0.49) | 1 (0–8) | 1.76 (0.77–4.17) |
| 12-hydroxy nevirapine | 0.32 (0.14–0.72) | 12 (2–24) | 61 (43–1,221) | 3.12 (1.10–5.16) | 27.11 (12.47–54.92) | 34.92 (14.17–78.17) | 0.51 (0.18–2.67) | 1.00 (0.50–3.81) | 3 (1–12) | 9.53** (4.18–34.42) |
| 4-Carboxynevirapine | 0.03 (0.02–0.12) | 12 (2–24) | 48 (25–81) | 0.23 (0.12–0.46) | 2.37 (0.82–6.88) | 2.77 (0.90–7.24) | 0.08 (0.03–0.21) | 0.13 (0.09–0.27) | 3 (1–14) | 1.12** (0.75–2.65) |
Values are medians (ranges).
, P < 0.001 for the AUC12 at steady state compared to the AUCinf after a single dose;
, P < 0.01 for the AUC12 at steady state compared to the AUCinf after a single dose.
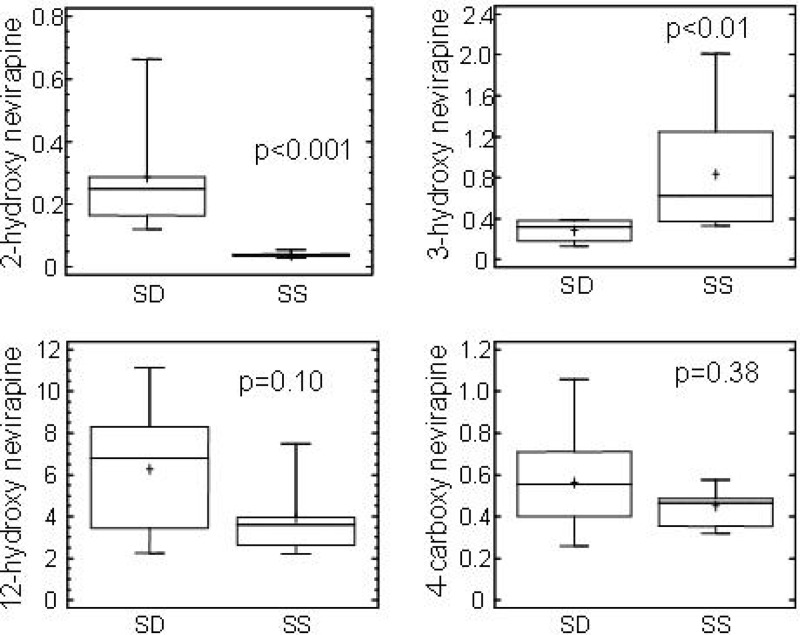
The nevirapine AUC12 in group B was significantly less than the AUCinf in group A, and the CL/F was significantly greater in group B than in group A (0.83 ml/min/kg and 0.29 ml/min/kg, respectively; P < 0.001), indicating nonlinear pharmacokinetics and autoinduction. The 3-hydroxynevirapine AUC12 in group B was greater than the 3-hydroxynevirapine AUCinf in group A, although this difference was not significant. In contrast, the 2-hydroxynevirapine AUC12 was 95% lower in group B than the 2-hydroxynevirapine AUCinf in group A (P < 0.001). The median AUC12 of 8-hydroxynevirapine was very low (0.76 μM · h) at steady state in patients of group B but could not be calculated in patients of group A. In contrast, 12-hydroxynevirapine and 4-carboxynevirapine AUC12 values were significantly lower in group B, representing approximately 30 and 40% of group A AUCinf values, respectively. There was a correlation between AUCs of 4-carboxynevirapine and 12-hydroxynevirapine (r = 0.82, P = 0.0003). Metabolite indexes were compared between group A and group B (Fig. 3). The 2-hydroxynevirapine metabolite index was lower in group B than in group A (P = 0.0002), while the 3-hydroxynevirapine metabolite index was higher in group B than in group A (P = 0.007). In contrast, for 12-hydroxynevirapine and 4-carboxynevirapine metabolic indexes, there were no significant differences between group A and group B. In analyses involving all 20 study subjects, there were correlations between the apparent clearance of nevirapine and the metabolite indexes for 2-hydroxynevirapine (r = −0.73, P = 0.0014), 3-hydroxynevirapine (r = 0.63, P = 0.006), and 12-hydroxynevirapine (r = −0.47, P = 0.03).
