LETTER
Iron is an essential nutrient for almost all living cells, and pathogenic bacteria are faced with severe iron limitation in the mammalian host (1). To make iron available during infection, most microorganisms produce and transport into the cell specific iron chelators (siderophores) or acquire iron bound to exogenous iron carriers, like xenosiderophores, heme, or the host's transferrins (2). Due to their capacity to promote microbial growth, siderophores act as virulence factors in several models of infection (3). In a recent article published in Antimicrobial Agents and Chemotherapy, Thompson et al. investigated the susceptibilities of common nosocomial pathogens (Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus) to iron chelators that have recently been developed for treatment of iron overload in humans, as well as to two U.S. Food and Drug Administration-approved drugs, namely, deferoxamine (Desferal; Novartis) and deferiprone (3-hydroxy-1,2-dimethylpyridin-4(1H)-one; Sigma-Aldrich) (4). In the same issue of the journal, de Léséleuc et al. assessed the activity of these two chelators against A. baumannii (5). Thompson et al. emphasize the potential of deferiprone as an antibacterial agent, but they overlook the problem that some chelators behave as Janus-faced molecules; on one face, chelators are essential to treat iron overload in humans, while on the other, they can act as growth promoters for invading pathogens (reviewed in reference 6). A paradigmatic example of this duality is offered by deferoxamine, whose xenosiderophore activity has been proven for several pathogenic bacteria, including P. aeruginosa (7, 8), consistent with clinical evidence indicating that prolonged administration to humans, as in cases of β-thalassemia, increases susceptibility to infection (6).
Thompson et al. showed that high deferiprone concentrations inhibit P. aeruginosa growth in RPMI medium and ascribed the antibacterial activity to iron withholding. Deferiprone MICs were 128 to 512 μg/ml, equivalent to 0.92 to 3.67 mM, depending on the strain (4). However, no evidence that iron could reverse growth inhibition was provided, raising concern about the mechanism of deferiprone activity and its intrinsic toxicity. Conversely, no inhibition was reported for deferoxamine up to 512 μg/ml, equivalent to 0.78 mM (4). Since deferiprone plasma levels in human therapy are ∼0.1 mM (http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021825lbl.pdf), the above findings do not rule out the possibility that deferiprone acts as an iron carrier to P. aeruginosa at lower, yet physiologically meaningful, concentrations.
P. aeruginosa is endowed with an impressively broad iron uptake capability, producing two endogenous siderophores, pyoverdine and pyochelin, and is capable of acquiring iron via a multiplicity of exogenous chelators (9). Consistently, P. aeruginosa has the coding potential for up to 34 TonB-dependent siderophore receptors, whose ligand specificities have only in part been determined (10). Siderophore receptor redundancy denotes the importance of iron acquisition for P. aeruginosa metabolism and suggests the existence of still-unexplored uptake capabilities in this species.
Here, we tested the antimicrobial activities of unsaturated and iron-saturated deferiprone against three prototypic P. aeruginosa strains: PAO1 (ATCC 15692 type strain isolated from a wound), PA14 (burn isolate [11]), and TR1 (cystic fibrosis isolate [12]). The deferiprone MICs in the iron-poor media M9-succinate (M9-S) and RPMI 1640 were 0.92 mM for all three strains, in line with results by Thompson et al. (4). However, saturation of deferiprone with iron (1:3 Fe[III]/deferiprone ratio) reversed but did not abrogate the antibacterial activity, resulting in MIC values of 1.84 to 3.67 mM, depending on the strain. At these or higher ferric-deferiprone concentrations, growth was still inhibited. Thus, while the anti-P. aeruginosa activity of deferiprone can partly be ascribed to its iron-chelating properties, some intrinsic, chelation-independent toxicity should be taken into account at high concentrations (≥3.67 mM).
