Abstract
Ureaplasma respiratory tract colonization is associated with bronchopulmonary dysplasia (BPD) in preterm infants. Previously, we demonstrated that a single intravenous (i.v.) dose of azithromycin (10 mg/kg of body weight) is safe but inadequate to eradicate Ureaplasma spp. in preterm infants. We performed a nonrandomized, single-arm open-label study of the pharmacokinetics (PK) and safety of intravenous 20-mg/kg single-dose azithromycin in 13 mechanically ventilated neonates with a gestational age between 24 weeks 0 days and 28 weeks 6 days. Pharmacokinetic data from 25 neonates (12 dosed with 10 mg/kg i.v. and 13 dosed with 20 mg/kg i.v.) were analyzed using a population modeling approach. Using a two-compartment model with allometric scaling of parameters on body weight (WT), the population PK parameter estimates were as follows: clearance, 0.21 liter/h × WT(kg)0.75 [WT(kg)0.75 indicates that clearance was allometrically scaled on body weight (in kilograms) with a fixed exponent of 0.75]; intercompartmental clearance, 2.1 liters/h × WT(kg)0.75; central volume of distribution (V), 1.97 liters × WT (kg); and peripheral V, 17.9 liters × WT (kg). There was no evidence of departure from dose proportionality in azithromycin exposure over the tested dose range. The calculated area under the concentration-time curve over 24 h in the steady state divided by the MIC90 (AUC24/MIC90) for the single dose of azithromycin (20 mg/kg) was 7.5 h. Simulations suggest that 20 mg/kg for 3 days will maintain azithromycin concentrations of >MIC50 of 1 μg/ml for this group of Ureaplasma isolates for ≥96 h after the first dose. Azithromycin was well tolerated with no drug-related adverse events. One of seven (14%) Ureaplasma-positive subjects and three of six (50%) Ureaplasma-negative subjects developed physiologic BPD. Ureaplasma was eradicated in all treated Ureaplasma-positive subjects. Simulations suggest that a multiple-dose regimen may be efficacious for microbial clearance, but the effect on BPD remains to be determined.
INTRODUCTION
Bronchopulmonary dysplasia (BPD), a disease of multifactorial etiology, causes significant morbidity and mortality in infants born preterm. Respiratory tract colonization with the genital mycoplasma species Ureaplasma parvum and Ureaplasma urealyticum is associated with risk for development of BPD in extremely low gestation infants (1, 2). It is unknown whether eradicating Ureaplasma spp. from the respiratory tracts of preterm infants with appropriate antibiotic therapy will prevent Ureaplasma infection-mediated lung injury. Our first step to address this question has been to conduct studies in the at-risk population to determine the optimal dose, safety, and in vivo anti-infective efficacy of specific antibiotics in preparation for future phase III randomized, placebo-controlled trials.
Azithromycin, an azalide antibiotic has anti-inflammatory properties (3, 4) and antimicrobial activity against Ureaplasma spp. in vitro (5–7) and in in vivo experimental models (8). Azithromycin exhibits 2-fold greater potency than erythromycin against Ureaplasma isolates in vitro (5, 9). Azithromycin therapy may enhance Ureaplasma clearance in infected infants and inhibit the pulmonary inflammatory response in both infected and noninfected infants, possibly contributing to a decreased risk for BPD (10).
Although the efficacy of azithromycin and a related macrolide, clarithromycin, to prevent BPD has been assessed in single-center studies of at-risk preterm infants (11, 12), the optimal dosing regimens for these antibiotics have not been determined in pharmacokinetic and pharmacodynamic studies. Recently, we characterized the pharmacokinetics (PK) of a single dose of intravenous (i.v.) azithromycin (10 mg/kg of body weight) in preterm infants (13). We demonstrated that a single 10-mg/kg azithromycin i.v. dose in mechanically ventilated infants with a gestational age of 24 to 28 weeks was safe but inadequate to eradicate Ureaplasma from the respiratory tract (13). Since increasing the dose is the most effective strategy to increase the area under the concentration-time curve over 24 h in the steady state divided by the MIC90 (AUC24/MIC90) (14), the pharmacodynamic parameter that predicts azithromycin efficacy for clearance of other organisms in preclinical models (15) and human studies (16, 17), the current study was designed to collect additional data on azithromycin PK in preterm infants at a higher dose level (20 mg/kg i.v.) to assess the safety of such a regimen and to determine the microbiological and clinical outcomes of the higher single dose. We hypothesized that intravenous azithromycin therapy will prevent BPD in Ureaplasma-colonized preterm infants by accelerating pathogen clearance and/or downregulating the pulmonary inflammatory response.
MATERIALS AND METHODS
Eligibility criteria.
We conducted a phase IIa nonrandomized, open-label, single-dose 20-mg/kg PK and safety study of intravenous azithromycin in mechanically ventilated preterm neonates with a gestational age from 24 weeks 0 days (240) to 28 weeks 6 days (286). Study subjects were recruited from the University of Maryland Medical Center, Baltimore, MD (UMB) and the University of Virginia, Charlottesville, VA (UVA) from June 2009 to June 2010. The institutional review board of each institution approved the study, and parental informed consent was obtained. Infants between 240 to 286 weeks admitted to the neonatal intensive care unit (NICU) less than 72 h of age were screened for study eligibility. Inclusion criteria were size appropriate for gestational age, mechanical ventilation for any duration during the first 48 h of life, and presence of indwelling intravenous and arterial lines. Exclusion criteria included major lethal congenital anomalies, nonviability or planned withdrawal of life support, hypotension for greater than 6 continuous hours, corrected QT interval (QTc) of >0.45 s, significant renal impairment, significant hepatic impairment, exposure to any other macrolide antibiotic, delivery for maternal indications, maternal receipt of a macrolide within 7 days prior to delivery, and participation in other clinical trials.
