Abstract
Eight Klebsiella pneumoniae clinical strains with high-level aminoglycoside resistance were collected from eight hospitals in São Paulo State, Brazil, in 2010 and 2011. Three of them produced an RmtD group 16S rRNA methyltransferase, RmtD1 or RmtD2. Five strains were found to produce a novel 16S rRNA methyltransferase, designated RmtG, which shared 57 to 58% amino acid identity with RmtD1 and RmtD2. Seven strains coproduced KPC-2 with or without various CTX-M group extended-spectrum β-lactamases, while the remaining strain coproduced CTX-M-2.
TEXT
The production of 16S rRNA methyltransferases (16S-RMTases) has emerged as a mechanism of high-level aminoglycoside resistance among Gram-negative pathogens in the last decade (1). Eight groups of such enzymes have been reported to date. Seven of them (ArmA and RmtA through RmtF) confer high-level resistance to 4,6-disubstituted deoxystreptamine (DOS) aminoglycosides, including gentamicin, tobramycin, and amikacin, by posttranscriptional methylation of position N7 at residue G1405 of 16S rRNA (1–3). N7 G1405 16S-RMTases have a global distribution and are often coproduced with carbapenamases or extended-spectrum β-lactamases (ESBLs). The other 16S-RMTase, NpmA, confers high-level resistance to 4,6-disubstituted DOS aminoglycosides, as well as 4,5-disubstituted DOS aminoglycosides, such as neomycin. NpmA has been shown to methylate position N1 at residue A1408 and has only been found in a single Escherichia coli strain in Japan (4).
Worldwide, ArmA and RmtB are the most commonly encountered 16S-RMTases, having been identified in Enterobacteriaceae, as well as Pseudomonas aeruginosa and Acinetobacter baumannii (1). The epidemiology appears to be distinct in South America, however, where RmtD, which includes the two closely related enzymes RmtD1 and RmtD2, predominates. RmtD1 was initially identified in P. aeruginosa clinical strains which were collected from hospitals in São Paulo, Brazil, in 2005 (5). The majority of these strains coproduced SPM-1 metallo-β-lactamase (6). RmtD1 was then identified in multiple species of Enterobacteriaceae from Brazil, Argentina, and Chile (7). Subsequently, RmtD2 was reported in Enterobacter and Citrobacter spp. from Argentina as the first variant of RmtD, differing from RmtD1 by nine amino acids (8).
The present study was conducted to investigate the 16S-RMTase contents among aminoglycoside-resistant Klebsiella pneumoniae clinical strains collected at Instituto Adolfo Lutz (IAL) from hospitals across the state of São Paulo. IAL serves as a state reference laboratory and receives multidrug-resistant Gram-negative pathogens on an ongoing basis. In 2010 and 2011, eight K. pneumoniae strains with high-level resistance to amikacin, gentamicin, and tobramycin (MIC of >256 μg/ml) were identified, all collected from different hospitals in São Paulo State. The sources of the strains included tracheal aspirate (2), rectal swab (2), bone (1), catheter tip (1), urine (1), and unknown (1) samples (Table 1).
Table 1.
