Abstract
To understand the process of Candida biofilm development and the effects of antifungal agents on biofilms, we analyzed real-time data comprising time-lapse images taken at times separated by brief intervals. The growth rate was calculated by measuring the change of biofilm thickness every hour. For the antifungal study, 5-h-old biofilms of Candida albicans were treated with either micafungin (MCFG) or fluconazole (FLCZ). MCFG began to suppress biofilm growth a few minutes after the initiation of the treatment, and this effect was maintained over the course of the observation period. In contrast, the suppressive effects of FLCZ on biofilm growth took longer to manifest: biofilms grew in the first 5 h after treatment, and then their growth was suppressed over the next 10 h, finally producing results similar to those observed with MCFG. MCFG was also involved in the disruption of cells in the biofilms, releasing string-like structures (undefined extracellular component) from the burst hyphae. Thus, MCFG inhibited the detachment of yeast cell clusters from the tips of hyphae. In contrast, FLCZ did not disrupt biofilm cells. MCFG also showed fast antifungal activity against Candida parapsilosis biofilms. In conclusion, our results show that inhibition of glucan synthesis due to MCFG contributed not only to fungicidal activity but also to the immediate suppression of biofilm growth, while FLCZ suppressed growth by inhibiting ergosterol synthesis. Therefore, those characteristic differences should be considered when treating clinical biofilm infections.
INTRODUCTION
Pathogenic fungi in the genus Candida can cause both superficial and serious systemic diseases and are now widely recognized as important agents of hospital-acquired infection (1). Candida albicans is known as a biofilm former. Bloodstream infections are frequently associated with the use of a catheter, and the catheter can be a scaffold for biofilms (2). Once biofilms are formed, the biofilms continuously supply detaching cells as a source of infection; thus, biofilm-related infection is associated with a poor prognosis (2, 3). Recently, research into molecular mechanisms related to biofilm formation has revealed transcriptional regulation (4); however, the majority of these related studies lack real-time observation of the development process, and therefore, the fate of the biofilm and the effects of antifungals against biofilms are not clearly understood. Knowing the mechanism of antifungal action against biofilms would provide us with key information when considering therapeutic strategy against clinical infections related to biofilms. In this study, we analyzed images obtained by time-lapse photography to investigate the developmental process and detachment of biofilms, as well as the antibiofilm effects of the echinocandin micafungin (MCFG), which inhibits cell wall glucan synthesis, and the azole fluconazole (FLCZ), which inhibits cell membrane ergosterol synthesis.
MATERIALS AND METHODS
Chemicals.
All general chemicals used in this study were purchased from Wako Chemicals (Tokyo, Japan), unless otherwise indicated, and were of the highest purity available. Ultrapure water dispensed by a Milli-Q water system (Millipore, Bedford, MA) was used for the preparation of buffers and solvents. MCFG (Astellas Pharma Inc., Tokyo, Japan) and FLCZ were dissolved in double-distilled water (ddW) at 1 mg/ml for stock solutions, and both were stored at −20°C prior to use.
Strains and growth conditions.
Clinical isolates of Candida albicans 21004 (Ca21004) and Candida parapsilosis 20007 (Cp20007) used in this study were stored in 20% glycerol at −80°C prior to use. Silicone disks were obtained from Dainichikougyo (Saitama, Japan) and self-manufactured to produce small pieces (approximately 1-mm by 1-mm squares, 0.3 mm thick).
Cells were grown in RPMI 1640–0.165 M morpholinepropanesulfonic acid (MOPS) (Sigma-Aldrich), and the overnight cultures were adjusted as an inoculation suspension. The small pieces of silicon disks were placed in an originally developed chamber and pretreated with fetal bovine serum at 37°C for 24 h, immersed in a cell suspension of 2 × 107 CFU/ml, and incubated at 37°C for 1 h. Following a brief wash, cells were grown in RPMI 1640–0.165 M MOPS with 20 ml/h flow using AC-2120 precision Perista pumps (ATTO, Tokyo, Japan), which were placed on both the influx and efflux sides. Biofilms were observed both from the top and the side, and those views were recorded by time-lapse images for 24 h at a rate of 1 frame per min, unless otherwise indicated. Time-lapse images were captured using a DXC-950 charge-coupled-device (CCD) color video camera (Sony, Tokyo, Japan) equipped with a Diaphot microscope (Nikon, Tokyo, Japan).
Scanning electron microscopy.
