Abstract
The main aim of this study was to develop a standardized experimental assay to enable differential antimicrobial comparisons of test biocidal aerosols. This study represents the first chlorine-matched comparative assessment of the antimicrobial activities of aerosolized sodium hypochlorite, chlorine dioxide, and electrochemically activated solution (ECAS) to determine their relative abilities to decontaminate various surface-associated health care-relevant microbial challenges. Standard microbiological challenges were developed by surface-associating typed Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis spores, or a clinical methicillin-resistant S. aureus (MRSA) strain on stainless steel, polypropylene, or fabric. All test coupons were subjected to 20-min biocidal aerosols of chlorine-matched (100 ppm) sodium hypochlorite, chlorine dioxide, or ECAS within a standard aerosolization chamber using a commercial humidifier under defined conditions. Biocidal treatment type and material surface had a significant effect on the number of microorganisms recovered from various material surfaces following treatment exposure. Under the conditions of the assay, the order of antimicrobial efficacy of biocidal aerosol treatment was as follows: ECAS > chlorine dioxide > sodium hypochlorite. For all biocides, greater antimicrobial reductions were seen when treating stainless steel and fabric than when treating plastic-associated microorganisms. The experimental fogging system and assay protocol designed within this study were shown capable of differentiating the comparative efficacies of multiple chlorine-matched biocidal aerosols against a spectrum of target organisms on a range of test surface materials and would be appropriate for testing other biocidal aerosol treatments or material surfaces.
INTRODUCTION
Biocides have a key role to play in decontamination within health care environments through disinfection and sterilization. There is a known linkage between the use of antibiotics and the emergence and spread of antibiotic resistance (1), and the most recent Health Protection Agency (United Kingdom) report on health care-associated infection and antimicrobial resistance indicates the continued rise in both selected antibiotic-resistant strains (e.g., Panton-Valentine leukocidin methicillin-resistant Staphylococcus aureus [MRSA]) and resistance factors (e.g., carbapenemases) (2). Hence there is an ever-greater need to ensure biocides are being used appropriately to help reduce the level of microbial contamination within the health care environment to acceptably safe levels and consequently reduce the risk of infection (3). There are numerous classes of biocides currently utilized for this purpose, with the exact formulation for a given practical application being dependent on convention and/or local standard operating procedures (4).
Effective biocidal action of any given active agent is reliant on concentration, exposure time, composition of the contact surface (with rough and/or absorbent surfaces requiring a longer contact time), and presence of organic loading (5). It is important to utilize a delivery mechanism that will maximize the antimicrobial potential of a biocide, and they are conventionally applied in liquid form. Aerosol delivery technology utilizes solid particles or liquid droplets suspended in a gas (i.e., biocide droplets carried in air) (6), in contrast to vapors, in which the substance itself is in the gas phase. Early research into aerosol delivery mechanisms focused mainly on disinfection of airborne bacteria and spores (7–10). More-recent research studies have assessed the use of aerosolized biocides for environmental decontamination, although the literature is relatively mixed in terms of its assessment of the benefit of aerosols (6). Nonetheless, the antimicrobial potential of certain soluble biocidal agents delivered as an aerosol, including sodium hypochlorite (11), peroxyacetic acid (12), quaternary ammonium compounds (6), lactic acid (13), hydrogen peroxide (14–17), and premixed combinations (peroxyacetic acid and hydrogen peroxide), has been proven (18). Advancements in aerosolization technology as a means of biocide delivery have found application in the decontamination of material surfaces (12) and fresh produce (19) in the food processing industry, commercial and residential building materials (11), and potential utility within health care environments (14).
For environmental decontamination applications within habitable spaces, clearly certain biocides are too toxic (e.g., phenolics and glutaraldehyde) or flammable (e.g., alcohols) or have the potential to leave unwanted residues on surfaces (e.g., iodophors). Chlorine-containing biocides are widely used for the decontamination of surfaces, usually in the form of hypochlorite (ClO−), being inexpensive to produce and having proven antimicrobial activity (20). Chlorine dioxide (ClO2) is a highly oxidative biocide originally developed for water disinfection applications; its disinfection activity is known to be less influenced by pH and results in less-harmful by-products than traditional chlorine treatment (21). Chlorine dioxide has been effectively used for environmental decontamination, particularly within food-processing environments, being generated on site using “sachet”-based systems (22). Interestingly, although gaseous or liquid systems are widely used, there is little/no literature on the delivery of aqueous chlorine dioxide as a biocidal aerosol. Electrochemically activated solutions (ECAS) are currently emerging as novel chlorine-containing biocides with numerous potential applications, including potable water disinfection (23), within the food industry (24) and within the health care sector (25). ECAS are produced by electrolysis of typically low-concentration salt (NaCl) solutions within an electrochemical cell, with the resultant anolyte solution (ECASa) usually having a high redox (oxidizing) potential, a low pH (which can be neutralized by internally reconfiguring the electrochemical cell), and a variable chlorine concentration depending on set operating parameters (25). ECAS have broad spectrum activity, including spores (26), and have been shown to be extremely fast acting, even in comparison to other commonly used biocides (27). The antimicrobial potential of aerosolized ECAS has been previously investigated using a portable electrostatic aerosol applicator which delivers large volumes of liquid in a spray, whereby sufficient wetting occurred for a mop to be required to dry the laboratory floor posttreatment (28, 29). Significant reductions in the microbial load of ceramic tiles were observed posttreatment (28); however, since no appropriate control (nonbiocidal fog) was described, the effects of physical removal and kill cannot be disentangled.
