Abstract
Coccidioidomycosis is a systemic mycosis caused by the dimorphic fungi Coccidioides spp. The treatment for chronic and/or disseminated coccidioidomycosis can be prolonged and complicated. Therefore, the search for new drugs is necessary. Farnesol is a precursor in the sterol biosynthesis pathway that has been shown to present antifungal activity. Thus, the objective of this study was to evaluate the in vitro antifungal activity of farnesol alone and in combination with antifungal agents against clinical and environmental strains of Coccidioides posadasii as well as to determine their effect on the synthesis of ergosterol and on cell permeability. This study employed the broth macrodilution method to determine the MIC of farnesol against 18 strains of C. posadasii. Quantification of ergosterol was performed with 10 strains of C. posadasii after exposure to subinhibitory concentrations of farnesol. Finally, the activity of farnesol was evaluated in the presence of osmotic stress, induced by the addition of NaCl to the culture medium, during the susceptibility tests. The results showed that farnesol exhibited low MICs (ranging from 0.00171 to 0.01369 mg/liter) against all tested strains. The combination of farnesol with the antifungals showed synergistic effects (fractional inhibitory concentration index [FICI] ≤ 0.5). As for the ergosterol quantification, it was observed that exposure to subinhibitory concentrations of farnesol decreased the amount of ergosterol extracted from the fungal cells. Furthermore, farnesol also showed lower MIC values when the strains were subjected to osmotic stress, indicating the action of this compound on the fungal membrane. Thus, due to the high in vitro antifungal activity, this work brings perspectives for the performance of in vivo studies to further elucidate the effects of farnesol on the host cells.
INTRODUCTION
Coccidioidomycosis is a systemic mycosis caused by the dimorphic and geophilic fungi Coccidioides immitis and Coccidioides posadasii (1) which usually is a benign infection with spontaneous resolution. However, a small proportion of infected individuals develop the progressive form, which is potentially lethal and can affect not only the lungs but other organs as well through hematogenous dissemination (2).
The treatments for chronic and disseminated coccidioidomycosis can be prolonged and are often complicated (3). Thus, there is a need for studies that seek new therapeutic options to treat coccidioidomycosis, since amphotericin B, considered the drug of choice for the treatment of the disease, is a potentially nephrotoxic drug (4) and only 50 to 60% of these patients are responsive to treatment with itraconazole and fluconazole (3).
Farnesol is a sesquiterpene that acts as a precursor in the biosynthesis of sterols and isoprenoids in Candida albicans (5, 6). Studies have shown that it acts as a quorum-sensing molecule and is involved in the inhibition of filamentation (5, 6) and the formation of biofilms (7, 8). More recently, it has been demonstrated that this compound also has an important role in the resistance to oxidative stress (9). However, farnesol has a cytotoxic effect on C. albicans at certain concentrations and under some environmental conditions (10) as well as on other microorganisms, inducing apoptosis (11).
Some studies have confirmed the inhibitory effect of farnesol on the growth of microorganisms (12–16). It was shown that farnesol can act as an antifungal agent against the dimorphic fungus Paracoccidioides brasiliensis (16). More recently, our group demonstrated its inhibitory activity against Cryptococcus spp. (17) and Candida spp. (18).
Thus, the objective of this study was to evaluate the in vitro antifungal activity of farnesol alone and in combination with antifungal agents against clinical and environmental strains of Coccidioides posadasii as well as to determine its effect on the synthesis of ergosterol and on cell permeability.
MATERIALS AND METHODS
Fungal culture.
This study included 18 strains (15 clinical and 3 environmental) of Coccidioides posadasii from northeastern Brazil. All strains belonged to the fungal collection of the Specialized Medical Mycology Center (CEMM, Federal University of Ceará, Brazil). The procedures for identification of the fungi included classic mycological analysis, as described by Brilhante et al. (19), and a PCR assay (20). All procedures were performed in a class II biological safety cabinet in a biosafety level 3 laboratory.
Inoculum preparation for antifungal susceptibility testing.