Samples for electron microscopy were prepared by a method previously described (5). Briefly, 24-h-old biofilms were fixed with 2.5% glutaraldehyde–2% paraformaldehyde–0.1 M phosphate-buffered saline (PBS) for 30 min and washed with 0.1 M PBS 3 times. After dehydration through an ascending alcohol series, the specimens were processed using a freeze dryer (model ES-2030; Hitachi, Tokyo, Japan) and tert-butyl ethanol, coated with osmium, and examined with a scanning electron microscope (SU6600; Hitachi, Tokyo, Japan).
Calculation of growth rate.
Time-lapse images of the side view of the biofilms were recorded for 24 h at a rate of 1 frame per min. Each frame was approximately 600 μm wide and was divided into 6 areas 100 μm in width. The peak height of each area was measured, and the values of the 6 areas were averaged to define the biofilm thickness. The biofilm thickness was calculated every hour. The growth rate was further calculated by measuring hourly changes in the biofilm thickness.
Treatment of antifungal agents.
Treatment with MCFG or FLCZ was performed on 5-h-old biofilms. Ca21004 and Cp20007 were treated with 1 μg/ml and 16 μg/ml of MCFG, respectively, and Ca21004 was treated with 25 μg/ml of FLCZ. MIC values were determined by the Clinical and Laboratory Standards Institute (CLSI) reference method for broth dilution antifungal susceptibility testing of yeasts (approved standard, second edition; CLSI document M27-A3) (6). The MIC values for MCFG were ≤0.002 and 0.5 μg/ml for Ca21004 and Cp20007, respectively, and the MIC value for FLCZ was 0.25 μg/ml for Ca21004.
Treatment doses were determined based on (i) the trough value (approximately 1 μg/ml) when MCFG was administered at 50 mg/day and (ii) the value for the maximum concentration of drug in serum (Cmax) (approximately 25 μg/ml) when fosfluconazole was administered at 1 g (800 mg FLCZ equivalent) of the loading dose (2 days) (7, 8).
Statistics.
Data were analyzed by unpaired t tests. Statistical significances are shown as P values. Data are presented as the means ± standard errors (SE). Error bars represent the SE.
RESULTS
Biofilms grew at constant rate, and cell detachment occurred in clusters.
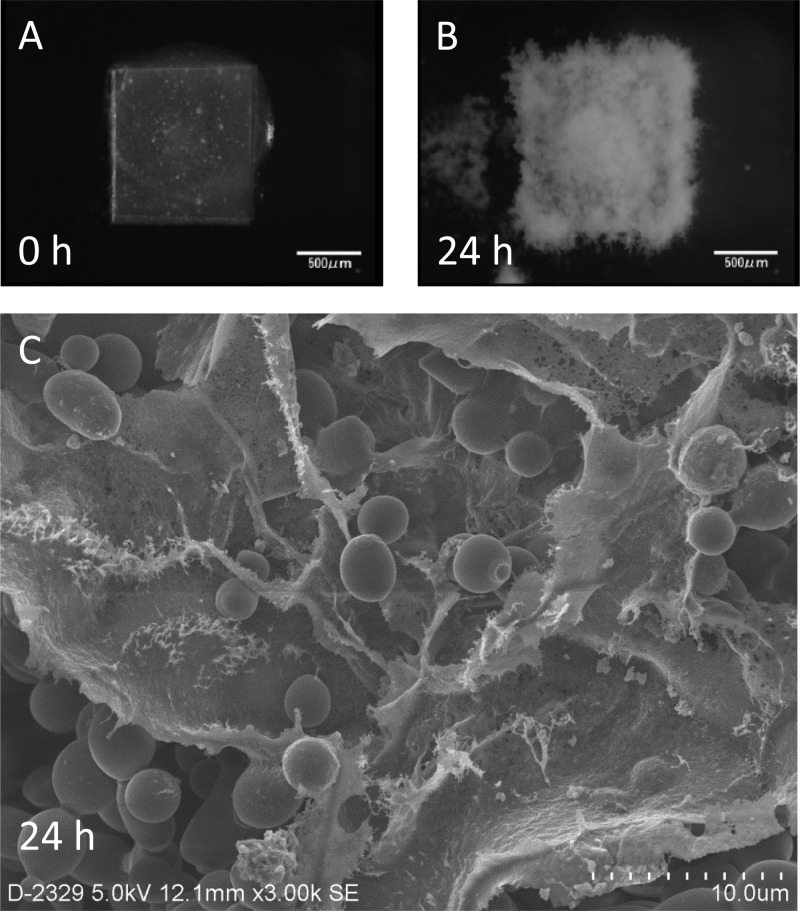
Biofilms of C. albicans Ca21004 were allowed to develop on silicon disks in flow, beginning with the attachment phase at 0 h until significant mass was achieved at 24 h (Fig. 1A and B). Electron microscopy revealed that the 24-h-old biofilms included dense extracellular matrices covering aggregated cells, indicative of matured biofilms (Fig. 1C).
