Abstract
The development of high-level daptomycin resistance (HLDR; MIC of ≥256 mg/liter) after exposure to daptomycin has recently been reported in viridans group streptococcus (VGS) isolates. Our study objectives were as follows: to know whether in vitro development of HLDR after exposure to daptomycin was common among clinical isolates of VGS and Streptococcus bovis; to determine whether HLDR also developed during the administration of daptomycin to treat experimental endocarditis caused by the daptomycin-susceptible, penicillin-resistant Streptococcus mitis strain S. mitis 351; and to establish whether combination with gentamicin prevented the development of HLDR in vitro and in vivo. In vitro studies were performed with 114 VGS strains (mitis group, 92; anginosus group, 10; mutans group, 8; and salivarius group, 4) and 54 Streptococcus bovis strains isolated from 168 consecutive patients with infective endocarditis diagnosed between 1995 and 2010. HLDR was only observed after 24 h of exposure to daptomycin in 27% of the mitis group, including 27% of S. mitis isolates, 47% of S. oralis isolates, and 13% of S. sanguis isolates. In our experimental model, HLDR was detected in 7/11 (63%) and 8/12 (67%) isolates recovered from vegetations after 48 h of daptomycin administered at 6 mg/kg of body weight/24 h and 10 mg/kg/24 h, respectively. In vitro, time-kill experiments showed that daptomycin plus gentamicin was bactericidal against S. mitis 351 at tested concentrations of 0.5 and 1 times the MIC and prevented the development of HLDR. In vivo, the addition of gentamicin at 1 mg/kg/8 h to both daptomycin arms prevented HLDR in 21 out of 23 (91%) rabbits. Daptomycin plus gentamicin was at least as effective as vancomycin plus gentamicin. In conclusion, HLDR develops rapidly and frequently in vitro and in vivo among mitis group streptococci. Combining daptomycin with gentamicin enhanced its activity and prevented the development of HLDR in most cases.
INTRODUCTION
Viridans group streptococci (VGS) are commensal flora of the oral cavity, vagina, and gastrointestinal tract. Their role as a major cause of bacteremia in neutropenic patients is a growing problem (1, 2), and their involvement in septic shock, adult respiratory distress syndrome, and endocarditis is well known (26% in native valve and 16% in prosthetic valve endocarditis) (3). VGS and Streptococcus bovis cause almost one-quarter of all cases of infective endocarditis (IE) (3). Beta-lactams are the drugs of choice; however, penicillin-resistant strains are increasingly being isolated in Europe and the United States and have become a matter for concern (4–6), since their management is challenging (7). Vancomycin is the recommended antibiotic for endocarditis in cases of resistance to penicillin or allergy to beta-lactams (8–10). Although clinical data on the treatment of VGS and S. bovis infections with daptomycin are lacking, the potential of this agent as a good alternative to vancomycin-based regimens should be studied.
Daptomycin is a cyclic lipopeptide antibiotic with bactericidal activity against staphylococci and streptococci (11). It is efficacious in the treatment of right-sided endocarditis caused by methicillin-susceptible and methicillin-resistant Staphylococcus aureus (MSSA and MRSA) (12, 13) and has been approved for these indications and for staphylococcal bacteremia and skin and soft tissue infections (14). Daptomycin offers some advantages over vancomycin, namely, less renal toxicity, single daily dose, and easy administration as outpatient parenteral antimicrobial therapy (15, 16).
Our group began to study the efficacy of daptomycin in experimental endocarditis induced by a penicillin-resistant, daptomycin-susceptible strain of Streptococcus mitis (S. mitis 351). We compared the activity of daptomycin at 6 mg/kg of body weight/24 h with that of vancomycin. Preliminary results showed that the microorganisms recovered from vegetations after 2 days of treatment had developed an elevated percentage of high-level daptomycin resistance (HLDR; MIC of ≥256 mg/liter) in the monotherapy daptomycin arm. This finding led us to design a study with the following objectives: first, to know whether HLDR was strain specific or whether it also developed in VGS and S. bovis isolates; second, to determine, using a human-like rabbit pharmacokinetic model, whether HLDR also developed during the administration of daptomycin at 10 mg/kg/24 h to treat experimental endocarditis caused by the daptomycin-susceptible and penicillin-resistant S. mitis strain (S. mitis 351); and third, to evaluate whether combination with gentamicin prevented the in vitro and in vivo development of HLDR.
(Presented in part at the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 17 to 20 September 2011 [17–19].)
MATERIALS AND METHODS
Microorganisms.
We studied 114 VGS and 54 S. bovis strains isolated from 168 consecutive patients with IE diagnosed in our center between 1995 and 2010. S. mitis 351, a penicillin-resistant isolate from our collection, was selected for the in vivo studies. None of the patients had previously received daptomycin.
Antibiotics.
Daptomycin powder was supplied by Cubist Pharmaceuticals (Lexington, MA) (20). Vancomycin and gentamicin were purchased from Sigma (St. Louis, MO).
Susceptibility testing.
The MICs of daptomycin, penicillin, ceftriaxone, and vancomycin were tested using the Etest method following the manufacturer's recommendations (bioMérieux S.A., Marcy l'Etoile, France). For S. mitis 351, the MICs of daptomycin, vancomycin, and gentamicin and the minimum bactericidal concentrations (MBCs) were determined using the microdilution method in cation-adjusted Mueller-Hinton broth (CAMHB) (Oxoid Ltd., Hampshire, England) supplemented with 5% lysed horse blood, according to the recommended procedures of the Clinical and Laboratory Standards Institute (CLSI) (21). Susceptibility to daptomycin was tested in Mueller-Hinton broth adjusted to 50 mg/liter of calcium using standard methods. Streptococcus pneumoniae ATCC 49619 was the control strain.
Synergy studies.