Fig 3.
Box plots of metabolic indexes of nevirapine metabolites after single-dose administration of 200 mg of nevirapine (SD) and at steady state during a 12-h dosing interval after administration of 200 mg of nevirapine twice daily (SS).
The 2 subjects carrying the CYP2B6 *6*6 genotype had nevirapine and metabolite concentrations in the same range as the other subjects either after single-dose administration or at steady state.
DISCUSSION
This is the first study to describe the pharmacokinetics of nevirapine and its phase I metabolites after a single 200-mg dose of nevirapine and at steady state with the recommended 200-mg twice-daily dosing. The most important findings from this study are, first, the much lower concentrations of nevirapine metabolites compared to nevirapine and, second, the differential disposition of hydroxylated metabolites, possibly in keeping with the different CYPs involved in the nevirapine metabolic pathways (12). Interestingly, the effect of nevirapine induction on CYP2B6 activity leads to an increased concentration of 3-hydroxynevirapine and a lower concentration of the 2-hydroxynevirapine metabolite formed through the CYP3A pathway. The pharmacokinetic characteristics of parent nevirapine in the present study agree with those of previous studies conducted in patients or volunteers after a single dose or at steady state, a finding which clearly demonstrates the autoinducing properties of nevirapine (10, 19–27). Indeed, the two populations studied herein differ by their ethnicity, demographics, and HIV infection status; the CYP2B6 *1*6 genetic polymorphism frequency is very close, but the significant difference remains when clearances are weight normalized (0.83 ml/min/kg versus 0.29 ml/min/kg). Low apparent nevirapine clearance suggests that biotransformation occurs mainly in the liver and that the intestinal first-pass effect is negligible. Consequently, as previously mentioned, the higher nevirapine clearance observed at steady state was due to a change in nevirapine metabolism rather than to altered bioavailability.
This study provides new information regarding unconjugated nevirapine metabolite disposition in plasma. After single dose or at steady state, concentrations are considerably less than those of nevirapine. On one hand, the volume of distribution of polar metabolites should be close to or smaller than that of nevirapine, and on the other hand, a very high rate of glucuronide conjugate elimination is limited by their rate of formation from nevirapine or from 12-hydroxynevirapine for 4-carboxynevirapine. Consequently, different concentrations may be related to different rates of formation. This is in keeping with the previous findings of Riska et al. (14), who demonstrated that after autoinduction and administration of a single oral dose (solution) of 50 mg containing 100 mCi of [14C]NVP, glucuronide conjugation and urinary excretion of glucuronidated metabolites represent the primary route of nevirapine biotransformation and elimination in humans. 2-Hydroxynevirapine glucuronide (18.6%), 3-hydroxynevirapine glucuronide (25.7%), and 12-hydroxynevirapine glucuronide (23.7%) were the major metabolites recovered in urine. They also showed that disposition of radioactivity was rate limited by biotransformation of nevirapine to its hydroxylated metabolites rather than by excretion of the metabolites into feces and urine, consistent with our results. Our plasma samples were not hydrolyzed; therefore, the plasma ratio of hydroxynevirapine to its glucuronide is not available in our study. A comparison of steady-state trough plasma concentrations in our patients with concentrations measured in HIV-infected patients with mild liver fibrosis (13) showed that nonconjugated 2-hydroxynevirapine and 3-hydroxynevirapine concentrations are well below those of the glucuronides, which remained lower than those of nevirapine, roughly 2 ng/ml versus 177 ng/ml and 12 ng/ml versus 759 ng/ml, respectively. In contrast, 8-hydroxynevirapine and 12-hydroxynevirapine concentrations are closed whether plasma was hydrolyzed or not (29 versus 31 ng/ml and 504 versus 142 ng/ml, respectively), indicating that glucuronide concentrations of these metabolites in plasma are low. Concentrations of 4-carboxynevirapine were low and in the same range, which is explained by the elimination of this metabolite unchanged in urine (14).