Fig 1.
Development of Candida albicans biofilms on a silicon disk in flow, observed from the top, and the electron microscopic image. (A and B) Attachment phase (A) and 24-h-old mature biofilms (B). (C) Mature biofilms were also observed under a scanning electron microscope, revealing a dense extracellular matrix covering the accumulated Candida cells.
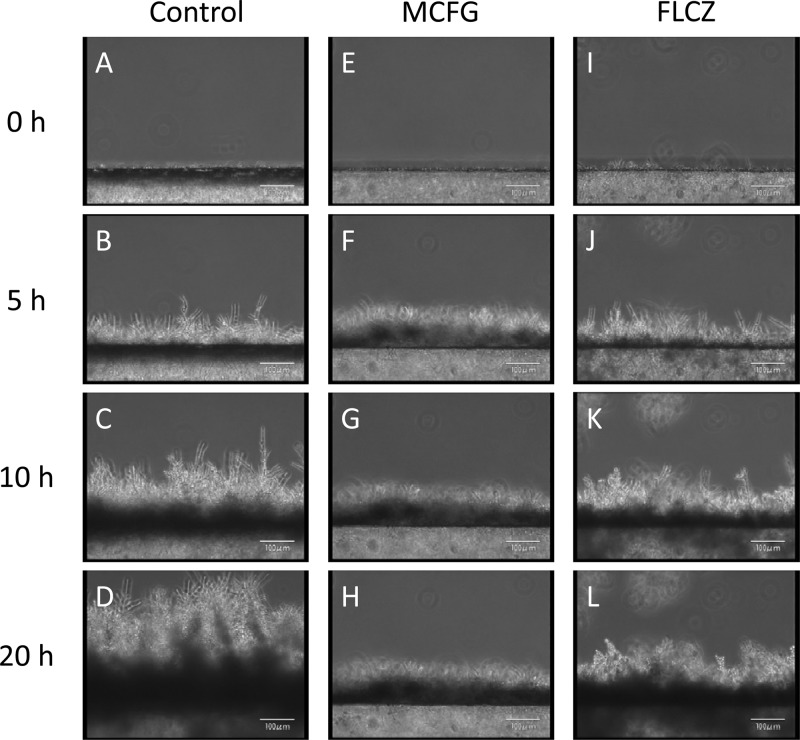
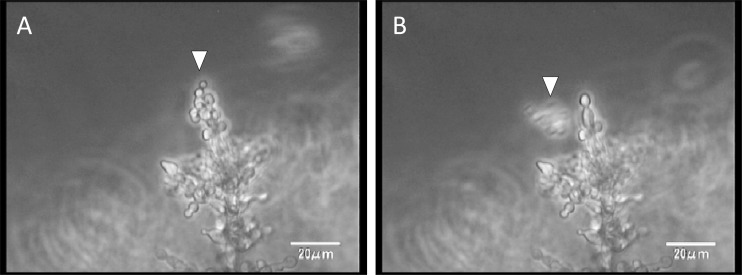
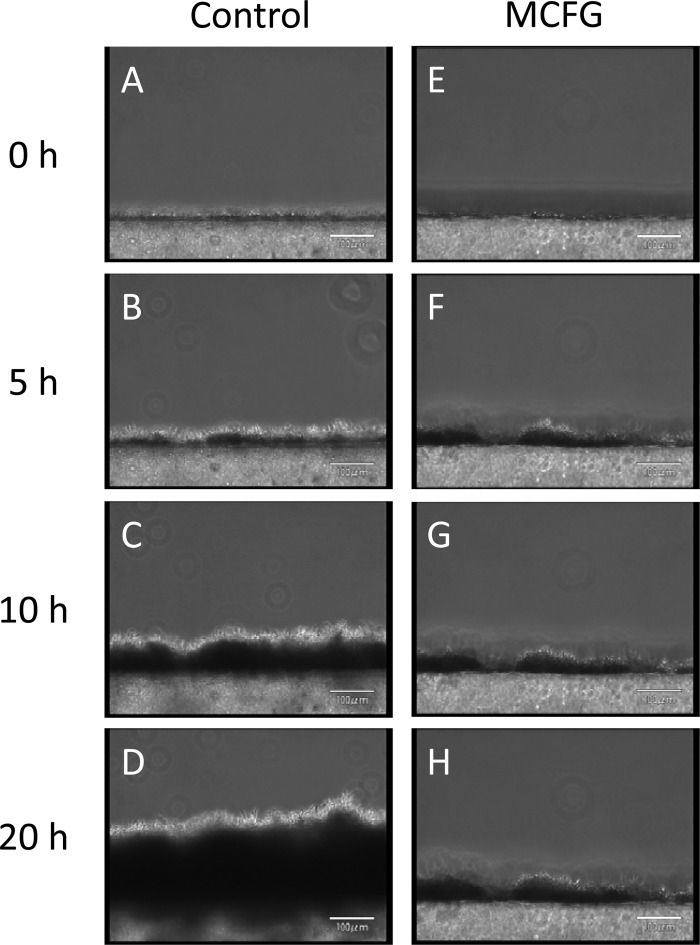
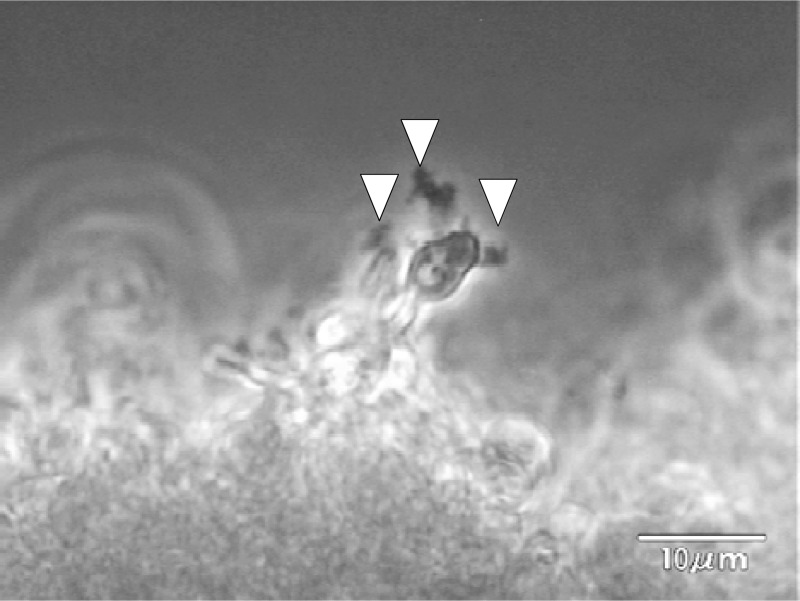
Observation of the continuously developing biofilms revealed that the silicon side of the biofilms became dense (dark), while the flow side consisted mainly of hyphae (Fig. 2A to D; see also movie S1A in the supplemental material). In addition, we observed detachment of small pieces of clustered cells, which had been loosely attached to the tips of hyphae in the matured biofilm (Fig. 3 and data not shown).
Fig 2.
Development of Candida albicans biofilms observed from a side view, and the effects of FLCZ and MCFG. (A to D) Biofilms developed continuously; the silicon sides of biofilms were dense (dark), while the flow sides consisted mainly of hyphae. (E to L) Treatment with MCFG or FLCZ was initiated on 5-h-old biofilms. MCFG completely suppressed biofilm growth (E to H), while FLCZ partially suppressed biofilm growth (I to L).
Fig 3.
The moment of detachment from Candida albicans biofilms. In a close view from the side, a cell cluster (arrowhead) detaches from the tip of the biofilm.