Aminoglycoside susceptibilities of the K. pneumoniae clinical strains and E. coli experimental strains
| Strain | Origin | MIC (μg/ml) ofa: |
16S-RMTase | β-Lactamase | ST | Inc typeb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GEN | TOB | AMK | ABK | NEO | APR | CAZ | CTX | FEP | ETP | MEM | ||||||
| K. pneumoniae 64/11 | Tracheal aspirate | >256 | >256 | >256 | >256 | >256 | 8 | 128 | >256 | 128 | >32 | >32 | rmtD1 | KPC-2, CTX-M-15 | 437 | N, A/C |
| K. pneumoniae 368/10 | Unknown | >256 | >256 | >256 | >256 | 128 | 8 | 12 | 64 | >256 | >32 | >32 | rmtD2 | KPC-2 | 11 | A/C |
| K. pneumoniae 253/11 | Rectal swab | >256 | >256 | >256 | >256 | 32 | 8 | 8 | 256 | >256 | >32 | >32 | rmtD2 | KPC-2 | 11 | A/C |
| K. pneumoniae 145/11 | Rectal swab | >256 | >256 | >256 | >256 | 16 | 4 | 32 | 32 | 12 | >32 | 32 | rmtG | KPC-2, CTX-M-59, TEM-1 | 1046 | N, L/M |
| K. pneumoniae 1194/11 | Catheter tip | >256 | >256 | >256 | >256 | 16 | 4 | 192 | >256 | 48 | 8 | 4 | rmtG | KPC-2, CTX-M-15, TEM-1 | 340 | ND |
| K. pneumoniae 350/10 | Bone | >256 | >256 | >256 | >256 | 4 | 8 | 16 | 256 | 96 | 4 | 4 | rmtG | CTX-M-2, TEM-1 | 442 | ND |
| K. pneumoniae 84/11 | Tracheal aspirate | >256 | >256 | >256 | >256 | 16 | 2 | >256 | >256 | 32 | >32 | >32 | rmtG | KPC-2, CTX-M-59, TEM-1 | 442 | N, L/M |
| K. pneumoniae 922/11 | Urine | >256 | >256 | >256 | >256 | 16 | 8 | >256 | >256 | >256 | >32 | >32 | rmtG | KPC-2, CTX-M-2, TEM-1 | 442 | N, A/C |
| E. coli DH10B(pKp350/10H3) | >256 | >256 | >256 | >256 | 2 | 4 | rmtG | |||||||||
| E. coli DH10B(prmtG) | >256 | >256 | >256 | >256 | 2 | 4 | rmtG | |||||||||
| E. coli DH10B(pKp84/11) | >256 | >256 | >256 | >256 | 2 | 8 | rmtG | CTX-M-59 | N | |||||||
| E. coli DH10B[pBC-SK(−)] | 1 | 0.5 | 2 | 1 | 2 | 8 | ||||||||||
| E. coli DH10B | 1 | 1 | 2 | 1 | 4 | 4 | ||||||||||
The MICs of aminoglycosides were determined by the agar dilution method. The MICs of cephalosporins and carbapenems were determined by Etest (bioMérieux, Hazelwood, MO). GEN, gentamicin; TOB, tobramycin; AMK, amikacin; ABK, arbekacin; NEO, neomycin; APR, apramycin; CAZ, ceftazidime; CTX, cefotaxime; FEP, cefepime; ETP, ertapenem; MEM, meropenem.
ND, not determined.
We first screened for known 16S-RMTase genes as described previously (9). Three strains were positive for rmtD. Sequencing of the full structural genes identified them as rmtD1 (1 strain) or rmtD2 (2 strains). The other strains were negative for any of the previously reported genes. One of these five strains without a known 16S-RMTase gene, K. pneumoniae 350/10, was selected for further investigation. The genomic DNA of K. pneumoniae 350/10 was extracted, digested with HindIII (New England BioLabs, Ipswich, MA), and ligated with vector pBC-SK(−) (Agilent Technologies, Santa Clara, CA). Electrocompetent Escherichia coli DH10B was transformed with this genomic library, and transformants were selected on tryptic soy agar (TSA) plates containing chloramphenicol (30 μg/ml) and gentamicin (50 μg/ml). This procedure yielded several colonies, all of which grew readily on TSA plates containing 100 μg/ml of arbekacin, a phenotype suggestive of 16S-RMTase production (9). The recombinant plasmid harbored by one of these transformants (pKp350/10H3) was then fully sequenced. The sequencing revealed the presence of a 1.6-kb insert, which contained two overlapping open reading frames. The first open reading frame was partial and corresponded to a 252-amino-acid sequence showing 76% identity with a putative tRNA ribosyltransferase reported upstream from rmtD1 and rmtD2 (8, 10). The second open reading frame overlapped the first one by eight nucleotides and corresponded to a 264-amino-acid sequence, which showed 58% and 57% identity with RmtD1 and RmtD2, respectively, 36% with RmtA, RmtB2, and RmtF, 35% with RmtB1, 29% with RmtE, 23% with RmtC, and 22% with ArmA. This open reading frame encoded a novel 16S-RMTase, which was designated RmtG (Fig. 1 and 2). Given the relative sequence similarity between RmtG and the RmtD proteins, as well as an analogous alignment observed with a putative tRNA ribosyltransferase gene located upstream from rmtD1 and rmtD2, it appears likely that these 16S-RMTases originated from closely related but as-yet-unidentified nonpathogenic species. The G+C content of rmtG (60%) was also similar to that of rmtD1 and rmtD2 (59%).