Drug administration and blood sampling.
After baseline studies were completed, each infant enrolled in the study received 20 mg/kg of intravenous azithromycin in 2-mg/ml concentration over 1 h. Six blood samples (0.5 ml each) were collected from an existing indwelling arterial catheter inserted for clinical indications or heel stick at 0 to 1, 1 to 4, 6 to 8, 24 to 48, 48 to 96, and 96 to 144 h postdose. The sampling windows were selected to allow precise characterization of the distribution and elimination phases of azithromycin (13). The blood samples were centrifuged, and the plasma was aspirated and frozen at −80°C. The levels of azithromycin in plasma were measured using a validated high-performance liquid chromatography-tandem mass spectroscopy (HPLC/MS/MS) detection method (18). The lower limit of detection was 10 ng/ml. The precision determined by replicate injections of quality control samples was below 15% coefficient of variation (CV) for all concentrations, but the lowest concentration had a 19.7% CV.
Pharmacokinetic data analysis.
Pharmacokinetic (PK) data for the 20-mg/kg i.v. dose from the present study were combined with published data for the 10-mg/kg i.v. dose (13) and analyzed simultaneously using a population modeling approach. Overall, data from 25 neonates (12 dosed with 10 mg/kg i.v. and 13 dosed with 20 mg/kg i.v.) were available for the PK analysis. The nonlinear mixed-effects modeling software NONMEM (version 7) (ICON Development Solutions, Ellicott City, MD) was used for the analysis. The first-order conditional estimation method with interaction was used throughout the modeling procedure. The final model was a two-compartment structural model (ADVAN3 TRANS4 NONMEM subroutine) with first-order elimination. The model selection was based on evaluation of the objective function (OF) value, pharmacokinetic parameter estimates and their relative standard errors, physiologic plausibility of the parameter estimates, and inspection of goodness-of-fit plots. The likelihood ratio test was used for comparing rival hierarchical models where a decrease in OF (−2-log-unit likelihood) of 6.6 points was necessary to consider the improvement in model performance statistically significant at P = 0.01 and 1 degree of freedom (19). An exponential error model was used to describe the interindividual variability in the pharmacokinetic parameters as follows: Pi = TVPexp(ηi) where ηi is the proportional difference between the hypothetical true parameter estimate of the ith subject (Pi) and the typical population parameter value (TVP) and is assumed to be normally distributed with a mean of 0 and a variance of ω2. The residual error (which includes model misspecification, intrasubject variability, and errors in dosing, sampling times, and sample analysis) was described using a proportional error model as follows: Yobs = Ypred(1 + ε) where Yobs is the observed plasma drug concentration, Ypred is the model-predicted plasma drug concentration, and ε is a normally distributed parameter with a mean of 0 and variance of σ2. The pharmacokinetic parameters were allometrically scaled on body weight with fixed exponents of 0.75 for the clearance parameters and 1 for the volume parameters. Such scaling resulted in significant drop (∼64 points) in the NONMEM objective function, indicating significant improvement in the model fit to the data. There was no evidence of departure from dose proportionality in azithromycin exposure as the dose increased from 10 mg/kg i.v. to 20 mg/kg i.v. (i.e., dose was not a significant covariate for azithromycin clearance).
To evaluate the performance of the final model, the model was used to simulate 10-mg/kg and 20-mg/kg i.v. single-dose administration (500 simulated studies each with 12 neonates/arm with similar weight distribution as observed in the analyzed studies). The median, 5th and 95th percentiles of plasma drug concentrations at each time point were calculated for each study replicate. Subsequently, the medians of the aforementioned parameters were calculated across the 500 replicates and compared graphically to the observed data. Additionally, an i.v. azithromycin dosage regimen of 20 mg/kg/day for 3 days was simulated to evaluate whether this regimen will maintain azithromycin concentrations above the MIC50. All simulations were conducted using Pharsight Trial Simulator software (version 2.2.1; Pharsight Corporation, Mountain View, CA).
Ureaplasma culture and antibiotic susceptibility testing.
Two tracheal aspirate samples at least 2 h apart and one nasopharyngeal sample were obtained predose and subsequent samples were obtained at 2 and 4 or 5 days postdose and 21 days postnatal age (PNA) as previously described (13). Follow-up specimens at the later time points were obtained from the endotracheal tube if the infant remained intubated and from the nasopharynx if the infant was extubated. All culture specimens were immediately inoculated in 10B broth (20) and placed on ice until processed in the laboratory. One-half of each culture specimen was serially diluted with 10B broth at UMB and incubated as a fresh culture, and the other half of the original specimen was frozen for shipment on dry ice to the University of Alabama at Birmingham (UAB) Diagnostic Mycoplasma Laboratory for confirmation (20). Local cultures were incubated at 37°C and observed for broth color change indicative of urea hydrolysis. All presumptive-positive broth cultures from UMB and all original specimens collected at UVA were shipped to UAB frozen on dry ice for culture by quantitative 10B broth and A8 agar culture, species-specific PCR, and azithromycin susceptibility testing by broth microdilution as described previously (13). Ureaplasma eradication was defined as 3 negative cultures postdose confirmed by the UAB Diagnostic Mycoplasma Laboratory.