C. posadasii strains were grown on potato agar and incubated for 7 days at room temperature (25 to 28°C). To prepare the inoculum, 2 ml of sterile saline was added to each culture and, with the aid of a microbiological loop, the surface of the mycelium was scraped. The suspensions were transferred to sterile tubes and allowed to stand for 5 min. The supernatant was read in a spectrophotometer at 530 nm, and its transmittance was set to 95%. The suspensions containing arthroconidia and hyphal fragments were diluted to 1:10 with RPMI 1640 and buffered with morpholinepropanesulfonic acid (MOPS) (0.156 M) to pH 7.0 to obtain inocula of approximately 1 × 103 to 5 × 103 CFU/ml−1 (21).
Antimicrobial agents and in vitro susceptibility testing.
The solutions tested were prepared at the time of use from a commercial solution of 95% farnesol (mixture of isomers; Sigma-Aldrich), using 30% dimethyl sulfoxide (DMSO) as a solvent. For the susceptibility assay, farnesol was further diluted with RPMI 1640 with l-glutamine, buffered to pH 7.0 with 0.165 M MOPS, until reaching the concentration range of 0.00020 to 0.0548 mg/liter.
After determining the MIC of farnesol and the antifungal agents alone, we tested combinations of farnesol with amphotericin B, itraconazole, voriconazole, and caspofungin. The combinations were tested in the following concentration range: 0.00000667 to 0.0137 mg/liter for farnesol, 0.0039 to 0.125 mg/liter for amphotericin B, 0.0156 to 0.5 mg/liter for itraconazole, 0.0078 to 0.25 mg/liter for voriconazole, and 2 to 32 mg/liter for caspofungin. The initial concentrations of the antifungals and farnesol represented the MICs found for these compounds individually against each tested strain.
The susceptibility of C. posadasii strains to farnesol and the antifungals alone and in combination was determined through the broth macrodilution method, according to the M38-A2 protocol standardized by the CLSI (22). The results obtained were visually read after 48 h of incubation at 35°C. The MICs for farnesol (17), itraconazole, voriconazole, and caspofungin alone or in combination were defined as the lowest concentration of drug capable of inhibiting 80% of fungal growth, when compared to the drug-free control tube (23). As for amphotericin B alone, the MIC was the lowest concentration at which no fungal growth was observed. For quality control of the antifungal susceptibility tests, Candida parapsilosis ATCC 22019 was included.
The interaction between the combined drugs was evaluated by calculating the fractional inhibitory concentration index (FICI), according to Johnson et al., where FICI values of ≤0.5 indicate synergism, 0.5 < FICI ≤ 4.0 indicates indifferent interactions, and an FICI of >4.0 indicates antagonism (24). The differences between the MICs of drugs individually and in combination were evaluated by Student's t test. The obtained FICI values for each drug combination were compared through Student's t test. P values lower than 0.05 indicated statistically significant differences.
Extraction of ergosterol.
Cellular ergosterol was extracted as described by Arthington-Skaggs (25), with some modifications. The extraction was performed after the exposure of 10 strains (05-2-064, 05-2-066, 05-2-067, 05-2-068, 05-2-070, 01-6-091, 01-6-092, 01-6-101, 01-6-102, and 01-6-103) of C. posadasii to subinhibitory concentrations of farnesol and itraconazole (control drug), through the macrodilution technique. Seven concentrations of the compounds were tested, ranging from 0.0000133 to 0.003469 mg/liter for farnesol and from 0.00195 to 0.125 mg/liter for itraconazole. The mycelial pellet for each concentration was exposed to 0.5 ml of KOH-EtOH (20%/60%) and incubated at 95°C, for 1 h, in a water bath. After that, 0.5 ml of hexane was added in order to isolate the sterols. The solutions were centrifuged for 2 min (10,000 × g). Then, the top layer of hexane was transferred to microtubes and added to 0.5 ml of hexane. Ergosterol quantification was performed in a spectrophotometer at λ = 295.10 nm and compared to a predetermined standard curve. For positive control, ergosterol from the 10 evaluated strains of C. posadasii grown in drug-free RPMI medium was quantified.