Time-kill methodology was used to test the activity of daptomycin plus gentamicin against S. mitis 351 according to criteria described elsewhere (22). A final inoculum of between 5 × 105 and 1 × 106 CFU/ml was used. Prior to inoculation, each tube of fresh CAMHB, adjusted to 50 mg/liter of calcium, was supplemented with 5% lysed horse blood and with daptomycin or vancomycin. Concentrations of 0.5× MIC and 1× MIC were chosen for testing. A tube without antibiotic was used as a growth control. Viability counts were performed at 0, 4, and 24 h according to the recommendation of Isenberg (23). Drug carryover was prevented by using dilution. Bactericidal activity was defined as a ≥3-log10 reduction in CFU/ml at 24 h in comparison with the initial inoculum. Synergistic activity was defined as a ≥2-log10 reduction in CFU/ml at 24 h in comparison with the reduction by the more-active antibiotic (24). Time-kill studies were performed in duplicate.
Resistance screening methodology.
All strains were subcultured in the presence of daptomycin at 0.5 mg/liter and 1 mg/liter (0.5× MIC to 33× MIC depending on the strain) on Mueller-Hinton agar plates (Oxoid Ltd., Hampshire, England) supplemented with 50 mg/liter of calcium and 5% lysed horse blood. The final inoculum was 4 × 105 to 8 × 105 CFU/ml. Plates were incubated for up to 48 h in a 5% CO2 atmosphere. S. mitis 351 was used as a positive quality control strain. In plates with positive growth in the presence of daptomycin, CFU were harvested and retested by the Etest method to determine increases in the MIC of daptomycin.
Strains with a MIC of ≥2 mg/liter were considered not susceptible to daptomycin (daptomycin nonsusceptible [DNS]), following the CLSI recommendations. HLDR was defined as a MIC of ≥256 mg/liter.
Study animals.
Experimental aortic valve endocarditis was induced in New Zealand White rabbits (body weight, 2.5 kg; San Bernardo Farm, Pamplona, Spain) (25). This study was approved by the Ethics Committee for Experimental Animal Studies of the University of Barcelona.
Human pharmacokinetics simulation studies.
The in vivo experimental pharmacokinetics of vancomycin, daptomycin, and gentamicin have been described elsewhere (24, 26). Antibiotics were administered using a computer-controlled infusion pump system designed to reproduce human serum pharmacokinetics in rabbits after an intravenous (i.v.) infusion. Animal antibiotic doses were chosen to simulate the human pharmacokinetic profile of daptomycin at 2 different doses (the recommended dose [RD-daptomycin; 6 mg/kg i.v. once daily] or a higher dose [HD-daptomycin; 10 mg/kg i.v. once daily]. Vancomycin (30 mg/kg i.v. in 2 doses; for a 70-kg adult, 1 g i.v. every 12 h) (26) and gentamicin (1 mg/kg i.v. every 8 h) (24) doses simulating the doses recommended in the American Heart Association guidelines for the antibiotic treatment of VGS IE (8) were administered.
Endocarditis model.
Experimental aortic valve IE was induced in rabbits according to the method described by Garrison and Freedman (27). A catheter was inserted through the right carotid artery into the left ventricle, and the catheter for administration of antibiotics was placed into the inferior vena cava through the jugular vein (25). Twenty-four hours after placement of the intracardiac catheter, all animals were infected via the marginal ear vein with 1 ml of saline solution containing about 5 × 105 to 8 × 105 CFU/ml of the S. mitis 351 strain. One milliliter of blood was obtained 24 h after infection and immediately before the initiation of antimicrobial therapy to confirm the presence of bacteremia, which was interpreted to indicate IE. At the same time, control animals were anesthetized and sacrificed, and bacterial CFU were measured in vegetations. Antibiotics were administered for 48 h via the computer-controlled infusion pump system. After the completion of treatment, an additional 6 half-lives were allowed to elapse before the animals were sacrificed. This provided time for viable bacteria remaining within the endocardial vegetations to grow, except in the daptomycin plus gentamicin combination arms, where, given the longer half-life of daptomycin, 24 h was allowed to elapse. The gentamicin infusion continued during the first 16 h (2 more cycles).
Treatment groups.
The treatment groups were 1 control arm, 2 daptomycin arms (RD- or HD-daptomycin), 1 vancomycin arm, 2 daptomycin (RD or HD)-plus-gentamicin arms, and a vancomycin-plus-gentamicin arm.
Analysis of endocardial vegetations.
After antibiotic treatment, rabbits were anesthetized and sacrificed, and aortic valve vegetations were removed and processed (25). Colonies recovered from quantitative cultures on plain agar were isolated, and MICs were retested using the Etest to detect in vivo resistance to daptomycin. The results were expressed as the number of log10 CFU per gram of vegetation. The result was assigned a value of 2 if there was no growth on the quantitative plates but there was growth in the qualitative culture (the rest of the homogenate was cultured in tryptic soy broth). The result was assigned a value of 0 and the vegetation was considered sterile if there was no growth from the initial quantitative culture or from the homogenates cultured for a week.
Statistical analysis.
The HLDR rates according to the MIC and MBC endpoints in the in vitro studies were compared using the Fisher exact test. The results from vegetations were expressed as the median and interquartile range (IQR) of the number of log10 CFU per gram of vegetation. The Mann-Whitney rank sum test was used to compare the log10 CFU/g values of the vegetations between the different treatment groups. The Fisher exact test was used to compare the rate of sterilization of vegetations and to assess whether there were differences between treatment groups.
RESULTS
Microorganisms.