Then, we analyzed the growth response of P. aeruginosa to deferiprone. Supplementation of M9-S with increasing deferiprone concentrations (0 to 0.16 mM) was paralleled by an increase of P. aeruginosa PAO1 growth rates and a decrease of pyoverdine yields at the stationary phase, suggesting that deferiprone can act as an iron carrier, thereby promoting bacterial growth and reducing the need for endogenous siderophore production (Fig. 1A). To gain further insight into this activity, we generated a P. aeruginosa PAO1 ΔpvdA ΔpchD double-null mutant impaired in the synthesis of both endogenous siderophores, pyoverdine and pyochelin, and analyzed its growth response to the addition of endogenous or exogenous siderophores (20 μM). Compared with wild-type PAO1, the ΔpvdA ΔpchD mutant grew poorly in M9-S and even less well in M9-S containing 400 μM 2,2′-dipyridyl (a chelator of the intracellular Fe[II] pool), while growth was restored by the addition of 50 μM FeCl3, reflecting a severe defect in iron acquisition (Fig. 1B). As expected, growth of the mutant was strongly promoted by the addition of pyoverdine or deferoxamine mesylate salt to the medium. Pyochelin had a similar though less pronounced effect, likely due to its poor affinity for iron (9). Most remarkably, supplementation with deferiprone resulted in significant growth promotion of the ΔpvdA ΔpchD mutant, higher than that caused by the homologous siderophore pyochelin (Fig. 1B). Siderophores are actively transported in P. aeruginosa via specific receptors, namely, FpvA and FpvB for pyoverdine (13), FptA for pyochelin (14), and FoxA and FoxB for deferoxamine (7, 8). Consequently, the significant growth promotion of the mutant in response to siderophores, such as pyoverdine, pyochelin, and deferoxamine, and to the chemically synthesized chelator deferiprone is strongly suggestive of a still-uncharacterized iron transport activity of deferiprone in P. aeruginosa. A similar growth-promoting activity has recently been reported for deferiprone in A. baumannii, suggesting that such a compound can act as an iron carrier also to this bacterium (5).
Fig 1.
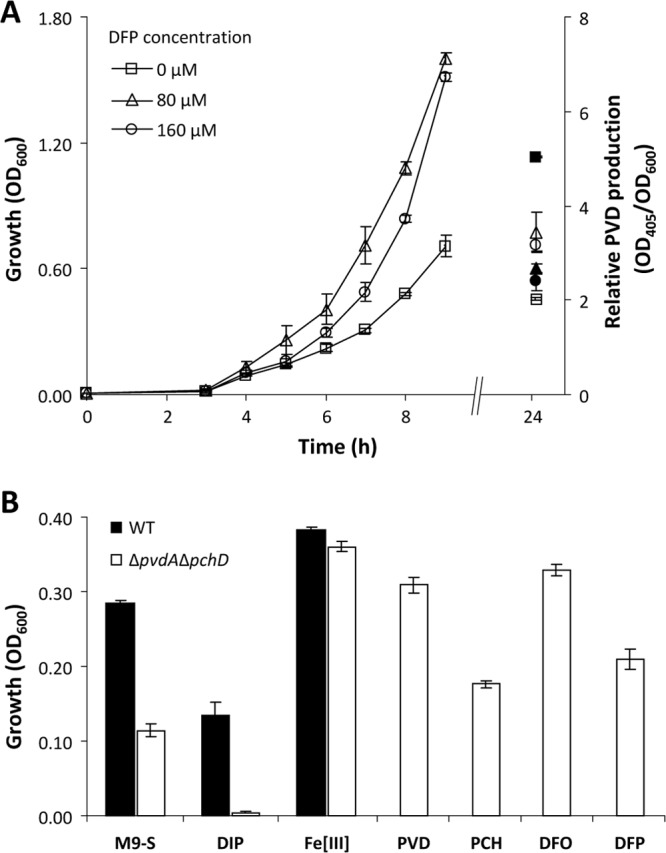
Growth-promoting activity of deferiprone. (A) Growth of P. aeruginosa PAO1 at 37°C in aerated 50-ml flasks containing 10 ml of M9-S supplemented with increasing deferiprone (DFP) concentrations (open symbols, left ordinate). Relative pyoverdine (PVD) levels were determined after 24 h of growth and are expressed as optical densities at 405 nm (OD405) of the bacterial culture supernatant normalized to the OD600 of the culture (filled symbols, right ordinate). (B) Growth response of wild-type P. aeruginosa PAO1 (WT) and of the ΔpvdA ΔpchD double mutant to iron starvation, and effects of different iron chelators on the growth of the ΔpvdA ΔpchD double mutant. M9-S was supplemented with 2,2′-dipyridyl (DIP; 400 μM) or FeCl3 (Fe[III]; 50 μM) to compare the responses to reduced or increased iron availability of the wild type and the siderophore null mutant. The growth-promoting activity of pyoverdine (PVD), pyochelin (PCH), deferoxamine (DFO), and deferiprone (DFP) was assessed by adding 20 μM each compound to M9-S. Cell density (OD600) was measured after 12 h of growth at 37°C in 96-well microtiter plates containing 200 μl of culture medium. An overnight P. aeruginosa culture in M9-S was used as the inoculum upon dilution at an OD600 of ∼0.01. Each value is the mean of eight independent measurements ± the standard deviation.