Ureaplasma species detection and species identification by real-time PCR assay.
In addition to culture, all clinical specimens were tested for U. urealyticum and U. parvum by a real-time PCR. Individual Ureaplasma isolates obtained by culture were also tested by PCR to determine the Ureaplasma species. Genomic DNA from clinical specimens and Ureaplasma isolates was extracted by the proteinase K method as described previously (21). A multiplex real-time PCR assay was used to detect and differentiate the 2 Ureaplasma species simultaneously using the Roche LightCycler 2.0 (Roche Diagnostics, Indianapolis, IN) as previously described (13, 22).
Clinical outcomes.
All infants were monitored for safety and tolerability of the drug during the study period as previously described for the 10-mg/kg cohort (13). For the BPD endpoint, the physiologic definition of BPD based on oxygen saturation monitoring was used (23). For neonates at 36 ± 1 week postmenstrual age (PMA) or predischarge who were on positive-pressure support or receiving >30% supplemental oxygen with oxygen saturations between 90% and 96%, the diagnosis of BPD was assigned with no further testing. Neonates at 36 ± 1 week PMA or predischarge who were receiving <30% oxygen with oxygen saturations between 90% and 96% or receiving ≥30% oxygen with oxygen saturations of >96% underwent a timed stepwise reduction by 2% increments every 5 min to room air and a period of observation in room air for 30 min by the method of Walsh et al. (23). For infants receiving supplemental oxygen by nasal cannula, the delivered fraction of inspired oxygen concentration or “effective FiO2” (23) was calculated by the technique of Benaron and Benitz (24) that was used in the supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP-ROP) trial (25), which is based on weight, oxygen liter flow, and oxygen concentration. An infant failed the room air challenge if the infant had oxygen saturations of 80 to 89% for 5 consecutive min or <80% for 15 s. An infant was considered to not have BPD if the infant had oxygen saturation of ≥90% in room air or if the infant passed the timed, oxygen reduction test. Study subjects who were transferred or discharged at ≤35 weeks PMA on supplemental oxygen were considered to have BPD.
RESULTS
Study enrollment.
Forty-four of 102 infants (43%) with a gestational age of 24 weeks 0 days (240) to 28 weeks 6 days (286) less than 72 h age upon NICU admission who were screened at both study sites were eligible for the study. Parents of 41 infants were approached for consent, 3 were missed, and 13 consented to the study. Reasons for noneligibility in this study were as follows: death or considered nonviable (n = 13), delivery for maternal indications (n = 10), no arterial access (n = 7), maternal receipt of macrolide (n = 6), not intubated within the first 48 h (n = 5), major anomalies (n = 4), or other factors (n = 13). All enrolled subjects received the single intravenous 20-mg/kg dose of azithromycin.
Subject characteristics.
Seven (54%) of the 13 enrolled subjects in the 20-mg/kg cohort were Ureaplasma positive prior to receiving study drug. Four subjects were colonized with U. parvum alone (57%), 2 subjects were colonized with U. urealyticum alone (29%), and 1 subject was colonized with both species (14). As shown in Table 1, the gestational age, birth weight, and race distribution of the Ureaplasma-positive and -negative groups were similar. The Ureaplasma-positive infants experienced longer duration of mechanical ventilation at the time of entry into the study, but there was no difference between the 2 subgroups for postnatal age at the time of azithromycin dosing.
Table 1.
Comparison of characteristics of the 20-mg/kg azithromycin single-dose combined cohort and stratified by Ureaplasma respiratory tract colonization status
| Variablea | No. of subjects (%) or parameter value (mean ± SD) for: |
P valueb | ||
|---|---|---|---|---|
| All subjects (n = 13) | Ureaplasma-positive subjects (n = 7) | Ureaplasma-negative subjects (n = 6) | ||
| Gestational age (wk) | 25.6 ± 1.2 | 25.6 ± 1.3 | 25.7 ± 1.2 | 0.893 |
| Birth weight (g) | 870 ± 116 | 914 ± 129 | 818 ± 80 | 0.143 |
| Sex (male) | 10 (77) | 4 (57) | 6 (100) | 0.067 |
| Race | ||||
| White | 6 (46) | 3 (43) | 3 (50) | |
| African-American | 6 (46) | 4 (57) | 2 (33) | 0.450 |
| Asian | 1 (8) | 0 | 1 (17) | |
| Mixed | 0 | 0 | 0 | |
| Ethnicity (Hispanic) | 0 | 0 | 0 (0) | 1.0 |
| Positive-pressure support at study entry | ||||
| CPAP | 4 (31) | 3 (43) | 1 (17) | 0.308 |
| IMV/HFOV | 9 (69) | 4 (57) | 5 (83) | |
| IMV duration at study entry | 27.7 ± 15.8 | 36.1 ± 16.2 | 17.8 ± 8.2 | 0.030 |
| FiO2 at study entry | 0.24 ± 0.01 | 0.22 ± 0.02 | 0.26 ± 0.04 | 0.073 |
| Postnatal age at dose (h) | 64.9 ± 30.3 | 60.2 ± 26.0 | 70.3 ± 36.5 | 0.573 |
CPAP, continuous positive airway pressure; IMV, intermittent mandatory ventilation; HFOV, high-frequency oscillatory ventilation; FiO2, delivered fraction of inspired oxygen concentration.