We studied 168 consecutive strains from our collection and identified the following species: mitis group, 92 isolates (55%) (51 Streptococcus mitis, 19 Streptococcus oralis, 15 Streptococcus sanguis, 4 Streptococcus gordonii, and 3 Streptococcus parasanguinis isolates); anginosus group, 10 isolates (6%) (6 Streptococcus anginosus, 1 Streptococcus constellatus, and 3 isolates not identified to species level); mutans group, 8 isolates (5%) (8 Streptococcus mutans isolates); salivarius group, 4 isolates (2%) (4 S. salivarius isolates); and bovis group, 54 isolates (32%) (54 Streptococcus bovis isolates).
Susceptibility testing.
The results of in vitro susceptibility testing with penicillin, ceftriaxone, and vancomycin are summarized in Table 1. All the strains tested were uniformly susceptible to vancomycin. The rates of resistance to penicillin ranged from 34% in the mitis group to 4% and 7% in the bovis and anginosus groups, respectively. Resistance to ceftriaxone was 11% in the mitis group. All microorganisms from the mutans and salivarius groups were susceptible to penicillin and ceftriaxone. Susceptibility to daptomycin is summarized in Table 2. The results are expressed as MIC50/MIC90 values, MBC50/MBC90 values, and range values for the 168 isolates tested by the microdilution method, and the MIC50/MIC90 values for those analyzed by Etest.
Table 1.
In vitro susceptibilities of isolates to penicillin, ceftriaxone, and vancomycin by Etest
| Microorganisms (no. of strains [n = 168]) | MIC50/MIC90 (mg/liter) valuesa (MIC range) of: |
||
|---|---|---|---|
| Penicillin | Ceftriaxone | Vancomycin | |
| Mitis group (n = 92) | 0.094/1 (0.007–4) | 0.125/1 (0.007–2) | 0.5/1 (0.125–1) |
| Bovis group (n = 54) | 0.094/0.125 (0.007–0.25) | 0.125/0.25 (0.03–0.5) | 0.38/0.5 (0.25–1) |
| Anginosus group (n = 10) | 0.06/0.125 (0.007–0.5) | 0.25/0.5 (0.015–1) | 1/1.5 (0.5–1.5) |
| Mutans group (n = 8) | 0.015–0.047 | 0.03–0.125 | 0.5–1.5 |
| Salivarius group (n = 4) | 0.03–0.5 | 0.015–1 | 0.25–1 |
Results are expressed as a range when there were fewer than 10 isolates.
Table 2.
In vitro susceptibilities of isolates to daptomycin by broth microdilution and Etest
| Microorganisms (no. of strains [n = 168]) | Etest MIC50/MIC90 (mg/liter) (range) | Broth microdilution |
|
|---|---|---|---|
| MIC50/MIC90 (mg/liter) (range) | MBC50/MBC90 (mg/liter) (range) | ||
| Mitis group (n = 92) | 0.25/0.5 (0.03–1.5) | 0.5/1 (0.12–2) | 2/16 (0.25–32) |
| Bovis group (n = 54) | 0.023/0.047 (0.023–0.12) | 0.06/0.12 (0.03–0.5) | 0.5/1 (0.03–2) |
| Anginosus group (n = 10) | 0.38/0.38 (0.12–0.5) | 0.5/0.5 (0.12–0.5) | 4/4 (0.5–4) |
| Mutans group (n = 8)a | 0.25–0.5 | 0.25–0.5 | 0.25–1 |
| Salivarius group (n = 4)a | 0.016–0.047 | 0.06–0.12 | 0.06–1 |
Results are expressed as a range when there were fewer than 10 isolates.
The strain selected for in vivo study was S. mitis 351, which, according to the CLSI standards for MIC breakpoints, is resistant to penicillin (MIC/MBC, 8/8 mg/liter) and susceptible to vancomycin, daptomycin, and gentamicin (MICs of 0.5 mg/liter, 0.5 mg/liter, and 8 mg/liter, respectively, and MBCs of >32 mg/liter, 8 mg/liter, and 16 mg/liter, respectively).
Results of screening for DNS strains and HLDR.
Table 3 shows the overall frequency of DNS and HLDR for the different VGS strains after subculture of strains with inhibitory concentrations of daptomycin. The species included in the mitis group are detailed, as they were the only ones that developed HLDR. DNS strains were identified from the mitis group in 61 cases (66%) and from the anginosus group in 5 cases (50%), i.e., 39% (66/168) of the total strains tested. The highest rates of resistance were observed in the mitis group: 74/92 (80%) strains were resistant, 61/74 (82%) were DNS, and 25/61 (41%) developed HLDR, i.e., 15% (25/168) of the strains tested, all of which belonged to the mitis group.
Table 3.
Rates of selection of resistance and high-level resistance after exposure to daptomycin
| Microorganism(s) | No. of strains | No. (%) screening positivea | No. (%) that wereb: |
|
|---|---|---|---|---|
| DNS (MIC, ≥2 mg/liter) | HLDR (MIC, ≥256 mg/liter) | |||
| Mitis group | 92 | 74 (80) | 61 (66) | 25 (27) |
| S. mitis | 51 | 35 (69) | 30 (59) | 14 (27) |
| S. oralis | 19 | 18 (95) | 14 (74) | 9 (47) |
| S. sanguis | 15 | 15 (100) | 11 (73) | 2 (13) |
| S. gordonii | 4 | 4 (100) | 4 (100) | 0 (0) |
| S. parasanguis | 3 | 2 (67) | 2 (67) | 0 (0) |
| Bovis group | 54 | 2 (4) | 0 | 0 |
| Anginosus group | 10 | 5 (50) | 5 (50) | 0 |
| Mutans group | 8 | 0 | 0 | 0 |
| Salivarius group | 4 | 0 | 0 | 0 |
Screening was considered positive if the microorganism grew in the presence of 0.5 mg or 1 mg/liter daptomycin.
DNS, daptomycin nonsusceptible; HLDR, high-level daptomycin resistance.