Fig 1.
Amino acid alignment of RmtG with RmtD1 and RmtD2, the 16S-RMTase group with the highest similarity with RmtG (produced with Clustal W [http://www.ebi.ac.uk/Tools/msa/clustalw2/]). Part of the nucleotide sequence preceding rmtG is also shown, with the −10 and −35 regions of the putative promoter and potential ribosomal binding site (RBS) underlined.
Fig 2.
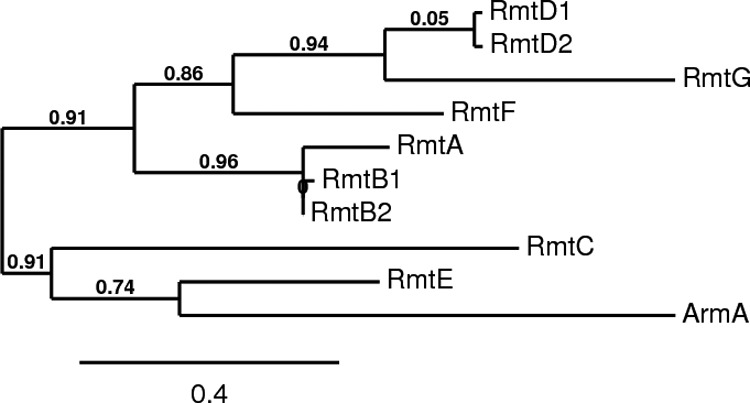
Dendrogram of confirmed and putative acquired N7 G1405 16S-RMTases. The dendrogram was generated using the tools available at http://www.phylogeny.fr (27). GenBank protein sequence accession numbers are as follows: ArmA, AAP50754.1; RmtA, BAD12551.1; RmtB1, BAC81971.1; RmtB2, AFC75738.1; RmtC, BAE48305.1; RmtD1, ABJ53409.1; RmtD2, ADW66527.1; RmtE, ADA63498.1; RmtF, AFJ11385.1. The numbers represent branch support values. The scale bar shows length in proportional difference.
We then amplified the rmtG structural gene by PCR using primers rmtG-F-XbaI (5′-GCTCTAGAATGCGTGATCCGTTGTTT-3′) and rmtG-R-BamHI (5′-GCGGATCCTCATTCAGATTCCCGATG-3′) (the restriction sites are underlined). The product was digested with XbaI and BamHI, ligated with pBC-SK(−), and used to transform E. coli DH10B. The recombinant plasmid from a colony which grew on a TSA plate containing chloramphenicol and gentamicin (prmtG) was found to contain rmtG, which was confirmed to be intact by sequencing. E. coli DH10B(prmtG) displayed high-level resistance to 4,6-disubstituted DOS aminoglycosides but not 4,5-disubstituted DOS ones (Table 1). We therefore speculate that RmtG is an N7 G1405 16S-RMTase (1).
We then designed detection primers rmtG-F (5′-AAATACCGCGATGTGTGTCC-3′) and rmtG-R (5′-ACACGGCATCTGTTTCTTCC-3′) to screen the four remaining K. pneumoniae strains, which were negative for known 16S-RMTase genes. The PCR conditions were the following: initial denaturation at 95°C for 2 min; 30 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 60 s; and final incubation for 7 min at 72°C. The results showed that they were all positive for the presence of rmtG. Sequencing of the entire genes confirmed them as identical to the originally identified rmtG from K. pneumoniae 350/10. Furthermore, the upstream sequence of rmtG was identical for all five strains for the 0.7-kb region captured in pKp350/10H3. Of the five RmtG-producing K. pneumoniae strains, transfer of rmtG by transformation to E. coli DH10B was successful only for K. pneumoniae 84/11(pKp84/11), suggesting a plasmidic location of rmtG for this strain. rmtG was cotransferred with blaCTX-M-59 but not blaKPC-2. Transfer of rmtG to E. coli J53 by broth mating was not successful for any of the five strains despite repeated attempts. We therefore conducted pulsed-field gel electrophoresis (PFGE) of S1 nuclease-treated genomic DNA (11). This was followed by DNA hybridization using an rmtG-specific probe and methodology described previously (12). As shown in Fig. 3, the size of pKp84/11 was estimated to be approximately 200 kb. While they did not transfer to E. coli, the rmtG genes in the other four K. pneumoniae strains also appeared to be carried on plasmids, which ranged in size between 200 and 400 kb. Replicon typing using a previously described method (13) revealed pKp84/11 to be an IncN plasmid (Table 1). However, two of the rmtG-harboring K. pneumoniae strains were negative for any replicon, including IncN, based on this protocol.