Statistical comparison between ureaplasma-positive subjects and ureaplasma-negative subjects.
Pharmacokinetic analysis.
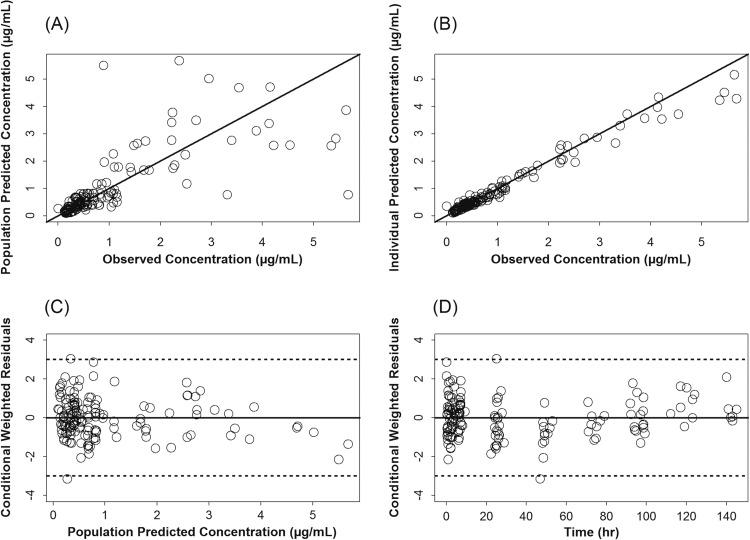
Plasma azithromycin concentrations (n = 149) from 25 neonates (12 dosed with 10 mg/kg i.v. and 13 dosed with 20 mg/kg i.v.) were available for the PK analysis. Diagnostic plots for the updated population PK model of azithromycin are presented in Fig. 1. These plots indicate that the model described the data well with no systematic bias. The population PK parameters of azithromycin in preterm infants are presented in Table 2. These parameters were in agreement with the parameter estimates of the model built based on the 10-mg/kg i.v. single-dose data only (13).
Fig 1.
Diagnostic scatter plots for the population PK model, including data from the 10- and 20-mg/kg single doses of azithromycin (AZI) given i.v. (A) Population-predicted versus observed AZI plasma concentrations; (B) individual-predicted versus observed AZI plasma concentrations; (C) conditional weighted residuals versus population-predicted plasma AZI concentrations; (D) conditional weighted residuals versus time. (The solid black lines represent the lines of identity in panels A and B and zero residuals in panels C and D. Dashed lines represent ±3 standard deviations.)
Table 2.
Parameter estimates of the population modela
| Parameterb | Estimate (% RSE) | % ISV (% RSE) |
|---|---|---|
| CL (liter/h) | 0.21 × WT(kg)0.75 (6) | 31 (27) |
| V1 (liter) | 1.97 × WT(kg) (19) | 86 (39) |
| Q (liter/h) | 2.1 × WT(kg)0.75 (9) | 39 (39) |
| V2 (liter) | 17.9 × WT(kg) (9) | 39 (31) |
| Residual error (%) | 24.5 (29) |
ISV, intersubject variability, calculated as the square root of the estimated variance of intersubject variability × 100; RSE, relative standard error; WT(kg), body weight (in kilograms); WT(kg)0.75, body weight (in kilograms) allometrically scaled with a fixed exponent of 0.75.
CL, clearance; V1, volume of distribution in the central compartment; Q, blood flow; V2, volume of distribution in the peripheral compartment.
In this study, we defined the concentration-time profile of azithromycin (20 mg/kg). Following intravenous administration, azithromycin achieved a high serum concentration above MIC90 and rapidly decreased to a level below MIC50 (1 μg/ml) within a few hours (Fig. 2). Based on the updated model, for the average observed weight of 0.87 kg for a neonate administered azithromycin in a 20-mg/kg i.v. dose, the estimated area under the plasma drug concentration-time curve from 0 h to infinity (AUC0–∞) is 105 μg · h/ml, and the AUC from time zero to 24 h postdose is approximately 30 μg · h/ml. In adults, 30% of the azithromycin dose is bound to plasma proteins, but the percent binding in newborns is unknown. Without correction for possible plasma protein binding, the calculated AUC24/MIC90 for the single azithromycin 20-mg/kg dose is 7.5 h, using a MIC90 of 4 μg/ml (13).
Fig 2.