A comparison of concentrations of nevirapine metabolites or metabolic indexes after single-dose administration and at steady state provides insight into nevirapine disposition. Dispositions of the four hydroxylated metabolites differ, a result which may be related to different CYPs involved in their formation. In vitro data demonstrated that CYP3A and CYP2B6 were responsible for the formation of 2- and 3-hydroxynevirapine, respectively, while CYP3A and CYP2D6 participated to the formation of 8- and 12-hydroxynevirapine, respectively (12). Hepatic CYP2B genes represent the most-inducible CYP isoforms by phenobarbital-type compounds in most mammalian species (28, 29). Consequently, induction of CYP2B6 may explain the relative increase in 3-hydroxymetabolite concentrations compared to nevirapine and a significant increase in the metabolic index at steady state compared to the single dose. Surprisingly, the 2 subjects from groups A and B carrying CYP2B6 *6*6 did not have decreased concentrations of 3-hydroxynevirapine, suggesting that this variant may be inducible by nevirapine. CYP3A was identified as the unique CYP involved in the formation of 2-hydroxynevirapine, which surprisingly is decreased at steady state compared to the single dose. A recent investigation conducted in human liver microsomes revealed the formation of a quinine methide reactive intermediate which is subsequently attacked by glutathione to yield a sulfhydryl conjugate of nevirapine (30). This reactive intermediate was catalyzed primarily by CYP3A and possibly by CYP2D6, CYP2C19, and CYP2A6 and was shown to inactivate CYP3A with a Ki of 31 μM (about 8,000 ng/ml), not far from the average concentration of nevirapine at steady state. These in vitro data correspond to the clinical observation of potent attenuation of CYP3A induction and possibly to the decreased formation of 2-hydroxynevirapine. Such a dual mechanism of inhibition/inactivation and induction has been demonstrated for other drugs, such as ritonavir or nelfinavir (31, 32), although the net effect of those antiretroviral drugs is potent CYP3A inhibition, in contrast to nevirapine, for which induction of CYP2B6 is likely the primary pathway. Such a hypothesis is not supported by many drug-drug interaction studies (33). However, a 20% increase in rifabutin and desacetyl-rifabutin (CYP3A substrates) concentrations has been reported when coadministered with nevirapine (Viramune product information; Boehringer Ingelheim). Another explanation may be induction of 3-hydroxynevirapine-glucuronide formation. Whether such induction may be specific to one glucuronide pathway remains to be further explored. The exact contribution of different CYPs to the formation of 8-hydroxynevirapine and 12-hydroxynevirapine is unknown. Based on in vitro data, 8- and 12-hydroxynevirapine formation is predicted to involve CYP3A and CYP2D6. 8-Hydroxynevirapine was detected in plasma only at steady state. We would expect induction of this metabolic pathway by nevirapine, which is not supported by in vitro data, or, alternatively, a low rate of formation by noninducible CYP2D6 and accumulation at steady state (34, 35). A decreased AUC12 compared to the AUCinf but no difference in metabolic indexes suggests a major contribution of noninducible CYP2D6 in the formation of 12-hydroxynevirapine.