In conclusion, while iron metabolism appears a suitable target for development of novel antimicrobial strategies, the therapeutic use of iron chelators to suppress microbial growth should prudently be assessed to avoid the risk posed by bacterial and fungal species that gain advantage from using such compounds as growth promoters. This is mandatory for those bacteria, like P. aeruginosa, that have the potential to prey upon a multiplicity of still-uncharacterized iron sources.
REFERENCES
- 1. Nairz M, Schroll A, Sonnweber T, Weiss G. 2010. The struggle for iron—a metal at the host-pathogen interface. Cell. Microbiol. 12:1691–1702 [DOI] [PubMed] [Google Scholar]
- 2. Wandersman C, Delepelaire P. 2004. Bacterial iron sources: from siderophores to hemophores. Annu. Rev. Microbiol. 58:611–647 [DOI] [PubMed] [Google Scholar]
- 3. Ratledge C. 2007. Iron metabolism and infection. Food Nutr. Bull. 28(Suppl 4):S515–S523 [DOI] [PubMed] [Google Scholar]
- 4. Thompson MG, Corey BW, Si Y, Craft DW, Zurawski DV. 2012. Antibacterial activities of iron chelators against common nosocomial pathogens. Antimicrob. Agents Chemother. 56:5419–5421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Léséleuc L, Harris G, KuoLee R, Chen W. 2012. In vitro and in vivo biological activities of iron chelators and gallium nitrate against Acinetobacter baumannii. Antimicrob. Agents Chemother. 56:5397–5400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kontoghiorghes GJ, Kolnagou A, Skiada A, Petrikkos G. 2010. The role of iron and chelators on infections in iron overload and non-iron loaded conditions: prospects for the design of new antimicrobial therapies. Hemoglobin 34:227–239 [DOI] [PubMed] [Google Scholar]
- 7. Banin E, Vasil ML, Greenberg EP. 2005. Iron and Pseudomonas aeruginosa biofilm formation. Proc. Natl. Acad. Sci. U. S. A. 102:11076–11081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cuív PO, Keogh D, Clarke P, O'Connell M. 2007. FoxB of Pseudomonas aeruginosa functions in the utilization of the xenosiderophores ferrichrome, ferrioxamine B, and schizokinen: evidence for transport redundancy at the inner membrane. J. Bacteriol. 189:284–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poole K, McKay GA. 2003. Iron acquisition and its control in Pseudomonas aeruginosa: many roads lead to Rome. Front. Biosci. 8:661–686 [DOI] [PubMed] [Google Scholar]
- 10. Cornelis P, Matthijs S. 2002. Diversity of siderophore-mediated iron uptake systems in fluorescent pseudomonads: not only pyoverdines. Environ. Microbiol. 4:787–798 [DOI] [PubMed] [Google Scholar]
- 11. He J, Baldini RL, Déziel E, Saucier M, Zhang Q, Liberati NT, Lee D, Urbach J, Goodman HM, Rahme LG. 2004. The broad host range pathogen Pseudomonas aeruginosa strain PA14 carries two pathogenicity islands harboring plant and animal virulence genes. Proc. Natl. Acad. Sci. U. S. A. 101:2530–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bragonzi A, Paroni M, Nonis A, Cramer N, Montanari S, Rejman J, Di Serio C, Döring G, Tümmler B. 2009. Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. Am. J. Respir. Crit. Care Med. 180:138–145 [DOI] [PubMed] [Google Scholar]
- 13. Bodilis J, Ghysels B, Osayande J, Matthijs S, Pirnay JP, Denayer S, De Vos D, Cornelis P. 2009. Distribution and evolution of ferripyoverdine receptors in Pseudomonas aeruginosa. Environ. Microbiol. 11:2123–2135 [DOI] [PubMed] [Google Scholar]
- 14. Cobessi D, Celia H, Pattus F. 2005. Crystal structure at high resolution of ferric-pyochelin and its membrane receptor FptA from Pseudomonas aeruginosa. J. Mol. Biol. 352:893–904 [DOI] [PubMed] [Google Scholar]