Table 4 shows the possible relationship between in vitro susceptibility parameters, such as MIC and MBC, and the development of HLDR. Microorganisms from the mitis group with MICs of >0.5 mg/liter had a relative risk (95% confidence interval [CI]) of 2.81 (range, 1.41 to 5.61) for developing HLDR. The parameters of a MIC of >0.5 mg/liter (by microdilution) and an MBC of >2 mg/liter were statistically significantly associated with HLDR.
Table 4.
Rates of high-level daptomycin resistance according to baseline MIC or MBC of isolates from the mitis group
| Test, parameter | No. of isolates with result/total no. of isolates (%) |
P value | |
|---|---|---|---|
| HLDR | Not HLDR | ||
| Microdilution, MIC50 = 0.5 (mg/liter) | |||
| MIC ≤ 0.5 | 9/55 (16) | 46/55 (84) | 0.004 |
| MIC > 0.5 | 17/37 (46) | 20/37 (54) | |
| Etest, MIC50 = 0.25 (mg/liter) | |||
| MIC ≤ 0.25 | 11/55 (20) | 44/55 (80) | 0.056 |
| MIC > 0.25 | 15/37 (40) | 22/37 (60) | |
| Microdilution, MBC50 = 2 (mg/liter) | |||
| MBC ≤ 2 | 9/56 (16) | 47/56 (84) | 0.006 |
| MBC > 2 | 16/36 (44) | 20/36 (56) | |
| Microdilution, MBC/MIC ≥ 8 (mg/liter) | |||
| MBC/MIC ≤ 4 | 12/57 (21) | 45/57 (79) | 0.085 |
| MBC/MIC ≥ 8 | 14/35 (40) | 21/35 (60) | |
In vitro time-kill experiments.
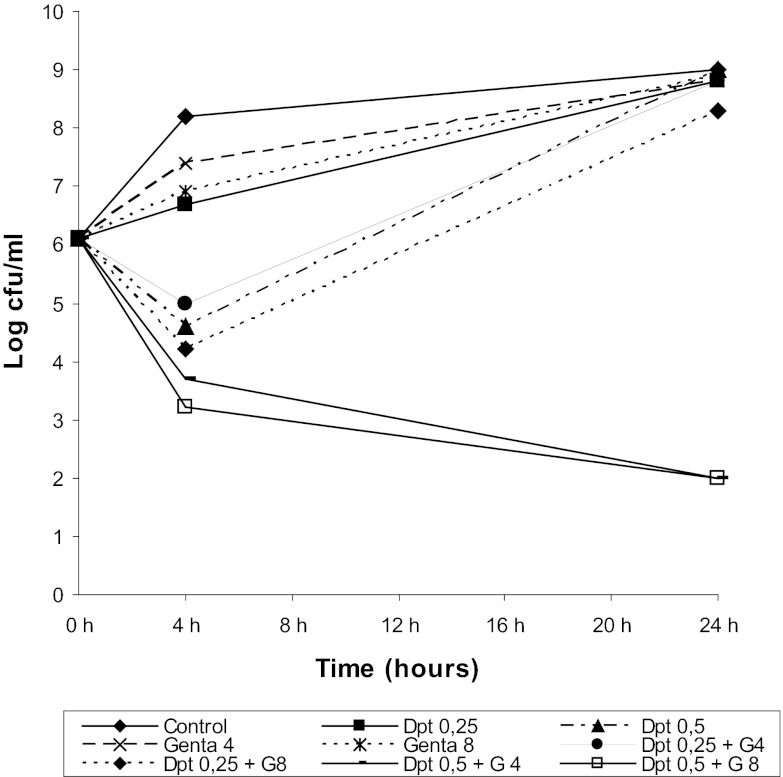
Daptomycin plus gentamicin demonstrated synergy and bactericidal activity (Fig. 1) at 1× MIC for daptomycin and 0.5× MIC and 1× MIC for gentamicin. None of the isolates recovered developed HLDR.
Fig 1.
Results of time-kill experiments for S. mitis 351 incubated with daptomycin (Dpt) plus gentamicin (Genta) at concentrations of 0.5× MIC and 1× MIC for both antibiotics. MICs for daptomycin and gentamicin were 0.5 mg/liter and 8 mg/liter, respectively.
Established endocarditis treatment.
The relative effectiveness of drugs in monotherapy and combination therapy is shown in Table 5. All control rabbits had infected aortic valve vegetations, with a median bacterial titer of ≥9 log10 CFU per gram of vegetation. Comparisons between treated groups revealed that after 48 h of treatment, HD-daptomycin showed the same activity as RD-daptomycin in sterilizing vegetations and in reducing the median number of CFU. In the monotherapy arms, vancomycin significantly (P < 0.001) reduced the density of microorganisms in the vegetations. Isolates recovered from endocardial vegetations from animals treated with daptomycin (RD- or HD-daptomycin arms) showed HLDR in 7/11 (63%) isolates from the RD-daptomycin arm and 8/12 (67%) from the HD-daptomycin arm. The addition of gentamicin to daptomycin or vancomycin reduced the median number of CFU in the vegetations of treated animals to a greater extent than monotherapy. These differences were statistically significant (Table 5).
Table 5.
Treatment of experimental endocarditis caused by S. mitis 351 strain
| Treatment group | No. of sterile vegetations/total no. of vegetations (%) | Log10 CFU/g vegetation [median (IQR)] | No. of recovered isolates with HLDR MIC/total no. of animals treated (%)l |
|---|---|---|---|
| Controla | 0/15 (0) | 9.1 (9–9.6) | 0 |
| RD-daptomycin | 1/11 (9)b | 6.7 (5.9–7.8)c | 7/11 (63)d |
| HD-daptomycin | 1/12 (8)e | 6.1 (5.2–7.2)f,g | 8/12 (67)h |
| Vancomycin | 0/12 (0)i | 3.4 (2–4)g,j | NA |
| RD-daptomycin + gentamicin | 10/11 (91)b,k | 0 (0–0)c | 1/11 (9)d |
| HD-daptomycin + gentamicin | 8/12 (67)e | 0 (0–2)f | 1/12 (8)h |
| Vancomycin + gentamicin | 6/12 (50)i,k | 1 (0–2.2)j | NA |
The control animals were sacrificed 24 h after the infection was started.