Nevirapine use has been associated with severe skin rash and/or liver toxicity in some patients (36). It has been suggested from in vitro experiments and animal models that the 12-hydroxynevirapine pathway may be involved in nevirapine toxicity. An ultimate reactive metabolite for both liver toxicity and skin rash may be the quinone methide formed by CYP3A as previously mentioned (7, 8, 30). Conversely, recent toxicogenomics of nevirapine suggest fundamentally different mechanisms of adverse events: cutaneous, most likely major histocompatibility complex (MHC) class I-mediated and influenced by the CYP2B6 nevirapine slow-metabolizer genotype, and hepatic, most likely MHC class II-mediated and unaffected by the CYP2B6 genotype (37). Our study, involving a limited number of subjects, was not designed to compare metabolite exposure and occurrence of side events. Reactive metabolites may form in the liver or skin, so plasma 12-hydroxynevirapine concentrations may not be directly related to toxicity.
This study had several limitations. The studied populations have different characteristics, as the subjects represented different races/ethnicities, HIV statuses, and body mass indexes. The timing of blood sample collection differed, with intensive sampling following a single dose or sparse sampling at steady state. However, results of nevirapine pharmacokinetic parameters are consistent with those of previous studies, allowing comparison of metabolite pharmacokinetics. We did not characterize the glucuronidated metabolites either in plasma or urine, so we could not fully characterize the disposition of phase 1 and phase 2 metabolism of nevirapine.
In conclusion, this study demonstrates different dispositions of unconjugated plasma nevirapine metabolites after single-dose administration of nevirapine and at steady state. All concentrations are well below those of nevirapine, with 12-hydroxynevirapine being the highest. Concentrations of 2-hydroxynevirapine, whose formation is CYP3A mediated, decreased from single dose to steady state, while those of 3-hydroxynevirapine, CYP2B6 mediated, increased from single dose to steady state. Clinical consequences of such findings are presently unknown and warrant further investigation.
ACKNOWLEDGMENTS
This work was funded by the French Agence Nationale de Recherche contre le VIH/SIDA et les Hepatitis Virales (ANRS), who sponsored the ANRS12154 trial, and National Institutes of Health grant no. 5U 01 AI069474. This work was supported in part by NIH grants AI-077505, AI-054999, and TR-000445 (to D.W.H.).
We acknowledge ESTHER, Mérieux foundation, and Institut Pasteur du Cambodge for their support to the ANRS12154 study. We thank all Cambodian health care providers who take care of the patients and the patients who agreed to participate in the study. We acknowledge Michael F. Para, Susan L. Koletar, Lori Mong Kryspin, Julie Kronenberger, and Brian Greenfelder for their generous support at the ACTU Laboratory facility.
The ANRS12154 trial group is constituted as follows. The principal investigators are Monidarin Chou (Phnom Penh, Cambodia) and Anne-Marie Taburet (Paris, France). Coinvestigators are Julie Bertrand, France Mentré, Olivier Segeral, and Céline Verstuyft (Paris, France) and Laurence Borand and Ouk Vara (Phnom Penh, Cambodia).
D. W. Haas has been the principal investigator on research grants to Vanderbilt University from Boehringer Ingelheim, Merck, and Gilead Sciences. None of the other authors have conflicts of interest related to the present study.