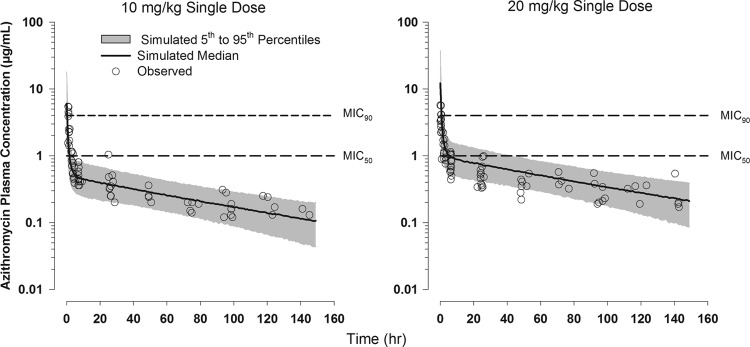
Observed and model-simulated plasma azithromycin concentration-versus-time profiles following administration of single 10- and 20-mg/kg doses of azithromycin given i.v. to preterm neonates relative to in vitro MIC90 (4 μg/ml) and MIC50 (1 μg/ml). Data for the 10-mg/kg dose were previously published (13).
The updated PK model was used to predict azithromycin AUC24/MIC90 and plasma azithromycin concentration-versus-time profiles with administration of 20 mg/kg for 3 days in preterm infants (Fig. 3). Simulations suggest that 3-day dosing will provide an AUC24/MIC90 of 7 h and, on average, maintain plasma drug concentrations above the MIC50 for more than 96 h after the first dose.
Fig 3.
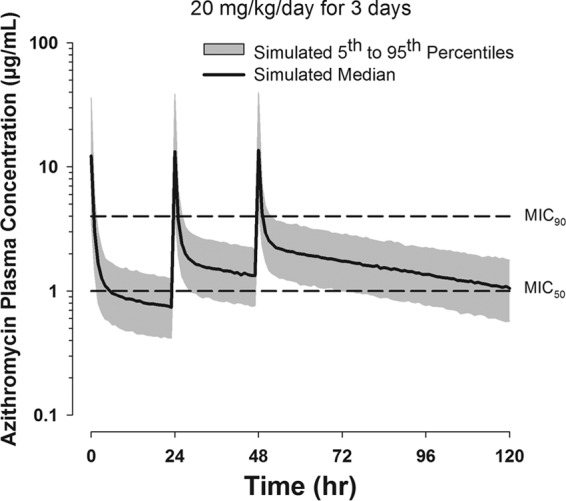
Simulated plasma azithromycin concentration-versus-time profiles in preterm neonates with administration of a regimen of 20 mg/kg/day for 3 days based on the updated PK model developed with the 10- and 20-mg single-dose data. The MIC90 and MIC50 are depicted by the dashed lines.
Ureaplasma culture, clearance, and azithromycin susceptibility.
Predose, 6 subjects were Ureaplasma culture and PCR positive and 1 subject was culture negative but PCR positive (Fig. 4). All subjects in the 20-mg/kg cohort were culture negative at all follow-up time points. Four of the seven original PCR-positive subjects remained PCR positive 2 days postdose, and one subject remained PCR positive 5 days postdose. All samples at postnatal day 21 were PCR negative. Therefore, there were no treatment failures. The MICs for Ureaplasma isolates for the combined 10- and 20-mg/kg single-dose cohorts ranged from 0.5 to 8 μg/ml.
Fig 4.
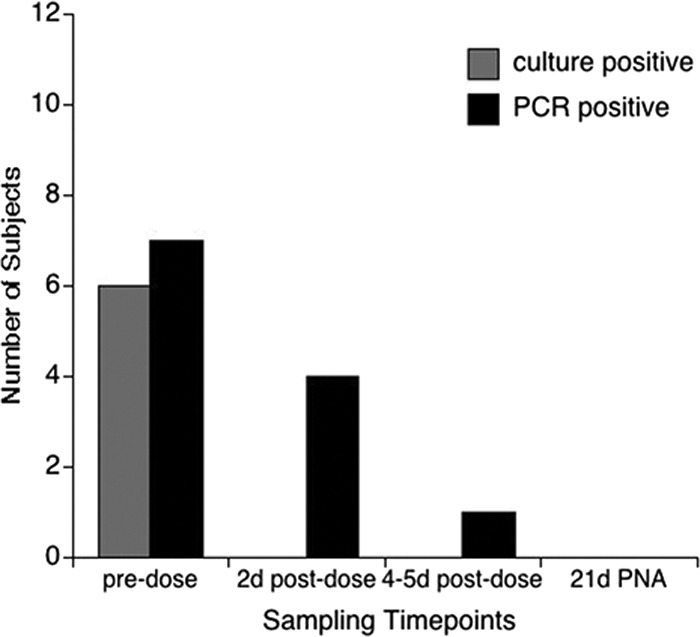
Microbial clearance following a single 20-mg/kg dose of AZI. Seven subjects were Ureaplasma positive prior to AZI dosing by culture were confirmed by A8 agar colonial morphology and/or PCR. Two days (2d) postdose, one subject remained culture and PCR positive, and 5 were PCR positive only. All respiratory samples were culture and PCR negative 5 days postdose and 21 days postnatal age (21d PNA).
Clinical outcomes.
Four of 13 (31%) infants developed physiologic BPD. One of seven (14%) Ureaplasma-positive subjects developed BPD compared to three of six (50%) Ureaplasma-negative infants (P = 0.164). All study subjects survived to discharge.