P < 0.001.
P < 0.001.
P = 0.004.
P = 0.003.
P = 0.002.
P = 0.001.
P = 0.004.
P = 0.005.
P = 0.002.
P = 0.03.
NA, not applicable.
RD-daptomycin plus gentamicin sterilized more vegetations than vancomycin plus gentamicin (P = 0.03). No statistically significant differences were detected between the two daptomycin-plus-gentamicin arms. In combination therapy with daptomycin, only 1 isolate from each arm showed HLDR, namely, 1/11 (9%) for RD-daptomycin plus gentamicin and 1/12 (8%) for HD-daptomycin plus gentamicin.
Stability of daptomycin resistance.
We conducted stability studies by subculturing two isolates presenting HLDR that were recovered from the treated animals (an isolate from the 6 mg/kg daptomycin arm [D6-6] and an isolate from the 10 mg/kg daptomycin-plus-gentamicin combined-therapy arm [D6+G-14]). Daily passes were carried out on daptomycin-free agar plates, and the daptomycin MIC was tested every day using the Etest. All tests were performed in triplicate. In all cases, we observed stability for both isolates, with median results (IQRs) of 25 (19 to 32) days and 27 (20 to 36) days for the strains isolated in D6-6 and D6+G-14, respectively. High-level resistance to daptomycin was maintained at a MIC of ≥256 mg/liter throughout testing. The loss of resistance was fast, and in one or two passes, the microorganism recovered the baseline MIC of 0.5 mg/liter.
Penicillin MIC seesaw effect.
The penicillin MIC was retested in the 15 strains with HLDR recovered from both daptomycin arms. The pretreatment penicillin MIC was 8 mg/liter, which decreased in all but 2 strains to a median (IQR) of 1 (0.5 to 4) mg/liter after 2 days of therapy with daptomycin.
DISCUSSION
VGS are considered uniformly susceptible to daptomycin. The baseline pattern of susceptibility to daptomycin that we observed by Etest and broth microdilution in the 114 VGS and 54 S. bovis strains of our collection reproduced the general conclusions of in vitro studies (11, 28–30). The prevalence of resistance to daptomycin in VGS and S. bovis bloodstream infection has been found to be very low in larger series (11, 28–30), with approximately 99.8% of MICs ranging from 0.03 to 1 mg/liter. After testing daptomycin in 915 bloodstream isolates of VGS and S. bovis, Streit et al. concluded that daptomycin was active against the 8 species of VGS and S. bovis tested, with MIC values of ≤2 mg/liter (11). Very soon after exposure to daptomycin inhibitory concentrations (for most strains, within the first 24 h), 39% of the strains tested proved to be DNS (present in the mitis and anginosus groups); HLDR was detected in 27% of the isolates from the mitis group, namely, 27% of S. mitis isolates, 47% of S. oralis isolates, and 13% of S. sanguis isolates (Table 3). None of the 4 S. gordonii isolates in our collection developed HLDR; all 4 were DNS. Isolates from the mitis group with daptomycin MICs of >0.5 mg/liter developed HLDR almost 3-fold more frequently (relative risk, 2.81; 95% CI, 1.41 to 5.61) than those with MICs of <0.5 mg/liter. Further studies are needed to elucidate the influence of the daptomycin MIC on the development of resistance against this antimicrobial.
In our experimental model, the frequency of in vivo microbiological failure and development of HLDR against daptomycin in both monotherapy arms (RD-daptomycin and HD-daptomycin) was statistically significant. The addition of gentamicin enhanced the activity of daptomycin and vancomycin and prevented the development of HLDR in most cases. Daptomycin plus gentamicin showed at least the same efficacy as vancomycin plus gentamicin and was effective against experimental endocarditis caused by the penicillin-resistant S. mitis strain. Indeed, this combination could be very relevant for clinical practice, since both doses (6 mg/kg and 10 mg/kg) proved to be efficacious. In addition, the high rate of prevention of HLDR after combination with gentamicin for both doses could justify combination therapy in humans, although HLDR is not prevented in 100% of cases. Akins et al. (31) studied the efficacy and sensitivity of daptomycin at doses of 6 mg/kg and 8 mg/kg in monotherapy against 5 isolates of different species of VGS from the mitis group (3 S. oralis, 1 S. mitis, and 1 S. gordonii isolate) with high baseline susceptibility to daptomycin (0.5 to 2 μg/ml) in a pharmacodynamic model with simulated endocardial vegetations and time-kill curves. All 5 strains developed HLDR in 72 h (MIC of >256 mg/liter) at both doses, except for 2 when treated at 6 mg/kg. In 2 strains (one treated at 6 mg/kg and the other at 8 mg/kg), the number of CFU/g increased during the experiment. Li et al. (32) tested the efficacy of daptomycin against 3 clinical VGS isolates (1 S. constellatus, 1 S. oralis, and 1 S. salivarius isolate) in the rat fibrin clot model. One isolate had a MIC of 4 mg/liter, and the other 2 had MICs of 1 mg/liter. Daptomycin was tested at 3 different doses, which simulated the area under the curve obtained at 4, 6, and 8 mg/kg in humans. A bactericidal effect was shown in 2 of 3 isolates at 6 mg/kg, and 8 mg/kg was needed to achieve a 3-log10 killing in 1 strain (not the strain with a MIC of 4 mg/liter). In this study, no resistance to daptomycin was detected. In the absence of larger studies to compare with, our results are consistent with those of Akins et al. We detected no differences between rates of HLDR in experiments using the S. mitis 351 strain, regardless of the dose of daptomycin used in monotherapy. Although both our study and that of Akins et al. have limitations, together they provide sufficient evidence that HLDR is not a rare phenomenon in clinical VGS strains and that high-level resistance can develop rapidly. Thus, clinical failures may be observed when using daptomycin against VGS, even when the strains are susceptible.