Footnotes
Published ahead of print 4 March 2013
REFERENCES
- 1. Calmy A, Pinoges L, Szumilin E, Zachariah R, Ford N, Ferradini L. 2006. Generic fixed-dose combination antiretroviral treatment in resource-poor settings: multicentric observational cohort. AIDS 20:1163–1169 [DOI] [PubMed] [Google Scholar]
- 2. Laurent C, Kouanfack C, Koulla-Shiro S, Nkoue N, Bourgeois A, Calmy A, Lactuock B, Nzeusseu V, Mougnutou R, Peytavin G, Liegeois F, Nerrienet E, Tardy M, Peeters M, Andrieux-Meyer I, Zekeng L, Kazatchkine M, Mpoudi-Ngole E, Delaporte E. 2004. Effectiveness and safety of a generic fixed-dose combination of nevirapine, stavudine, and lamivudine in HIV-1-infected adults in Cameroon: open-label multicentre trial. Lancet 364:29–34 [DOI] [PubMed] [Google Scholar]
- 3. Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, Allen M, Nakabiito C, Sherman J, Bakaki P, Owor M, Ducar C, Deseyve M, Mwatha A, Emel L, Duefield C, Mirochnick M, Fowler MG, Mofenson L, Miotti P, Gigliotti M, Bray D, Mmiro F. 2003. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet 362:859–868 [DOI] [PubMed] [Google Scholar]
- 4. Lallemant M, Jourdain G, Le Coeur S, Mary JY, Ngo-Giang-Huong N, Koetsawang S, Kanshana S, McIntosh K, Thaineua V. 2004. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N. Engl. J. Med. 351:217–228 [DOI] [PubMed] [Google Scholar]
- 5. Lockman S, Shapiro RL, Smeaton LM, Wester C, Thior I, Stevens L, Chand F, Makhema J, Moffat C, Asmelash A, Ndase P, Arimi P, van Widenfelt E, Mazhani L, Novitsky V, Lagakos S, Essex M. 2007. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N. Engl. J. Med. 356:135–147 [DOI] [PubMed] [Google Scholar]
- 6. Chantarangsu S, Cressey TR, Mahasirimongkol S, Capparelli E, Tawon Y, Ngo-Giang-Huong N, Jourdain G, Lallemant M, Chantratita W. 2009. Influence of CYP2B6 polymorphisms on the persistence of plasma nevirapine concentrations following a single intra-partum dose for the prevention of mother to child transmission in HIV-infected Thai women. J. Antimicrob. Chemother. 64:1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antunes AM, Duarte MP, Santos PP, da Costa GG, Heinze TM, Beland FA, Marques MM. 2008. Synthesis and characterization of DNA adducts from the HIV reverse transcriptase inhibitor nevirapine. Chem. Res. Toxicol. 21:1443–1456 [DOI] [PubMed] [Google Scholar]
- 8. Chen J, Mannargudi BM, Xu L, Uetrecht J. 2008. Demonstration of the metabolic pathway responsible for nevirapine-induced skin rash. Chem. Res. Toxicol. 21:1862–1870 [DOI] [PubMed] [Google Scholar]
- 9. Almond LM, Edirisinghe D, Dalton M, Bonington A, Back DJ, Khoo SH. 2005. Intracellular and plasma pharmacokinetics of nevirapine in human immunodeficiency virus-infected individuals. Clin. Pharmacol. Ther. 78:132–142 [DOI] [PubMed] [Google Scholar]
- 10. Gandhi M, Benet LZ, Bacchetti P, Kalinowski A, Anastos K, Wolfe AR, Young M, Cohen M, Minkoff H, Gange SJ, Greenblatt RM. 2009. Nonnucleoside reverse transcriptase inhibitor pharmacokinetics in a large unselected cohort of HIV-infected women. J. Acquir. Immune Defic. Syndr. 50:482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamson MJ, Sabo JP, MacGregor TR, Pav JW, Rowland L, Hawi A, Cappola M, Robinson P. 1999. Single dose pharmacokinetics and bioavailability of nevirapine in healthy volunteers. Biopharm. Drug Dispos. 20:285–291 [DOI] [PubMed] [Google Scholar]
- 12. Erickson DA, Mather G, Trager WF, Levy RH, Keirns JJ. 1999. Characterization of the in vitro biotransformation of the HIV-1 reverse transcriptase inhibitor nevirapine by human hepatic cytochromes P-450. Drug Metab. Dispos. 27:1488–1495 [PubMed] [Google Scholar]