Inhibitory effect of farnesol in the presence of osmotic stress.
The ability of farnesol to alter the permeability of the fungal membrane was also evaluated by macrodilution. To induce osmotic stress, we used the method described by Coleman et al. with modifications, where the RPMI 1640 medium (buffered to pH 7.0) was added to 0.175 M NaCl (26). The concentration of NaCl was previously determined through macrodilution in the range of 7 to 0.0021 M. Concentrations ≤ 0.175 M did not interfere with the growth of C. posadasii compared to the drug-free salt-free control. Lastly, sub-MICs of farnesol were tested. The results were visually read after 48 h of incubation at 35°C.
RESULTS
MIC of farnesol alone and in combination with antifungal drugs.
All strains of C. posadasii were inhibited by low farnesol concentrations, with MICs ranging from 0.00171 to 0.01369 mg/liter (0.0078 to 0.0616 μM) and a geometric mean of 0.00634 mg/liter (0.285 μM). For the antifungal drugs in clinical use, the MIC intervals found (in mg/liter) were 0.0625 to 0.125 for amphotericin B, 0.125 to 0.5 for itraconazole, 0.125 to 0.25 for voriconazole, and 16 to 32 for caspofungin. In addition, all drug combinations tested were able to inhibit growth of C. posadasii at lower doses and a significant reduction was found for all tested drugs (caspofungin P < 0.0001, itraconazole P = 0.0016, amphotericin B P < 0.0001, and voriconazole P = 0.0002), with a statistically significant synergistic effect for the combinations of farnesol with amphotericin B (P = 0.0124) and caspofungin (P = 0.0003), as shown in Table 1. The antifungal MICs obtained against C. parapsilosis ATCC 22019 were 0.5 mg/liter for itraconazole and caspofungin and 1.0 and 0.03 mg/liter for amphotericin B and voriconazole, respectively.
Table 1.
MIC and FICI values for the combinations of farnesol with the antifungal agents caspofungin, voriconazole, and itraconazole against strains of C. posadasiia
| MIC range (mg/liter) of indicated drug alone |
MIC range (mg/liter) of indicated drug in combination |
FICI (range) | No. (%) of strains showing synergistic effects | ||||
|---|---|---|---|---|---|---|---|
| FNZ | Antifungal | FNZ | Antifungal | ||||
| 0.00171–0.01369 | ITR | 0.125–0.5 | 0.000107–0.003424 | ITR | 0.0078–0.0312 | 0.125–1 | 12 (66.6) |
| VRZ | 0.125–0.25 | 0.000428–0.001712 | VRZ | 0.0156–0.125 | 0.125–2 | 15 (83.3) | |
| CAS | 32–16 | 0.000107–0.003424 | CAS | 1–8 | 0.125–1 | 17 (94.4) | |
| AMB | 0.0625–0.125 | 0.000428–0.003424 | AMB | 0.0078–0.0312 | 0.25–1 | 17 (94.4) | |
FNZ, farnesol; AMB, amphotericin B; ITR, itraconazole; VRZ, voriconazole; CAS, caspofungin.
Ergosterol quantification.
The results showed that the exposure of the 10 strains of C. posadasii to subinhibitory concentrations of farnesol altered the amount of ergosterol extracted from each sampled strain. Higher concentrations of farnesol resulted in the extraction of smaller amounts of ergosterol from the fungal cells. Similar results were observed with itraconazole, which is known to inhibit ergosterol biosynthesis. Figure 1 shows the geometric means of the obtained results for the 10 evaluated isolates.
Fig 1.

Quantification of ergosterol from 10 strains of Coccidioides posadasii after exposure to different concentrations of farnesol (A) and itraconazole (B). The values represent the geometric means of the results obtained for all tested strains. R2 represents the coefficient of determination.
Osmotic stress.
The MIC values obtained by using RPMI medium supplemented with NaCl were significantly lower than the MICs found when standard RPMI medium was used, as shown in Fig. 2. The geometric means obtained for the itraconazole MICs were 0.25 and 0.00012 mg/liter for standard RPMI medium and RPMI medium supplemented with NaCl, respectively.