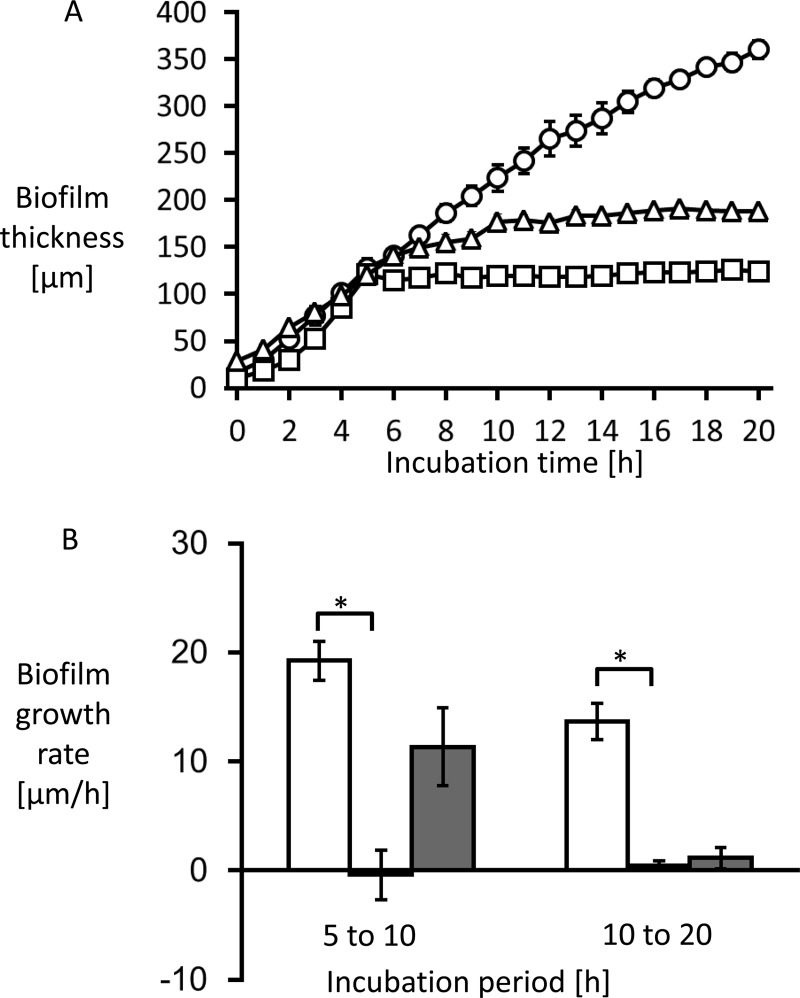
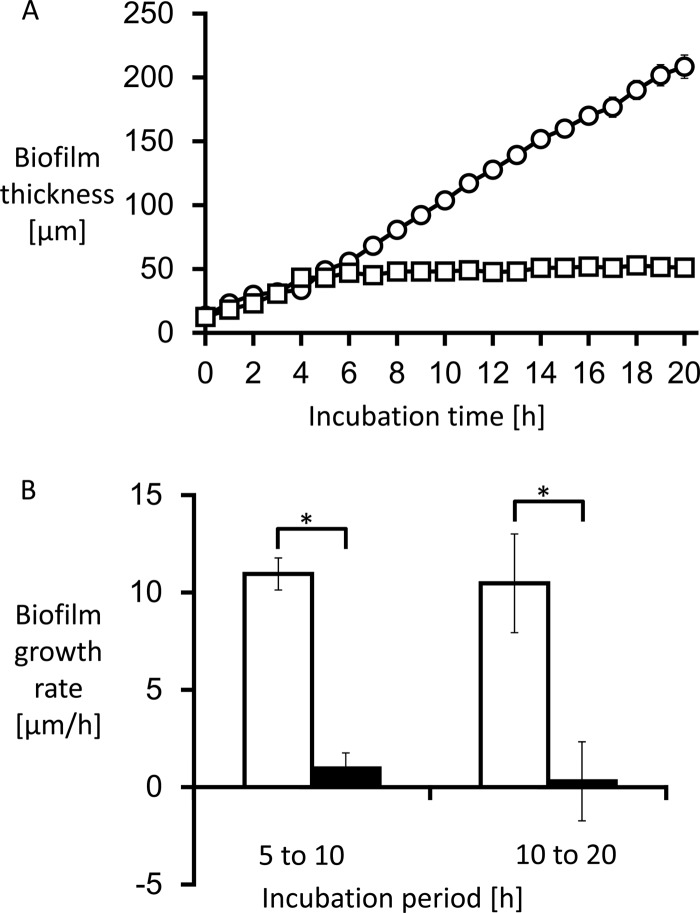
Biofilm thickness was measured hourly to calculate growth rate. The growth curve and growth rate are shown in Fig. 4. The average growth rate from 0 to 20 h without treatment was 17.2 ± 1.3 μm/h.
Fig 4.
The thickness of Candida albicans biofilms was measured (A), and the growth rate was calculated by measuring the change of biofilm thickness every hour (B). (A) The untreated biofilms exhibited linear growth (circles). (B) The average growth rate at 0 to 20 h without treatment was 17.2 ± 1.3 μm/h (white bars). MCFG began to suppress biofilm growth only minutes after the start of the treatment (A [squares]). The average growth rate for the first 5 h after addition was −0.4 ± 2.3 μm/h, followed by a growth rate of 0.5 ± 0.4 μm/h for the next 10 h (B [black bars]). In contrast, FLCZ suppressed biofilm growth at a lower rate (A [triangles]), with an average growth rate for the first 5 h of 11.3 ± 3.6 μm/h, followed by a growth rate of 1.1 ± 1.0 μm/h for the next 10 h (B [gray bars]). *, P < 0.01.