The single dose of azithromycin at 20 mg/kg was well tolerated, and there were no reported azithromycin-related side effects. Severe intraventricular hemorrhage (IVH) (≥grade 3) was observed on cranial ultrasounds in 3 subjects (1 Ureaplasma-positive subject and 2 Ureaplasma-negative subjects) predose and one Ureaplasma-negative subject between study days 3 and 7. Periventricular leukomalacia was noted in one Ureaplasma-negative infant on cranial ultrasound obtained within 2 days postdose. Three infants in the Ureaplasma-positive group failed the hearing screen in both ears at discharge. There were no reported occurrences of necrotizing enterocolitis in the 20-mg/kg single-dose group. Of the serious adverse events described above, none were attributed to the drug.
DISCUSSION
In the present population PK analysis of azithromyin in preterm infants, a two-compartment model with allometric scaling of all parameters on body weight (WT) provided the best fit to the data. Azithromycin fixed-effects pharmacokinetic parameter estimates from the current analysis, combining the 10- and 20-mg/kg i.v. single-dose data, were comparable to results from our previous analysis of 10-mg/kg data alone (13). In the present analysis, dose was tested as a covariate on azithromycin clearance, and inclusion of this covariate did not lead to any appreciable improvement in the model fit. Therefore, with the limited available data, there is no evidence of departure from dose proportionality in azithromycin exposure over the 10- to 20-mg/kg dose range in premature neonates. As such, the final PK model was a linear model that was able to reasonably predict the observed concentrations for both dose levels in simulations (Fig. 2). With the increased number of neonates included in the current analysis, the importance of allometric scaling all four PK parameters (elimination clearance, intercompartmental clearance, and volumes of the central and peripheral compartments) was more evident.
The increased number of neonates included in the current analysis allowed for more reliable estimation of residual between-subject variability in azithromycin PK. This has resulted in improvement in the model fit at the individual subject level compared to the previous analysis of the 10-mg/kg data (13). The model replicated the between-subject variability fairly well in simulation, indicating that estimation of the between-subject variability on all PK parameters in the current analysis was not due to overparameterization that would have caused variability to be overinflated in simulations. Overall, the good agreement between observed and simulated concentrations for single azithromycin intravenous doses suggest that the model may be a useful tool for simulating multiple-dose regimens for azithromycin in preterm neonates. Uncertainty remains due to lack of information regarding time linearity and impact of azithromycin accumulation with repeated dosing in premature neonates. Our next multidose study will address the sources of uncertainty.
In this study, we defined the concentration-time profile of azithromycin given in a single 20-mg/kg dose. We observed that following intravenous administration, azithromycin achieves a high serum drug concentration above MIC90 and rapidly decreases to a level below MIC50 within a few hours. This finding agrees with the predicted azithromycin plasma concentration-versus-time profile based on the PK model derived from the 10-mg/kg study (13). Previous pharmacodynamic studies indicate that the AUC24/MIC90 best predicts azithromycin efficacy. The target range of AUC24/MIC90 required for bacterial eradication varies from >5 for common respiratory tract infections (17) to 25 to 35 for Streptococcus pneumoniae-associated community-acquired pneumonia in adults (16), but the optimal target for Ureaplasma clearance from the preterm infant respiratory tract has not been established. In the current study, a single intravenous 20-mg/kg dose of azithromycin achieved an AUC24/MIC90 of 7.5 h. Despite the apparent clearance of ureaplasmas in all subjects treated with 20 mg/kg compared to the 43% failure rate in the 10-mg/kg group (13), simulations suggest that a multiple azithromycin dose regimen of 20 mg/kg for 3 days will achieve comparable AUC24/MIC90 with prolonged time above MIC50 that we speculate might contribute to more effective clearance, reduced pulmonary inflammation, and improved clinical outcomes. Efficacy of azithromycin for Ureaplasma clearance and impact on pulmonary inflammation will need to be evaluated in a larger randomized trial.
The importance of pharmacokinetic/pharmacodynamic studies is underscored by the failure of a previous low-dose regimen of azithromycin (11) and of the related macrolide erythromycin to eradicate Ureaplasma spp. from the newborn respiratory tract or prevent BPD (26–29). Clarithromycin treatment (10 mg/kg twice per day for 10 days) of Ureaplasma nasopharynx-colonized preterm infants with a birth weight of 750 to 1,250 g in a large center in Ankara, Turkey resulted in a reduced BPD rate in the treatment group compared to placebo (2.9% versus 36.4%; P < 0.001) but failed to eradicate Ureaplasma colonization in 31.5%. Follow-up cultures were not done in the placebo group to determine the rate of spontaneous clearance and hence the effective eradication rate of clarithromycin treatment. This study suggests that BPD may be reduced by macrolides without complete eradication of the organism, potentially due to immunomodulatory effects. However, neither systemic nor pulmonary inflammatory mediators were assessed in the clarithromycin study.
Both azithromycin and clarithromycin are proarrhythmic with prior reports of occurrences of QT interval prolongation and torsades de pointes (30). Recently, a retrospective study of a large Tennessee Medicaid cohort detected a small absolute increased risk of cardiovascular death (hazard ratio, 2.88; 95% confidence interval [95% CI], 1.79 to 4.63) in adults who took a 5-day course of azithromycin compared to individuals who took no antibiotics (30). There were 47 additional cardiovascular deaths per 1 million azithromycin courses. The increased risk for cardiovascular death was highest among patients with a high baseline risk for cardiovascular disease.