None of the patients from whom the 168 strains used in our study were obtained had been treated with daptomycin. Therefore, the rapid development of HLDR we recorded was not conditioned by previous clinical exposure to daptomycin. The level of resistance in the S. mitis strain studied in vivo is much closer to that of Enterococcus spp. than that of S. aureus. While the MICs for Enterococcus faecalis and Enterococcus faecium have been shown to increase 8-fold (from ≤4 to 32 mg/liter [33, 34]) in cases of daptomycin resistance, the highest MICs for S. aureus scarcely reach 4 mg/liter (with a MIC of ≤1 mg/liter as the clinical threshold for sensitivity). As with enterococci, we hypothesize that the molecular basis of daptomycin resistance in S. mitis 351 depends on the change in cell membrane charge due to modifications in lipoproteins, as a consequence of which, insertion of the daptomycin lipopeptide tail is prevented. This hypothesis is supported by the “seesaw” phenomenon observed in HLDR strains recovered from vegetations: the increase in resistance to daptomycin is accompanied by a concomitant fall in resistance to penicillin. Further studies are needed to elucidate the molecular mechanisms underlying resistance to daptomycin in VGS.
The clinical relevance of the emergence of HLDR among VGS treated with daptomycin remains to be demonstrated. HLDR has not yet been reported in patients with infections by VGS, probably because daptomycin is not a first-choice antimicrobial agent against these microorganisms. In any case, physicians should be aware of the possibility of resistance, because daptomycin is often used off-label instead of vancomycin in nonstaphylococcal Gram-positive infections in cases of beta-lactam allergy or penicillin resistance and in patients with febrile neutropenia who are also at risk of S. mitis infections. The phenomenon is well illustrated in two recently published case reports. The first involves a case of breakthrough bacteremia and septic shock caused by daptomycin-resistant S. anginosus in a patient treated with daptomycin because of several previous MRSA infections (35). Twenty-one days after the initiation of daptomycin therapy, the patient was admitted to the medical intensive care unit with septic shock, and the MIC for the S. anginosus isolated from positive blood cultures was 4 mg/liter. The second report (36) describes a patient diagnosed with native-valve S. oralis endocarditis. Daptomycin (500 mg/day iv) was started empirically and continued because of its favorable MIC (0.094 mg/liter). The daily dose of daptomycin was increased to 700 mg on day 7, and at day 15, daptomycin serum levels were determined. Despite appropriate daptomycin levels in serum (maximum concentration of drug in serum, 82 mg/liter, and minimum concentration of drug in serum, 15 mg/liter), an increase in the size of mitral valve vegetations was found after 15 days of daptomycin treatment, and the patient underwent surgical replacement. Culture of the vegetations remained positive for S. oralis, with a 4-fold increase in the MIC. Daptomycin vegetation levels were 26 μg/g of tissue. After surgery, antibiotic treatment was switched to intravenous ceftriaxone at the recommended dose, and the patient was considered cured at the 6-month follow-up visit. Therefore, when daptomycin is used as monotherapy to treat bacteremia or endocarditis caused by VGS, physicians should closely monitor the efficacy of daptomycin to ensure early detection of potential microbiological failure due to the development of resistance.
In conclusion, the rapid development of HLDR is not a trivial event and is a frequent finding in mitis group streptococci, although the clinical implications have yet to be defined. Combination with gentamicin enhances the activity of daptomycin and prevents the development of HLDR in most cases. Further studies are necessary to elucidate the molecular basis of daptomycin resistance in VGS and the clinical significance of this finding.
ACKNOWLEDGMENTS
José M. Miró has received honoraria for consultancy and/or research grants from Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Cubist, Novartis, GlaxoSmithKline (GSK), Gilead Sciences, Pfizer, Roche, and Theravance. Carlos Cervera has served as an advisory board member for Novartis and has received honoraria for lectures and travel grants from Pfizer, Gilead, Merck, and Roche. No other author reports any potential conflicts of interest.
Members of the Hospital Clínic Endocarditis Study Group, Hospital Clínic-IDIBAPS, University of Barcelona School of Medicine, Barcelona, Spain, include the following: J. M. Miró, A. Moreno, A. del Río, C. Cervera, X. Castañeda, J. M. Pericas, and J. M. Gatell (Infectious Diseases Service); F. Marco, C. García-de-la-Mària, Y. Armero, M. Almela, and J. Vila (Microbiology Service); C. A. Mestres, J. C. Paré, C. Falces, R. Cartañá, S. Ninot, M. Azqueta, M. Sitges, M. Heras, and J. L. Pomar (Cardiovascular Institute); G. Fita and I. Rovira (Anesthesia Service); J. Ramírez and T. Ribalta (Pathology Department); M. Brunet (Toxicology Service); D. Soy (Pharmacy Service); and J. Llopis and I. Pérez (Statistical Unit).
This work was supported by a medical school grant from Cubist Pharmaceuticals, Inc. (Lexington, MA), by the Spanish Network for Research in Infectious Diseases (REIPI RD06/0008), and by the Fundación Máximo Soriano Jiménez (Barcelona, Spain). José M. Miró received a research grant from the Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) (Barcelona, Spain).