MCFG immediately suppresses biofilm growth, while FLCZ takes longer to produce an antibiofilm effect.
Treatment with MCFG or FLCZ was performed on 5-h-old biofilms of C. albicans Ca21004. Although neither MCFG nor FLCZ eradicated biofilms from the surface of the disks, MCFG began to suppress the growth of biofilms only minutes after the start of the treatment, and the average growth rate for 5 h after addition was −0.4 ± 2.3 μm/h. The growth rate for the next 10 h was still only 0.5 ± 0.4 μm/h (Fig. 2E to H, Fig. 4). Thus, MCFG acts immediately and completely suppresses biofilm growth.
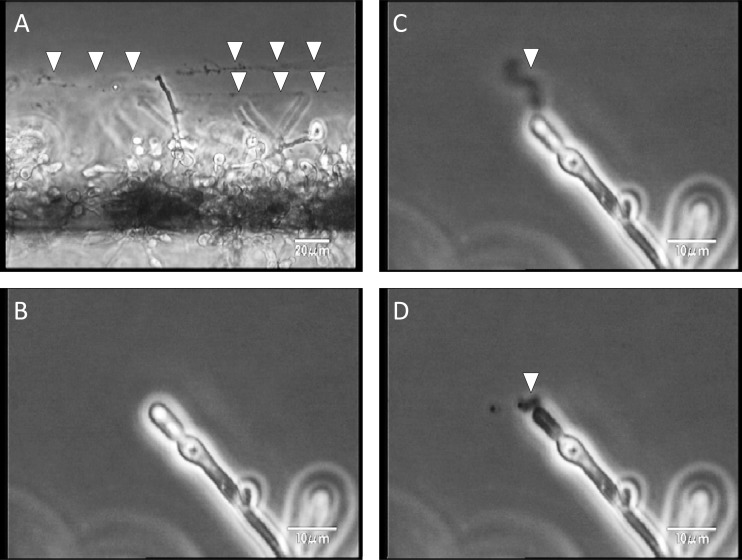
MCFG was also observed to disrupt cells in the biofilms and burst the tips of their hyphae, releasing string-like structures (undefined extracellular component) from the cells (Fig. 5).
Fig 5.
MCFG disrupted cells in the Candida albicans biofilms and burst the tips of their hyphae. (A) After MCFG disrupted the cells in the biofilms, string-like structures were released from the cells (arrowheads). (B to D) A closer view reveals the ejection of material from the bursting cells (arrowheads).
In addition, these same effects were observed in biofilms of C. parapsilosis Cp21007 (Fig. 6 to 8; see also movie S2A to C in the supplemental material), though the biofilm structures were slightly different. C. parapsilosis biofilms did not consist of typical hyphae, but MCFG acted immediately and burst cells in biofilms, resulting in release of the contents.
Fig 6.
Development of Candida parapsilosis biofilms observed from a side view with and without the effect of MCFG. (A to D) Biofilms developed continuously; the silicon sides of biofilms were dense (dark); but unlike Candida albicans, Candida parapsilosis did not form hyphae. (E to H) Treatment with MCFG was initiated on 5-h-old biofilms, and MCFG completely suppressed biofilm growth.
Fig 8.
MCFG disrupted cells in the Candida parapsilosis biofilms and burst the tips of their daughter cells. MCFG disrupted cells in the biofilms, and string-like contents of cells (arrowheads) were released from a bursting cell.
Fig 7.
The thickness of Candida parapsilosis biofilms was measured (A), and the growth rate was calculated by measuring the change of biofilm thickness every hour (B). (A) Untreated biofilms exhibited linear growth (circles). (B) The average growth rate at 5 to 10 h without treatment was 11.0 ± 1.0 μm/h (white bars). MCFG began to suppress biofilm growth only minutes after initiation of the treatment (A [squares]). The average growth rate for 5 h after addition was 1.0 ± 1.1 μm/h, with a growth rate of 0.3 ± 0.4 μm/h for the next 10 h (B [black bars]). *, P < 0.01.