The implications of that study for azithromycin use in newborns and children are unclear. There is a single case report in the literature of cardiac arrest with suspected prolonged QT in a 9 month old who inadvertently received 50 mg/kg azithromycin intravenously over 20 min (31). Prolonged QT interval is rare among newborns with an incidence of the heritable long QT syndromes estimated between 1 per 3,000 and 1 per 5,000 births (32). On the basis of a retrospective study of prolonged QT interval in neonates, Villain and coworkers (33) suggest that infants presenting with a corrected QT interval (QTc) of <0.5 s normalize their QTc over time, while infants with a QTc of >0.6 s are at risk for severe arrhythmias and sudden cardiovascular death. Prolonged QTc was not detected in any of the 171 preterm infants we screened for the 10-mg/kg and 20-mg/kg single-dose studies. However, careful screening of potential subjects for prolonged QT interval in subsequent studies of multidose azithromycin is recommended.
In conclusion, we did not observe any large threats to safety with a 20-mg/kg dose of azithromycin in the preterm population and developed a PK model that can be used for future studies. On the basis of findings from our two studies, we are prepared to conduct a phase IIa multicenter trial with a larger sample size to study the PK and efficacy of multidose azithromycin to eradicate Ureaplasma from the respiratory tracts of preterm infants.
ACKNOWLEDGMENTS
This study was supported by a grant NIH NICHD R21 HD056424.
Technical support for performance of mycoplasma cultures, antibiotic susceptibility testing, and PCR assays from Donna Crabb and Amy Ratliff is gratefully acknowledged.
Ahmed A. Othman is a former employee of the University of Maryland and is currently an employee of Abbott Laboratories.
Footnotes
Published ahead of print 25 February 2013
REFERENCES
- 1. Schelonka RL, Katz B, Waites KB, Benjamin DK., Jr 2005. Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr. Infect. Dis. J. 24:1033–1039 [DOI] [PubMed] [Google Scholar]
- 2. Viscardi RM, Hasday JD. 2009. Role of Ureaplasma species in neonatal chronic lung disease: epidemiologic and experimental evidence. Pediatr. Res. 65:84R–90R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amsden GW. 2005. Anti-inflammatory effects of macrolides–an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J. Antimicrob. Chemother. 55:10–21 [DOI] [PubMed] [Google Scholar]
- 4. Beigelman A, Gunsten S, Mikols CL, Vidavsky I, Cannon CL, Brody SL, Walter MJ. 2009. Azithromycin attenuates airway inflammation in a noninfectious mouse model of allergic asthma. Chest 136:498–506 [DOI] [PubMed] [Google Scholar]
- 5. Matlow A, Th'ng C, Kovach D, Quinn P, Dunn M, Wang E. 1998. Susceptibilities of neonatal respiratory isolates of Ureaplasma urealyticum to antimicrobial agents. Antimicrob. Agents Chemother. 42:1290–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duffy LB, Crabb D, Searcey K, Kempf MC. 2000. Comparative potency of gemifloxacin, new quinolones, macrolides, tetracycline and clindamycin against Mycoplasma spp. J. Antimicrob. Chemother. 45(Suppl 1):29–33 [DOI] [PubMed] [Google Scholar]
- 7. Waites KB, Crabb DM, Duffy LB. 2009. Comparative in vitro susceptibilities of human mycoplasmas and ureaplasmas to a new investigational ketolide, CEM-101. Antimicrob. Agents Chemother. 53:2139–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walls SA, Kong L, Leeming HA, Placencia FX, Popek EJ, Weisman LE. 2009. Antibiotic prophylaxis improves Ureaplasma-associated lung disease in suckling mice. Pediatr. Res. 66:197–202 [DOI] [PubMed] [Google Scholar]
- 9. Waites KB, Crabb DM, Duffy LB. 2003. In vitro activities of ABT-773 and other antimicrobials against human mycoplasmas. Antimicrob. Agents Chemother. 47:39–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Turner MA, Jacqz-Aigrain E, Kotecha S. 2012. Azithromycin, Ureaplasma and chronic lung disease of prematurity: a case study for neonatal drug development. Arch. Dis. Child. 97:573–577 [DOI] [PubMed] [Google Scholar]
- 11. Ballard HO, Shook LA, Bernard P, Anstead MI, Kuhn R, Whitehead V, Grider D, Crawford TN, Hayes D., Jr 2011. Use of azithromycin for the prevention of bronchopulmonary dysplasia in preterm infants: a randomized, double-blind, placebo controlled trial. Pediatr. Pulmonol. 46:111–118 [DOI] [PubMed] [Google Scholar]
- 12. Ozdemir R, Erdeve O, Dizdar EA, Oguz SS, Uras N, Saygan S, Karabulut E, Dilmen U. 2011. Clarithromycin in preventing bronchopulmonary dysplasia in Ureaplasma urealyticum-positive preterm infants. Pediatrics 128:e1496–e1501 [DOI] [PubMed] [Google Scholar]