- 13. Cammett AM, MacGregor TR, Wruck JM, Felizarta F, Miailhes P, Mallolas J, Piliero PJ. 2009. Pharmacokinetic assessment of nevirapine and metabolites in human immunodeficiency virus type 1-infected patients with hepatic fibrosis. Antimicrob. Agents Chemother. 53:4147–4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Riska P, Lamson M, MacGregor T, Sabo J, Hattox S, Pav J, Keirns J. 1999. Disposition and biotransformation of the antiretroviral drug nevirapine in humans. Drug Metab. Dispos. 27:895–901 [PubMed] [Google Scholar]
- 15. Hall DB, Macgregor TR. 2007. Case-control exploration of relationships between early rash or liver toxicity and plasma concentrations of nevirapine and primary metabolites. HIV Clin. Trials 8(6):391–399 [DOI] [PubMed] [Google Scholar]
- 16. Ren C, Fan-Havard P, Schlabritz-Loutsevitch N, Ling Y, Chan KK, Liu Z. 2010. A sensitive and specific liquid chromatography/tandem mass spectrometry method for quantification of nevirapine and its five metabolites and their pharmacokinetics in baboons. Biomed. Chromatogr. 24(7):717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haas DW, Gebretsadik T, Mayo G, Menon UN, Acosta EP, Shintani A, Floyd M, Stein CM, Wilkinson GR. 2009. Associations between CYP2B6 polymorphisms and pharmacokinetics after a single dose of nevirapine or efavirenz in African Americans. J. Infect. Dis. 199:872–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chou M, Bertrand J, Segeral O, Verstuyft C, Borand L, Comets E, Le Tiec C, Becquemont L, Ouk V, Mentre F, Taburet AM. 2010. Population pharmacokinetic-pharmacogenetic study of nevirapine in HIV-infected Cambodian patients. Antimicrob. Agents Chemother. 54:4432–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cressey TR, Jourdain G, Lallemant MJ, Kunkeaw S, Jackson JB, Musoke P, Capparelli E, Mirochnick M. 2005. Persistence of nevirapine exposure during the postpartum period after intrapartum single-dose nevirapine in addition to zidovudine prophylaxis for the prevention of mother-to-child transmission of HIV-1. J. Acquir. Immune Defic. Syndr. 38:283–288 [PubMed] [Google Scholar]
- 20. Dailly E, Billaud E, Reliquet V, Breurec S, Perre P, Leautez S, Jolliet P, Bourin M, Raffi F. 2004. No relationship between high nevirapine plasma concentration and hepatotoxicity in HIV-1-infected patients naive of antiretroviral treatment or switched from protease inhibitors. Eur. J. Clin. Pharmacol. 60:343–348 [DOI] [PubMed] [Google Scholar]
- 21. de Maat MM, Huitema AD, Mulder JW, Meenhorst PL, van Gorp EC, Beijnen JH. 2002. Population pharmacokinetics of nevirapine in an unselected cohort of HIV-1-infected individuals. Br. J. Clin. Pharmacol. 54:378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kunz A, Frank M, Mugenyi K, Kabasinguzi R, Weidenhammer A, Kurowski M, Kloft C, Harms G. 2009. Persistence of nevirapine in breast milk and plasma of mothers and their children after single-dose administration. J. Antimicrob. Chemother. 63:170–177 [DOI] [PubMed] [Google Scholar]
- 23. Marier JF, Dimarco M, Guilbaud R, Dodard C, Morelli G, Tippabhotla SK, Singla AK, Thudi NR, Monif T. 2007. Pharmacokinetics of lamivudine, zidovudine, and nevirapine administered as a fixed-dose combination formulation versus coadministration of the individual products. J. Clin. Pharmacol. 47:1381–1389 [DOI] [PubMed] [Google Scholar]