In contrast, FLCZ slowly suppressed biofilm growth, with an average growth rate of 11.3 ± 3.6 μm/h for the first 5 h, followed by a growth rate of 1.1 ± 1.0 μm/h for the next 10 h (Fig. 2I to L and Fig. 4). Thus, FLCZ can also suppress biofilm growth but does so at a lower rate than MCFG and without disruption of the biofilm cells. Furthermore, FLCZ did not inhibit detachment of yeast cell clusters from the tips of the hyphae.
DISCUSSION
The growth rate of biofilms has been conventionally calculated by flow eluate, a method that may produce inaccurate results but has never been evaluated by real-time observation (9). If cell division occurs at a constant rate, cell counts increase exponentially. However, our direct observation revealed the growth of biofilms to be linear, implying that cell division is heterogenous in biofilms. These results might support the notion of the existence of persisters, which may terminate division or inhibit growth (10).
In this study, detachment of yeast cell clusters from the tips of the hyphae was observed. In conventional models, such as Pseudomonas aeruginosa biofilms, planktonic cells have been shown to disperse from the centers of the biofilms (11). Moreover, a study by Blankenship et al. showed that quorum-sensing factors promote dispersal (12) and Uppuluri et al. reported that NRG1, a transcriptional regulator, controls the dispersion (13). However, our flow model shows that cell detachment occurs near the surface as a cluster. Therefore, cell detachment seems to occur passively in a cluster as a result of shear stress rather than by signal-induced dispersal.
The present study also clarified the antifungal effect of MCFG and FLCZ. MCFG is well known as a fungicidal for plankton because it can eradicate biofilm, while FLCZ is fungistatic for plankton and cannot eradicate biofilms even at extremely high concentrations (14, 15). In this study, MCFG suppressed biofilm growth within minutes of the start of the treatment, and these effects persisted throughout the observation period. This rapid effect by MCFGs presents clinical advantages for its use. In addition, MCFG was responsible for disruption of cells in the biofilms and for bursting the tips of their hyphae. Further evaluation is required to understand the entire effect of MCFG on biofilms, but these current results are consistent with those of previous studies demonstrating the biofilm-eradicating effects of MCFG (14, 16). Furthermore, the bursting activity we observed may further contribute to biofilm eradication. Similar effectiveness was observed in biofilms of C. parapsilosis, which is known frequently to cause biofilm-related infections such as line infections (17). Therefore, MCFG may be expected to shown efficacy against candidiasis, including such biofilm infections.
In a recent related study, Valentín et al. reported that voriconazole displayed the ability to reduce the formation of biofilms when it was present during biofilm formation or when biofilms were allowed to form on surfaces previously coated with the drug (18). Our results suggest that azoles are capable of suppressing biofilm growth at clinically achievable concentrations, although it should be noted that a lack of azole activity against preformed Candida biofilms has been reported (14, 15, 19). Methodological differences may contribute to the divergence between our data and other published data. Previous methodologies, for example, biofilm assays using XTT, are very easy to perform and thus useful when multiple samples need to be evaluated simultaneously but lack information about the growth rate and would not be able to evaluate FLCZ inhibition of Candida biofilm growth accurately. Our method is useful for finding the detailed mechanism of action, but it requires special apparatus and technique. Thus, it is not thought that our method will ever replace XTT or CFU counts, but real-time observation could provide us with novel findings about biofilm behavior. Thus, our data may show that the ineffectiveness of FLCZ against preformed Candida biofilms was due in part to the delayed activity. In addition, the delayed action of FLCZ against Candida biofilms might be due to preexistent ergosterol that was to some extent pooled in the cell.
When we evaluated the biofilm growth, other parameters, including biomass measuring, were also considered; however, biomass measurement requires special software and apparatus. Besides, this measurement of peak height was thought to reflect the intuitive observation of biofilm growth, and therefore we adopted this strategy.
In conclusion, our results show that inhibition of glucan synthesis due to MCFG contributes not only to fungicidal activity but also to the immediate suppression of biofilm growth, whereas FLCZ contributes only to suppression of biofilm growth through the inhibition of ergosterol synthesis. Therefore, those characteristic differences should be considered when treating clinical biofilm infections.
Supplementary Material
ACKNOWLEDGMENTS
This work was partly supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (KAKENHI 24791032), the Japan Society for the Promotion of Grants-in-Aid for Young Scientists (B) (24791032), the Ministry of Health, Labor and Welfare of Japan (H22shinkouippan008, H23shinkouippan007, and H23shinkouippan018), and the Takeda Science Foundation.
We thank Noriko Saito for performing scanning electron microscopy.
Y.K. contributed to the overall study design and manuscript preparation. S Miyagawa, OT, and MH performed time-lapse image analysis. H.O. generally advised for the entire manuscript. S. Matsumoto and Y.M. provided overall supervision. We all approved the manuscript prior to submission.