Fig 3.
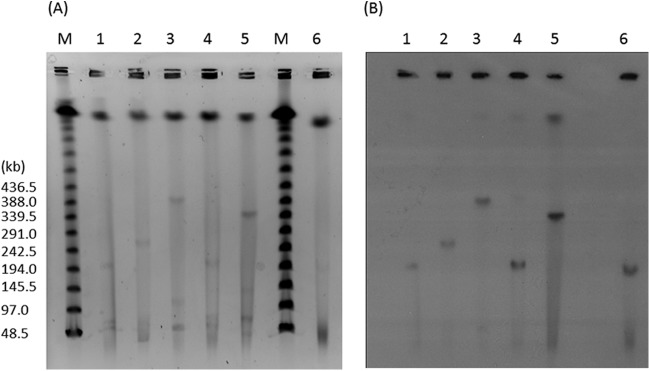
(A) PFGE of S1 nuclease-digested plasmids. (B) DNA hybridization with rmtG-specific probe. Lanes M, marker; lanes 1, strain 145/11; lanes 2, strain 1194/11; lanes 3, strain 350/10; lanes 4, strain 84/11; lanes 5, strain 922/11; lanes 6, E. coli DH10B transformant of strain 84/11.
K. pneumoniae strains producing various ESBLs and, more recently, KPCs (Klebsiella pneumoniae Carbapenemases), are reported from Brazil (14–19). We screened the eight 16S-RMTase-producing strains for KPC, CTX-M, SHV, and TEM group β-lactamases by PCR and sequencing as described previously (20). All but one strain were found to harbor blaKPC-2. In addition, six strains carried blaCTX-M (blaCTX-M-2, blaCTX-M-59, or blaCTX-M-15) (Table 1). KPC-producing K. pneumoniae strains in Brazil are predominantly clonal complex 258 (CC258), which includes sequence types (STs) such as ST11, ST258, and ST437 (16, 21). We determined the STs of the eight clinical strains using the standard protocol (22). The strains producing RmtD1 and RmtD2 belonged to ST11 or ST437 (Table 1). One of the RmtG-producing strains belonged to ST340, which is also part of CC258. The other four strains belonged to ST442 or ST1046. ST442 was reported in a clinical strain which was recovered from blood in the state of Goiás in Brazil in 2009 (21). ST1046 is a double-locus variant of ST961, which was recently registered as an environmental strain from Portugal. Therefore, the rmtD alleles were likely acquired by global epidemic strains from other Enterobacteraceae species or P. aeruginosa, whereas the strains carrying rmtG appeared to be of a more-local origin. Coproduction of KPC and 16S-RMTase has been reported for ArmA and RmtB in K. pneumoniae and Enterobacter cloacae (23–25). RmtG is thus the third 16S-RMTase to be described in KPC-producing Enterobacteriaceae. The production of 16S-RMTase by KPC-producing K. pneumoniae could further limit the treatment options for infection caused by this organism, the majority of which otherwise remain susceptible to one or more aminoglycosides, gentamicin in particular (26).
Nucleotide sequence accession number.
The sequence reported in this work has been deposited to the GenBank under accession number JX486113.
ACKNOWLEDGMENTS
M.F.C.B. and G.R.F. were supported through the Global Infectious Disease Research Training Program funded by the National Institute of Allergy and Infectious Diseases (grant D43TW006592; principal investigator, Lee H. Harrison) and a scholarship from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paul; grants 2012/06827-1 and 2012/06828-1). Part of this work was also supported by a research grant from FAPESP (grant 2009/53229-0) to D.D.O.G. Y.D. was supported in part through a Research Scholar Development Award funded by the National Institute of Allergy and Infectious Diseases (grant K22AI080584).