Fig 2.
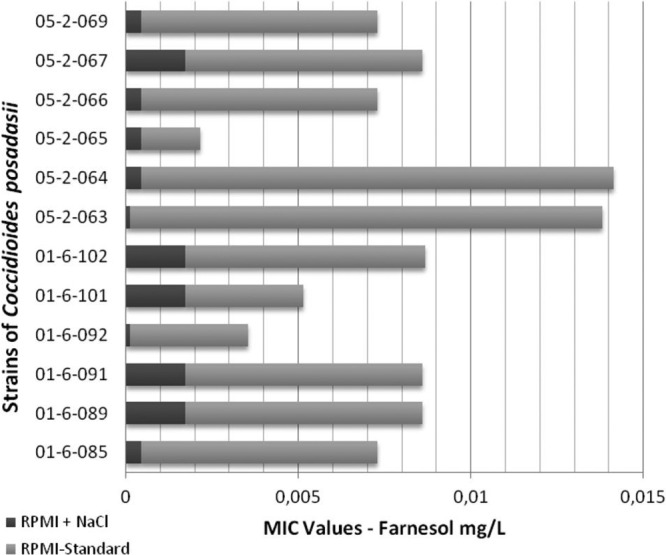
MIC values of farnesol against strains of Coccidioides posadasii in the presence and absence of osmotic stress.
DISCUSSION
Several studies have confirmed the inhibitory effect of farnesol on the growth of several bacteria (12, 13, 27). More recently, many studies have described the activity of farnesol against different species of fungi (15–18). In this study, for the first time, the antifungal activity of farnesol against the primary pathogen C. posadasii, the causative agent of coccidioidomycosis, was investigated.
The in vitro MIC values obtained showed high inhibitory activity of farnesol against all tested strains, with no differences between clinical and environmental strains. These results, ranging from 0.00171 to 0.01369 mg/liter (0.0078 to 0.0616 μM), are quite low compared to those previously described for other fungal species. Derengowski et al. demonstrated the in vitro activity of farnesol against the dimorphic fungus Paracoccidioides brasiliensis, which presented an average MIC of 25 μM (16). Moreover, recent studies from our group performed with the species Cryptococcus neoformans and C. gattii showed MIC values ranging from 0.29 to 75 μM for both species (17), while the farnesol MICs ranged from 9.37 to 150 μM against different Candida species (18).
In this study, we observed that farnesol significantly decreased the MICs for itraconazole and voriconazole and synergistically interacted with amphotericin B and caspofungin. These results corroborate reports previously described in the literature that suggest the potential use of farnesol as an adjuvant in antifungal therapy and its ability to promote the reversal of antimicrobial resistance (8, 28). Cordeiro et al. showed that farnesol, when combined with antifungal drugs, reversed the in vitro antifungal resistance of Candida strains and synergistically interacted with all tested drugs (18).
Regarding the mechanism of action of farnesol in the fungal cells, considering that it shares several precursors with ergosterol in the biosynthetic pathway, some authors have suggested that this compound acts by inhibiting the synthesis of ergosterol, causing alterations in the cell membrane (8). The results of this study confirm those inferences, since the concentration of ergosterol in the cell decreased when the strains of C. posadasii were exposed to farnesol, even at subinhibitory doses. The results also showed that exposure to the higher concentrations of farnesol led to the extraction of smaller amounts of ergosterol from fungal cells. A similar effect was also observed when cells were exposed to itraconazole, which is known to inhibit the synthesis of ergosterol. Thus, it can be suggested that the two agents act similarly.
Furthermore, when the strains were subjected to a medium with a high salt concentration, the obtained farnesol MICs considerably decreased, supporting the proposition that the mechanism of action of this compound is associated with degeneration of the fungal membrane, since under conditions of osmotic stress, the strains have more fragile cells. Derengowski et al. reported that farnesol does not appear to act in the cell wall, because the wall remains intact in cells of P. brasiliensis after exposure to this compound (16).