Footnotes
Published ahead of print 11 March 2013
REFERENCES
- 1. Bruckner L, Gigliotti F. 2006. Viridans group streptococcal infections among children with cancer and the importance of emerging antibiotic resistance. Semin. Pediatr. Infect. Dis. 17:153–160 [DOI] [PubMed] [Google Scholar]
- 2. Presterl E, Grisold AJ, Reichmann S, Hirschl AM, Georgopoulos A, Graninger W. 2005. Viridans streptococci in endocarditis and neutropenic sepsis: biofilm formation and effects of antibiotics. J. Antimicrob. Chemother. 55:45–50 [DOI] [PubMed] [Google Scholar]
- 3. Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG, Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falcó V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH, International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 169:463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knoll B, Tleyjeh IM, Steckelberg JM, Wilson WR, Baddour L. 2007. Infective endocarditis due to penicillin-resistant viridans group streptococci. Clin. Infect. Dis. 44:1585–1592 [DOI] [PubMed] [Google Scholar]
- 5. Seppälä H, Haanperä M, Al-Juhaish M, Järvinen Jalava HJ, Huovinen P. 2003. Antimicrobial susceptibility patterns and macrolide resistance genes of viridans group streptococci from normal flora. J. Antimicrob. Chemother. 52:636–644 [DOI] [PubMed] [Google Scholar]
- 6. Westling K, Julander I, Ljungman P, Heimdahl A, Thalme A, Nord CE. 2004. Reduced susceptibility to penicillin of viridans group streptococci in the oral cavity of patients with haematological disease. Clin. Microbiol. Infect. 10:899–903 [DOI] [PubMed] [Google Scholar]
- 7. Levy CS, Kogulan P, Gill VJ, Croxton MB, Kane JG, Lucey DR. 2001. Endocarditis caused by penicillin-resistant viridans streptococci: 2 cases and controversies in therapy. Clin. Infect. Dis. 33:577–579 [DOI] [PubMed] [Google Scholar]
- 8. Baddour LM, Wilson WR, Bayer AS, Fowler VG, Jr, Bolger AF, Levison ME, Ferrieri P, Gerber MA, Tani LY, Gewitz MH, Tong DC, Steckelberg JM, Baltimore RS, Shulman ST, Burns JC, Falace DA, Newburger JW, Pallasch TJ, Takahashi M, Taubert KA, Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association, Infectious Diseases Society of America 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:e394–e434 [DOI] [PubMed] [Google Scholar]
- 9. Gould FK, Denning DW, Elliott TS, Foweraker J, Perry JD, Prendergast BD, Sandoe JA, Spry MJ, Watkin RW. 2012. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 67:269–289 [DOI] [PubMed] [Google Scholar]
- 10. Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, Moreillon P, de Jesus Antunes M, Thilen U, Lekakis J, Lengyel M, Müller L, Naber CK, Nihoyannopoulos P, Moritz A, Zamorano JL. 2009. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009). Eur. Heart J. 30:2369–2413 [DOI] [PubMed] [Google Scholar]
- 11. Streit JM, Steenbergen JN, Thorne GM, Alder J, Jones RN. 2005. Daptomycin tested against 915 bloodstream isolates of viridans group streptococci (eight species) and Streptococcus bovis. J. Antimicrob. Chemother. 55:574–578 [DOI] [PubMed] [Google Scholar]
- 12. Cervera C, Castañeda X, Pericas JM, del Río A, García de la Mària C, Mestres C, Falces C, Marco F, Moreno A, Miró JM. 2011. Clinical utility of daptomycin in infective endocarditis caused by Gram-positive cocci. Int. J. Antimicrob. Agents 38:365–370 [DOI] [PubMed] [Google Scholar]
- 13. Fowler VG, Jr, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, Levine DP, Chambers HF, Tally FP, Vigliani GA, Cabell CH, Link AS, DeMeyer I, Filler SG, Zervos M, Cook P, Parsonnet J, Bernstein JM, Price CS, Forrest GN, Fätkenheuer G, Gareca M, Rehm SJ, Brodt HR, Tice A, Cosgrove SE. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355:653–665 [DOI] [PubMed] [Google Scholar]
- 14. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: Executive summary. Clin. Infect. Dis. 52:e18–e55 [DOI] [PubMed] [Google Scholar]
- 15. Larioza J, Girard A, Brown RB. 2011. Clinical experience with daptomycin for outpatient parenteral antibiotic therapy. Am. J. Med. Sci. 342:486–488 [DOI] [PubMed] [Google Scholar]
- 16. Nathwani D. 2009. Developments in outpatient parenteral antimicrobial therapy (OPAT) for gram-positive infections in Europe, and the potential impact of daptomycin. J. Antimicrob. Chemother. 64:447–453 [DOI] [PubMed] [Google Scholar]
- 17. Miró JM, García de la Mària C, Vila-Farrés X, Castañeda X, Espinal P, Armero Y, Cervera C, Soy D, del Río A, Falces C, Ninot S, Almela M, Mestres CA, Gatell JM, Jiménez de Anta MT, Moreno A, Vila J, Marco F, the Hospital Endocarditis Study Group 2011. Addition of gentamicin (GEN) to daptomycin (DAP) prevents the development of high-level DAP-resistance (HLDR) in the treatment of penicillin (PEN)-resistant Streptococcus mitis in a rabbit model of experimental endocarditis (EE), abstr B-051. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 18. Vila-Farrés X, Espinal P, García de de Maria C, Cervera C, del Río A, Almela M, Moreno A, Marco F, Miro JM, Vila J, the Hospital Clinic Endocarditis Study Group 2011. Analysis of the mechanism explaining the increase in the susceptibility to penicillin in a daptomycin-resistant mutant, which emerges from a penicillin-resistant/daptomycin-susceptible S. mitis strain after 48 hours of daptomycin treatment in a model of experimental endocarditis, abstr C1-1772. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 19. Miró JM, García de la Mària C, Vila-Farrés X, Castañeda X, Espinal P, Armero Y, Cervera C, Soy D, del Río A, Falces C, Ninot S, Almela M, Mestres CA, Gatell JM, Jiménez de Anta MT, Moreno A, Vila J, Marco F, the Hospital Endocarditis Study Group 2011. Development of high level daptomycin resistance (HLDR) in Streptococcus mitis group bloodstream isolates recovered from patients with infective endocarditis (IE), abstr E-1318. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 20. Cubist Pharmaceuticals 2008. Cubicin (daptomycin for injection) prescribing information. Cubist Pharmaceuticals, Inc., Lexington, MA [Google Scholar]