Footnotes
Published ahead of print 4 March 2013
REFERENCES
- 1. Wachino J, Arakawa Y. 2012. Exogenously acquired 16S rRNA methyltransferases found in aminoglycoside-resistant pathogenic Gram-negative bacteria: an update. Drug Resist. Updat. 15:133–148 [DOI] [PubMed] [Google Scholar]
- 2. Davis MA, Baker KN, Orfe LH, Shah DH, Besser TE, Call DR. 2010. Discovery of a gene conferring multiple-aminoglycoside resistance in Escherichia coli. Antimicrob. Agents Chemother. 54:2666–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galimand M, Courvalin P, Lambert T. 2012. RmtF, a new member of the aminoglycoside resistance 16S rRNA N7 G1405 methyltransferase family. Antimicrob. Agents Chemother. 56:3960–3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wachino J, Shibayama K, Kurokawa H, Kimura K, Yamane K, Suzuki S, Shibata N, Ike Y, Arakawa Y. 2007. Novel plasmid-mediated 16S rRNA m1A1408 methyltransferase, NpmA, found in a clinically isolated Escherichia coli strain resistant to structurally diverse aminoglycosides. Antimicrob. Agents Chemother. 51:4401–4409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doi Y, de Oliveira Garcia D, Adams J, Paterson DL. 2007. Coproduction of novel 16S rRNA methylase RmtD and metallo-β-lactamase SPM-1 in a panresistant Pseudomonas aeruginosa isolate from Brazil. Antimicrob. Agents Chemother. 51:852–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doi Y, Ghilardi AC, Adams J, de Oliveira Garcia D, Paterson DL. 2007. High prevalence of metallo-β-lactamase and 16S rRNA methylase coproduction among imipenem-resistant Pseudomonas aeruginosa isolates in Brazil. Antimicrob. Agents Chemother. 51:3388–3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fritsche TR, Castanheira M, Miller GH, Jones RN, Armstrong ES. 2008. Detection of methyltransferases conferring high-level resistance to aminoglycosides in enterobacteriaceae from Europe, North America, and Latin America. Antimicrob. Agents Chemother. 52:1843–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tijet N, Andres P, Chung C, Lucero C, Low DE, Galas M, Corso A, Petroni A, Melano RG. 2011. rmtD2, a new allele of a 16S rRNA methylase gene, has been present in Enterobacteriaceae isolates from Argentina for more than a decade. Antimicrob. Agents Chemother. 55:904–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doi Y, Arakawa Y. 2007. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin. Infect. Dis. 45:88–94 [DOI] [PubMed] [Google Scholar]
- 10. Doi Y, Adams-Haduch JM, Paterson DL. 2008. Genetic environment of 16S rRNA methylase gene rmtD. Antimicrob. Agents Chemother. 52:2270–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barton BM, Harding GP, Zuccarelli AJ. 1995. A general method for detecting and sizing large plasmids. Anal. Biochem. 226:235–240 [DOI] [PubMed] [Google Scholar]
- 12. Sidjabat HE, Paterson DL, Adams-Haduch JM, Ewan L, Pasculle AW, Muto CA, Tian GB, Doi Y. 2009. Molecular epidemiology of CTX-M-producing Escherichia coli isolates at a tertiary medical center in western Pennsylvania. Antimicrob. Agents Chemother. 53:4733–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228 [DOI] [PubMed] [Google Scholar]
- 14. de Oliveira Garcia D, Doi Y, Szabo D, Adams-Haduch JM, Vaz TM, Leite D, Padoveze MC, Freire MP, Silveira FP, Paterson DL. 2008. Multiclonal outbreak of Klebsiella pneumoniae producing extended-spectrum β-lactamase CTX-M-2 and novel variant CTX-M-59 in a neonatal intensive care unit in Brazil. Antimicrob. Agents Chemother. 52:1790–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tollentino FM, Polotto M, Nogueira ML, Lincopan N, Neves P, Mamizuka EM, Remeli GA, De Almeida MT, Rubio FG, Nogueira MC. 2011. High prevalence of blaCTX-M extended spectrum β-lactamase genes in Klebsiella pneumoniae isolates from a tertiary care hospital: first report of blaSHV-12, blaSHV-31, blaSHV-38, and blaCTX-M-15 in Brazil. Microb. Drug Resist. 17:7–16 [DOI] [PubMed] [Google Scholar]