Considering the high in vitro antifungal activity of farnesol against strains of C. posadasii and its low toxicity, as previously demonstrated by Navarathna et al. (29), its use in combination with other drugs as a possible therapeutic antifungal agent and an adjuvant in the treatment of coccidioidomycosis seems feasible. Thus, this work brings perspectives for the performance of in vivo studies to further elucidate the effects of farnesol on the host cells.
ACKNOWLEDGMENTS
This work was funded by the National Council for Scientific and Technological Development (CNPq, Brazil; process numbers 307402/2010-0, 562296/2010-7, 304779/2011-3, and 504189/2012-3) and by the Brazilian Federal Agency for the Support and Evaluation of Graduate Education (CAPES, Brazil; process numbers PNPD 2103/2009 and AE1-0052-000630100/11).
Footnotes
Published ahead of print 4 March 2013
REFERENCES
- 1. Fisher MC, Koenig GL, White TJ, Taylor JW. 2002. Molecular and phenotypic description Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 94:73–84 [PubMed] [Google Scholar]
- 2. Parish JM, Blair JE. 2008. Coccidioidomycosis. Mayo Clin. Proc. 83:343–348 [DOI] [PubMed] [Google Scholar]
- 3. Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Johnson RH, Stevens DA, Williams PL. 2005. Coccidioidomycosis. Clin. Infect. Dis. 41:1217–1223 [DOI] [PubMed] [Google Scholar]
- 4. Johnson RH, Einstein HE. 2007. Amphotericin B and coccidioidomycosis. Ann. N. Y. Acad. Sci. 1111:434–441 [DOI] [PubMed] [Google Scholar]
- 5. Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, Dussault P, Nickerson KW. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho T, Aoyama T, Toyoda M, Nakayama H, Chibana H, Kaminishi H, Calderone RA. 2008. Farnesol as a quorum-sensing molecule in Candida albicans. Nippon Ishinkin Gakkai Zasshi 49:281–286 (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 7. Ramage G, Saville SP, Wickes BL, López-Ribot JL. 2002. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 68:5459–5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jabra-Rizk MA, Shirtliff M, James C, Meiller T. 2006. Effect of farnesol on Candida dubliniensis biofilm formation and fluconazole resistance. FEMS Yeast Res. 6:1063–1073 [DOI] [PubMed] [Google Scholar]
- 9. Deveau A, Piispanen AE, Jackson AA, Hogan DA. 2010. Farnesol induces hydrogen peroxide resistance in Candida albicans yeast by inhibiting the Ras-cAMP signaling pathway. Eukaryot. Cell 9:569–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Langford ML, Hasim S, Nickerson KW, Atkin AL. 2010. Activity and toxicity of farnesol towards Candida albicans are dependent on growth conditions. Antimicrob. Agents Chemother. 54:940–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Semighini CP, Hornby JM, Dumitru R, Nickerson KW, Harris SD. 2006. Farnesol-induced apoptosis in Aspergillus nidulans reveals a possible mechanism for antagonistic interactions between fungi. Mol. Microbiol. 59:753–764 [DOI] [PubMed] [Google Scholar]
- 12. Inoue Y, Shiraishi A, Hada T, Hirose K, Hamashima H, Shimada J. 2004. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol. Lett. 237:325–331 [DOI] [PubMed] [Google Scholar]
- 13. Jabra-Rizk MA, Meiller TF, James CE, Shirtliff ME. 2006. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob. Agents Chemother. 50:1463–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koo H, Rosalen PL, Cury JA, Park YK, Bowen WH. 2002. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob. Agents Chemother. 46:1302–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Semighini CP, Murray N, Harris SD. 2008. Inhibition of Fusarium graminearum growth and development by farnesol. FEMS Microbiol. Lett. 279:259–264 [DOI] [PubMed] [Google Scholar]