Footnotes
Published ahead of print 4 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02290-12.
REFERENCES
- 1. Kuhn DM, Ghannoum MA. 2004. Candida biofilms: antifungal resistance and emerging therapeutic options. Curr. Opin. Investig. Drugs 5:186–197 [PubMed] [Google Scholar]
- 2. Zirkel J, Klinker H, Kuhn A, Abele-Horn M, Tappe D, Turnwald D, Einsele H, Heinz WJ. 2012. Epidemiology of Candida blood stream infections in patients with hematological malignancies or solid tumors. Med. Mycol. 50:50–55 [DOI] [PubMed] [Google Scholar]
- 3. Zilberberg MD, Kollef MH, Arnold H, Labelle A, Micek ST, Kothari S, Shorr AF. 2010. Inappropriate empiric antifungal therapy for candidemia in the ICU and hospital resource utilization: a retrospective cohort study. BMC Infect. Dis. 10:150 doi:10.1186/1471-2334-10-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, Tuch BB, Andes DR, Johnson AD. 2012. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 148:126–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seki N, Kasai S, Saito N, Komagata O, Mihara M, Sasaki T, Tomita T, Sasaki T, Kobayashi M. 2007. Quantitative analysis of proliferation and excretion of Bartonella quintana in body lice, Pediculus humanus L. Am. J. Trop. Med. Hyg. 77:562–566 [PubMed] [Google Scholar]
- 6. Clinical and Laboratory Standards Institute 2007. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard, 3rd ed CLSI document M27-A3. CLSI, Wayne, PA [Google Scholar]
- 7. Azuma J, Nakahara K, Kagayama A, Okuma T, Kawamura A, Mukai T. 2002. Pharmacokinetic study of micafungin. Jpn. J. Chemother. 50(S-1):155–184 [Google Scholar]
- 8. Sobue S, Tan K, Layton G, Eve M, Sanderson JB. 2004. Pharmacokinetics of fosfluconazole and fluconazole following multiple intravenous administration of fosfluconazole in healthy male volunteers. Br. J. Clin. Pharmacol. 58:20–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baillie GS, Douglas LJ. 1998. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob. Agents Chemother. 42:1900–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lafleur MD, Qi Q, Lewis K. 2010. Patients with long-term oral carriage harbor high-persister mutants of Candida albicans. Antimicrob. Agents Chemother. 54:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Purevdorj-Gage B, Costerton WJ, Stoodley P. 2005. Phenotypic differentiation and seeding dispersal in non-mucoid and mucoid Pseudomonas aeruginosa biofilms. Microbiology 151:1569–1576 [DOI] [PubMed] [Google Scholar]
- 12. Blankenship JR, Mitchell AP. 2006. How to build a biofilm: a fungal perspective. Curr. Opin. Microbiol. 9:588–594 [DOI] [PubMed] [Google Scholar]
- 13. Uppuluri P, Pierce CG, Thomas DP, Bubeck SS, Saville SP, Lopez-Ribot JL. 2010. The transcriptional regulator Nrg1p controls Candida albicans biofilm formation and dispersion. Eukaryot. Cell 9:1531–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaneko Y, Ohno H, Fukazawa H, Murakami Y, Imamura Y, Kohno S, Miyazaki Y. 2010. Anti-Candida-biofilm activity of micafungin is attenuated by voriconazole but restored by pharmacological inhibition of Hsp90-related stress responses. Med. Mycol. 48:606–612 [DOI] [PubMed] [Google Scholar]
- 15. Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaneko Y, Ohno H, Kohno S, Miyazaki Y. 2010. Micafungin alters the expression of genes related to cell wall integrity in Candida albicans biofilms. Jpn. J. Infect. Dis. 63:355–357 [PubMed] [Google Scholar]
- 17. Mukherjee PK, Zhou G, Munyon R, Ghannoum MA. 2005. Candida biofilm: a well-designed protected environment. Med. Mycol. 43:191–208 [DOI] [PubMed] [Google Scholar]
- 18. Valentín A, Canton E, Peman J, Martinez JP. 2012. Voriconazole inhibits biofilm formation in different species of the genus Candida. J. Antimicrob. Chemother. 67:2418–2423 [DOI] [PubMed] [Google Scholar]
- 19. Ramage G, VandeWalle K, Bachmann SP, Wickes BL, Lopez-Ribot JL. 2002. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob. Agents Chemother. 46:3634–3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.