- 16. Andrade LN, Curiao T, Ferreira JC, Longo JM, Climaco EC, Martinez R, Bellissimo-Rodrigues F, Basile-Filho A, Evaristo MA, Del Peloso PF, Ribeiro VB, Barth AL, Paula MC, Baquero F, Canton R, Darini AL, Coque TM. 2011. Dissemination of blaKPC-2 by the spread of Klebsiella pneumoniae clonal complex 258 clones (ST258, ST11, ST437) and plasmids (IncFII, IncN, IncL/M) among Enterobacteriaceae species in Brazil. Antimicrob. Agents Chemother. 55:3579–3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monteiro J, Santos AF, Asensi MD, Peirano G, Gales AC. 2009. First report of KPC-2-producing Klebsiella pneumoniae strains in Brazil. Antimicrob. Agents Chemother. 53:333–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abboud CS, Bergamasco MD, Doi AM, Zandonadi EC, Barbosa V, Cortez D, Saraiva CR, Doy C, de Oliveira Garcia D. 2011. First report of investigation into an outbreak due to carbapenemase-producing Klebsiella pneumoniae in a tertiary Brazilian hospital, with extension to a patient in the community. J. Infect. Prev. 12:150–153 [Google Scholar]
- 19. Pereira GH, Garcia DO, Mostardeiro M, Ogassavara CT, Levin AS. 2011. Spread of carbapenem-resistant Klebsiella pneumoniae in a tertiary hospital in Sao Paulo, Brazil. J. Hosp. Infect. 79:182–183 [DOI] [PubMed] [Google Scholar]
- 20. Kim YA, Qureshi ZA, Adams-Haduch JM, Park YS, Shutt KA, Doi Y. 2012. Features of infections due to Klebsiella pneumoniae carbapenemase-producing Escherichia coli: emergence of sequence type 131. Clin. Infect. Dis. 55:224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seki LM, Pereira PS, de Souza MDP, Conceicao MDS, Marques EA, Porto CO, Colnago EM, Alves CDF, Gomes D, Assef AP, Samuelsen O, Asensi MD. 2011. Molecular epidemiology of KPC-2-producing Klebsiella pneumoniae isolates in Brazil: the predominance of sequence type 437. Diagn Microbiol Infect Dis. 70:274–277 [DOI] [PubMed] [Google Scholar]
- 22. Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43:4178–4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Galani I, Souli M, Panagea T, Poulakou G, Kanellakopoulou K, Giamarellou H. 2012. Prevalence of 16S rRNA methylase genes in Enterobacteriaceae isolates from a Greek university hospital. Clin. Microbiol. Infect. 18:E52–E54 [DOI] [PubMed] [Google Scholar]
- 24. Zacharczuk K, Piekarska K, Szych J, Zawidzka E, Sulikowska A, Wardak S, Jagielski M, Gierczynski R. 2011. Emergence of Klebsiella pneumoniae coproducing KPC-2 and 16S rRNA methylase ArmA in Poland. Antimicrob. Agents Chemother. 55:443–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu Q, Liu Q, Han L, Sun J, Ni Y. 2010. Plasmid-mediated carbapenem-hydrolyzing enzyme KPC-2 and ArmA 16S rRNA methylase conferring high-level aminoglycoside resistance in carbapenem-resistant Enterobacter cloacae in China. Diagn. Microbiol. Infect. Dis. 66:326–328 [DOI] [PubMed] [Google Scholar]
- 26. Falagas ME, Karageorgopoulos DE, Nordmann P. 2011. Therapeutic options for infections with Enterobacteriaceae producing carbapenem-hydrolyzing enzymes. Future Microbiol. 6:653–666 [DOI] [PubMed] [Google Scholar]
- 27. Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36:W465–W469 [DOI] [PMC free article] [PubMed] [Google Scholar]