- 13. Hassan HE, Othman AA, Eddington ND, Duffy L, Xiao L, Waites KB, Kaufman DA, Fairchild KD, Terrin ML, Viscardi RM. 2011. Pharmacokinetics, safety, and biologic effects of azithromycin in extremely preterm infants at risk for ureaplasma colonization and bronchopulmonary dysplasia. J. Clin. Pharmacol. 51:1264–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon EM, Blumer JL. 2004. Rationale for single and high dose treatment regimens with azithromycin. Pediatr. Infect. Dis. J. 23:S102–S107 [DOI] [PubMed] [Google Scholar]
- 15. Girard D, Finegan SM, Dunne MW, Lame ME. 2005. Enhanced efficacy of single-dose versus multi-dose azithromycin regimens in preclinical infection models. J. Antimicrob. Chemother. 56:365–371 [DOI] [PubMed] [Google Scholar]
- 16. Noreddin AM, El-Khatib WF, Aolie J, Salem AH, Zhanel GG. 2009. Pharmacodynamic target attainment potential of azithromycin, clarithromycin, and telithromycin in serum and epithelial lining fluid of community-acquired pneumonia patients with penicillin-susceptible, intermediate, and resistant Streptococcus pneumoniae. Int. J. Infect. Dis. 13:483–487 [DOI] [PubMed] [Google Scholar]
- 17. Muto C, Liu P, Chiba K, Suwa T. 2011. Pharmacokinetic-pharmacodynamic analysis of azithromycin extended release in Japanese patients with common respiratory tract infectious disease. J. Antimicrob. Chemother. 66:165–174 [DOI] [PubMed] [Google Scholar]
- 18. Barrett B, Borek-Dohalsky V, Fejt P, Vaingatova S, Huclova J, Nemec B, Jelinek I. 2005. Validated HPLC-MS-MS method for determination of azithromycin in human plasma. Anal. Bioanal Chem. 383:210–217 [DOI] [PubMed] [Google Scholar]
- 19. Sheiner LB, Ludden TM. 1992. Population pharmacokinetics/dynamics. Annu. Rev. Pharmacol. Toxicol. 32:185–209 [DOI] [PubMed] [Google Scholar]
- 20. Waites KB, Bebear CM, Robertson JA, Talkington DF, Kenny GE. 2001. Cumitech 34, Laboratory diagnosis of mycoplasmal infections. Coordinating ed, Nolte FS. American Society for Microbiology, Washington, DC [Google Scholar]
- 21. Blanchard A, Hentschel J, Duffy L, Baldus K, Cassell GH. 1993. Detection of Ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amniotic fluid, and in the respiratory tract of newborns. Clin. Infect. Dis. 17(Suppl 1):S148–S153 [DOI] [PubMed] [Google Scholar]
- 22. Xiao L, Glass JI, Paralanov V, Yooseph S, Cassell GH, Duffy LB, Waites KB. 2010. Detection and characterization of human ureaplasma species and serovars by real-time PCR. J. Clin. Microbiol. 48:2715–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, Everette R, Peters N, Miller N, Muran G, Auten K, Newman N, Rowan G, Grisby C, Arnell K, Miller L, Ball B, McDavid G. 2004. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 114:1305–1311 [DOI] [PubMed] [Google Scholar]
- 24. Benaron DA, Benitz WE. 1994. Maximizing the stability of oxygen delivered via nasal cannula. Arch. Pediatr. Adolesc. Med. 148:294–300 [DOI] [PubMed] [Google Scholar]
- 25. STOP-ROP Multicenter Study Group 2000. Supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP-ROP), a randomized, controlled trial. I: Primary outcomes. Pediatrics 105:295–310 [DOI] [PubMed] [Google Scholar]
- 26. Bowman ED, Dharmalingam A, Fan WQ, Brown F, Garland SM. 1998. Impact of erythromycin on respiratory colonization of Ureaplasma urealyticum and the development of chronic lung disease in extremely low birth weight infants. Pediatr. Infect. Dis. J. 17:615–620 [DOI] [PubMed] [Google Scholar]
- 27. Lyon AJ, McColm J, Middlemist L, Fergusson S, McIntosh N, Ross PW. 1998. Randomised trial of erythromycin on the development of chronic lung disease in preterm infants. Arch. Dis. Child. 78:F10–F14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jonsson B, Rylander M, Faxelius G. 1998. Ureaplasma urealyticum, erythromycin and respiratory morbidity in high-risk preterm neonates. Acta Paediatr. 87:1079–1084 [DOI] [PubMed] [Google Scholar]
- 29. Baier RJ, Loggins J, Kruger TE. 2003. Failure of erythromycin to eliminate airway colonization with Ureaplasma urealyticum in very low birth weight infants. BMC Pediatr. 3:10 doi:10.1186/1471-2431-3-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. 2012. Azithromycin and the risk of cardiovascular death. N. Engl. J. Med. 366:1881–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tilelli JA, Smith KM, Pettignano R. 2006. Life-threatening bradyarrhythmia after massive azithromycin overdose. Pharmacotherapy 26:147–150 [DOI] [PubMed] [Google Scholar]
- 32. Hoffman JI. 2001. The prolonged QT syndrome. Adv. Pediatr. 48:115–156 [PubMed] [Google Scholar]
- 33. Villain E, Levy M, Kachaner J, Garson A., Jr 1992. Prolonged QT interval in neonates: benign, transient, or prolonged risk of sudden death. Am. Heart J. 124:194–197 [DOI] [PubMed] [Google Scholar]