- 16. Derengowski LS, De-Souza-Silva C, Braz SV, Mello-De-Sousa TM, Báo SN, Kyaw CM, Silva-Pereira I. 2009. Antimicrobial effect of farnesol, a Candida albicans quorum sensing molecule, on Paracoccidioides brasiliensis growth and morphogenesis. Ann. Clin. Microbiol. Antimicrob. 8:13 doi:10.1186/1476-0711-8-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cordeiro RA, Nogueira GC, Brilhante RSN, Teixeira CEC, Mourão CI, Castelo-Branco DSCM, Araujo Neto PM, Ribeiro JF, Monteiro AJ, Sidrim JJC, Rocha MFG. 2012. Farnesol inhibits in vitro growth of the Cryptococcus neoformans species complex with no significant changes in virulence-related exoenzymes. Vet. Microbiol. 159:375–380 [DOI] [PubMed] [Google Scholar]
- 18. Cordeiro RA, Teixeira CE, Brilhante RS, Castelo-Branco DS, Paiva MA, Giffoni Leite JJ, Lima DT, Monteiro AJ, Sidrim JJ, Rocha MF. 2013. Minimum inhibitory concentrations of amphotericin B, azoles and caspofungin against Candida species are reduced by farnesol. Med. Mycol. 51:53–59 [DOI] [PubMed] [Google Scholar]
- 19. Brilhante RS, Cordeiro RA, Rocha MF, Fechine MA, Furtado FM, Nagao-Dias AT, Camargo ZP, Sidrim JJ. 2008. Coccidioidal pericarditis: a rapid presumptive diagnosis by an in-house antigen confirmed by mycological and molecular methods. J. Med. Microbiol. 57:1288–1292 [DOI] [PubMed] [Google Scholar]
- 20. Umeyama T, Sano A, Kamei K, Niimi M, Nishimura K, Uehara Y. 2006. Novel approach to designing primers for identification and distinction of the human pathogenic fungi Coccidioides immitis and Coccidioides posadasii by PCR amplification. J. Clin. Microbiol. 44:1859–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cordeiro RA, Brilhante RSN, Rocha MFG, Fechine MAB, Costa AK, Camargo ZP, Sidrim JJC. 2006. In vitro activities of caspofungin, amphotericin B and azoles against Coccidioides posadasii strains from northeast Brazil. Mycopathologia 161:21–26 [DOI] [PubMed] [Google Scholar]
- 22. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi—2nd ed: approved standard M38-A2. CLSI, Wayne, PA [Google Scholar]
- 23. Cordeiro RA, Brilhante RSN, Rocha MFG, Medrano JDA, Monteiro AJ, Tavares JL, Lima RAC, Camargo ZP, Sidrim JJC. 2009. In vitro synergistic effects of antituberculous drugs plus antifungals against Coccidioides posadasii. Int. J. Antimicrob. Agents 34:278–280 [DOI] [PubMed] [Google Scholar]
- 24. Johnson MD, MacDougall C, Ostrosky-Zeichner L, Perfect JR, Rex JH. 2004. Combination antifungal therapy. Antimicrob. Agents Chemother. 48:693–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arthington-Skaggs BA, Jradi H, Desai T, Morrison CJ. 1999. Quantitation of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J. Clin. Microbiol. 37:3332–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coleman JJ, Okoli I, Tegos GP, Holson EB, Wagner FF, Hamblin MR, Mylonakis E. 2010. Characterization of plant-derived saponin natural products against Candida albicans. ACS Chem. Biol. 5:321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brilhante RS, Valente LG, Rocha MF, Bandeira TJ, Cordeiro RA, Lima RA, Leite JJ, Ribeiro JF, Pereira JF, Castelo-Branco DS, Monteiro AJ, Sidrim JJ. 2012. Sesquiterpene farnesol contributes to increased susceptibility to β-lactams in strains of Burkholderia pseudomallei. Antimicrob. Agents Chemother. 56:2198–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brehm-Stecher BF, Johnson EA. 2003. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob. Agents Chemother. 47:3357–3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Navarathna DH, Hornby JM, Krishnan N, Parkhurst A, Duhamel GE, Nickerson KW. 2007. Effect of farnesol on a mouse model of systemic candidiasis, determined by use of a DPP3 knockout mutant of Candida albicans. Infect. Immun. 75:1609–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]