- 21. CLSI 2006. Performance standards for antimicrobial susceptibility testing. Approved standard M100-S16. CLSI, Wayne, PA [Google Scholar]
- 22. Eliopoulos G, Moellering R. 1996. Antimicrobial combinations, p 330–396 In Lorian V. (ed), Antibiotics in laboratory medicine. William and Wilkins, Co., Baltimore, MD [Google Scholar]
- 23. Isenberg HD. 2004. Clinical microbiology procedures handbook. ASM Press, Washington, DC [Google Scholar]
- 24. Miró JM, García-de la-Mària C, Armero Y, Soy D, Moreno A, del Río A, Almela M, Sarasa M, Mestres CA, Gatell JM, Jiménez de Anta MT, Marco F, Hospital Clinic Experimental Endocarditis Study Group 2009. Addition of gentamicin or rifampin does not enhance the effectiveness of daptomycin in the treatment of experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:4172–4177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miró JM, García-de la-Mària C, Armero Y, de-Lazzari E, Soy D, Moreno A, del Rio A, Almela M, Mestres CA, Gatell JM, Jiménez-de-Anta MT, Marco F, Hospital Clinic Experimental Endocarditis Study Group 2007. Efficacy of televancin in the treatment of experimental endocarditis due to glycopeptide-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 51:2373–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marco F, García-de la-Mària C, Armero Y, Amat E, Soy D, Moreno A, del Río A, Almela M, Mestres CA, Gatell JM, Jiménez de Anta MT, Miró JM, Hospital Clinic Experimental Endocarditis Study Group 2008. Daptomycin is effective in treatment of experimental endocarditis due to methicillin-resistant and glycopeptide-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 52:2538–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garrison PK, Freedman LR. 1970. Experimental endocarditis. I. Staphylococcal endocarditis in rabbits resulting from placement of a polyethylene catheter in the right side of the heart. Yale J. Biol. Med. 42:394–410 [PMC free article] [PubMed] [Google Scholar]
- 28. Loza E, Morosini MI, Pascual A, Tubau F, Alcalá J, Liñares J, Hernández-Bello JR, Baquero F, Perea E, Martín R, Jones RN, Cantón R, Surveillance Program SENTRY, Spain (2002-2006) 2008. Comparative in vitro activity of daptomycin against gram-positive microorganisms: SENTRY surveillance program, Spain (2002-2006). Enferm. Infecc. Microbiol. Clin. 26:489–494 (In Spanish.) [PubMed] [Google Scholar]
- 29. Pfaller MA, Sader HS, Jones RN. 2007. Evaluation of the in vitro activity of daptomycin against 19615 clinical isolates of Gram-positive cocci collected in North American hospitals (2002-2005). Diagn. Microbiol. Infect. Dis. 57:459–465 [DOI] [PubMed] [Google Scholar]
- 30. Sader HS, Fritsche TR, Jones RN. 2008. Frequency of occurrence and daptomycin susceptibility rates of gram-positive organisms causing bloodstream infections in cancer patients. J. Chemother. 20:570–576 [DOI] [PubMed] [Google Scholar]
- 31. Akins R. 2011. In vitro development of high-level resistant viridans group streptococci upon exposure to daptomycin, abstr P-951. Abstr. 21st Eur. Conf. Microbiol. Infect. Dis., Milan, Italy, 7 to 10 May 2011 [Google Scholar]
- 32. Li T, Mortin LI, Zhang S, Van Praagh A, Chen L, Zhang X, Alder J. 2005. Bactericidal efficacy of daptomycin (DAP) in rat fibrin clot model versus clinical viridans group streptococcus (VGS) strains, abstr 534. Abstr. 43rd Annu. Meet. Infect. Dis. Soc. Am., San Francisco, CA, 6 to 9 October 2005 [Google Scholar]
- 33. Arias CA, Panesso D, McGrath DM, Quin X, Mojica MF, Miller C, Diaz L, Tran TT, Rinco S, Barbu ME, Reyes J, Roh JH, Lobos E, Sodergren E, Pasqualini R, Arap W, Quinn PJ, Shamoo Y, Murran BE, Winstock GM. 2011. Genetic basis of in vivo daptomycin resistance in enterococci. N. Engl. J. Med. 365:892–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palmer K, Daniel A, Hardy C, Siverman J, Gilmore M. 2011. Genetic basis for daptomycin resistance in enterococci. Antimicrob. Agents Chemother. 55:3345–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Palacios F, Lewis JS, Sadkowski L, Echevarria K, Jorgensen JH. 2011. Breakthrough bacteremia and septic shock due to Streptococcus anginosus resistant to daptomycin in a patient receiving daptomycin therapy. Antimicrob. Agents Chemother. 55:3639–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tascini C, Di Paolo A, Poletti R, Flammini S, Emdin M, Ciullo I, Tagliaferri E, Moter A, Menichetti F. 2013. Daptomycin concentrations in valve tissue and vegetation in patients with bacterial endocarditis. Antimicrob. Agents Chemother. 57:601–602 [DOI] [PMC free article] [PubMed] [Google Scholar]