- 24. Molto J, Valle M, Miranda C, Cedeno S, Miranda J, Santos JR, Negredo E, Vilaro J, Costa J, Clotet B. 2008. Once- or twice-daily dosing of nevirapine in HIV-infected adults: a population pharmacokinetics approach. J. Antimicrob. Chemother. 62:784–792 [DOI] [PubMed] [Google Scholar]
- 25. Muro E, Droste JA, Hofstede HT, Bosch M, Dolmans W, Burger DM. 2005. Nevirapine plasma concentrations are still detectable after more than 2 weeks in the majority of women receiving single-dose nevirapine: implications for intervention studies. J. Acquir. Immune Defic. Syndr. 39:419–421 [DOI] [PubMed] [Google Scholar]
- 26. Sabo JP, Lamson MJ, Leitz G, Yong CL, MacGregor TR. 2000. Pharmacokinetics of nevirapine and lamivudine in patients with HIV-1 infection. AAPS PharmSci 2:E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou XJ, Sheiner LB, D'Aquila RT, Hughes MD, Hirsch MS, Fischl MA, Johnson VA, Myers M, Sommadossi JP. 1999. Population pharmacokinetics of nevirapine, zidovudine, and didanosine in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 43:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mo SL, Liu YH, Duan W, Wei MQ, Kanwar JR, Zhou SF. 2009. Substrate specificity, regulation, and polymorphism of human cytochrome P450 2B6. Curr. Drug Metab. 10:730–753 [DOI] [PubMed] [Google Scholar]
- 29. Wang H, Tompkins LM. 2008. CYP2B6: new insights into a historically overlooked cytochrome P450 isozyme. Curr. Drug Metab. 9:598–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wen B, Chen Y, Fitch WL. 2009. Metabolic activation of nevirapine in human liver microsomes: dehydrogenation and inactivation of cytochrome P450 3A4. Drug Metab. Dispos. 37:1557–1562 [DOI] [PubMed] [Google Scholar]
- 31. Greenblatt DJ, von Moltke LL, Daily JP, Harmatz JS, Shader RI. 1999. Extensive impairment of triazolam and alprazolam clearance by short term low-dose ritonavir: the clinical dilemma of concurrent inhibition and induction. J. Clin. Psychopharmacol. 19:293–296 [DOI] [PubMed] [Google Scholar]
- 32. Kirby BJ, Collier AC, Kharasch ED, Whittington D, Thummel KE, Unadkat JD. 2011. Complex drug interactions of HIV protease inhibitors 1: inactivation, induction, and inhibition of cytochrome P450 3A by ritonavir or nelfinavir. Drug Metab. Dispos. 39:1070–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Back D, Gibbons S, Khoo S. 2003. Pharmacokinetic drug interactions with nevirapine. J. Acquir. Immune Defic. Syndr. 34(Suppl 1):S8–S14 [DOI] [PubMed] [Google Scholar]
- 34. Dixit V, Hariparsad N, Li F, Desai P, Thummel KE, Unadkat JD. 2007. Cytochrome P450 enzymes and transporters induced by anti-human immunodeficiency virus protease inhibitors in human hepatocytes: implications for predicting clinical drug interactions. Drug Metab. Dispos. 35:1853–1859 [DOI] [PubMed] [Google Scholar]
- 35. Kojima K, Nagata K, Matsubara T, Yamazoe Y. 2007. Broad but distinct role of pregnane X receptor on the expression of individual cytochrome p450s in human hepatocytes. Drug Metab. Pharmacokinet. 22(4):276–286 [DOI] [PubMed] [Google Scholar]
- 36. Murphy RL. 2003. Defining the toxicity profile of nevirapine and other antiretroviral drugs. J. Acquir. Immune Defic. Syndr. 34(Suppl 1):S15–S20 [DOI] [PubMed] [Google Scholar]
- 37. Yuan J, Guo S, Hall D, Cammett AM, Jayadev S, Distel M, Storfer S, Huang Z, Mootsikapun P, Ruxrungtham K, Podzamczer D, Haas DW. 2011. Toxicogenomics of nevirapine-associated cutaneous and hepatic adverse events among populations of African, Asian, and European descent. AIDS 25:1271–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]