The main study objective was to further our understanding of the antimicrobial potential of aerosolized biocides through the development of a standardized experimental model and defined microbiological challenge, enabling differential antimicrobial comparisons of various test biocides. This study represents the first chlorine-matched comparative assessment of the antimicrobial activities of aerosolized sodium hypochlorite, chlorine dioxide, and ECAS to determine their relative abilities to decontaminate various surface materials (stainless steel, polypropylene, and cellulose fabric) when loaded with a range of bacterial contaminants relevant to health care environments.
MATERIALS AND METHODS
Preparation of test biocides.
Liquid ClO2 (Selectrocide; Selective Micro Technologies, MA) was prepared at 500 ppm according to the manufacturer's instructions, and both sodium hypochlorite (Sigma-Aldrich, United Kingdom) and liquid ClO2 were diluted in deionized water to 100 ppm for experimental use. Acidic electrochemically activated solutions (ECASa) were generated by the electrolysis of 1% (wt/vol) NaCl solution within a commercial ECAS generator (Bridge Systems Ltd., Fife, United Kingdom). The free chlorine level of ECAS was determined using N,N-diethyl-p-phenylenediamine sulfate (DPD) no. 1 test (Palintest Ltd., Gateshead, United Kingdom) and standardized to 100 ppm by altering the operating parameters of the ECAS generator (specifically the flow rate and salinity of bulk solution passed through the electrolytic cell). The redox potential and pH of ECASa were measured during production using inline probes and validated using external probes before experimentation.
Growth and maintenance of target microorganisms.
Methicillin-sensitive Staphylococcus aureus (MSSA; ATCC 6538), methicillin-resistant Staphylococcus aureus (MRSA; SMH 11622; kind gift from Southmead Hospital, Bristol, United Kingdom), and Pseudomonas aeruginosa (ATCC 15442) were stored at −80°C and recovered onto tryptone soya agar (TSA; Oxoid Ltd., Basingstoke, United Kingdom) when required. Bacillus subtilis subsp. spizizenii (ATCC 6633) spores were prepared by using the protocol described in BS EN ISO 14347 (30). Briefly, spread plate cultures were prepared and then incubated for 3 days at 37°C followed by 7 days at 30°C. Plate cultures were scraped into sterile distilled water, and the resultant suspension was washed by centrifugation. Spore suspensions were washed in isopropanol for 3 h to kill any remaining vegetative cells and washed a further three times in distilled water to remove cell debris.
Aerosolization test chamber.
The chamber was constructed from clear acrylic polymer sheets held together by tongue and groove jointing and mounted on a plastic base plate supported on a metal frame. All edges were sealed using a silicone rubber compound, with the exception of the front door panel, which was hinged and sealed with metal latches to allow for introduction and removal of test equipment and sample material. The final internal dimensions of this chamber were 450 by 380 by 380 mm, which created a fogging space with a nominal volume of 65 liters. Fog was introduced via an inlet port cut into the bottom base plate (Ø = 55 mm).
Preparation of material test surfaces.
Three test surface materials were chosen. Stainless steel (type 1.4301 with grade 2 B finish; FC Hammonds, Bristol, United Kingdom) and plastic (polypropylene; Sirane, Telford, United Kingdom) sheets were cut into 15-mm-diameter coupons. Surfaces were cleaned and sterilized according to BS EN ISO 13697 (31). Briefly, coupons were soaked in 5% (vol/vol) alkaline detergent solution (Decon 90; Decon Laboratories Limited, East Sussex, United Kingdom) for 60 min and then immediately rinsed with deionized water. Coupons were disinfected by immersion in 70% (vol/vol) isopropanol for 15 min and then dried by evaporation within a laminar flow hood. Cotton gauze coupons (10 mm2) were cut from larger presterilized sheets (Velveteen; Bel-art Products, NJ).
Preparation of surface-associated standard microbiological challenge.
S. aureus (MSSA and MRSA) and P. aeruginosa cell suspensions were prepared by emulsifying fresh plate colonies (grown on tryptone soya agar incubated for <24 h) into diluent (0.1% [wt/vol] tryptone plus 0.85% [wt/vol] NaCl) and adjusting the cell density to 3 × 109 CFU per ml according to previously determined spectrophotometric calibration curves (optical density at 620 nm [OD620]). B. subtilis spore suspensions were prepared (as previously described), enumerated by viable counting, and adjusted to 1 × 109 spores per ml. Sterile coupons of each test surface (three coupons per species per test condition) were inoculated with 10 μl of bacterial test suspensions (deposited as a single drop). Inoculated coupons were left to dry (≤1 h) in a class II biological safety cabinet.
Aerosolization test procedure.
For each aerosolization experiment, three coupons of each test surface for each species were transferred to 10 ml Letheen broth (BD, Oxford, United Kingdom) immediately after inoculation to provide untreated control data. A separate set of coupons (n = 3 per species of each test surface) was placed on a sterile plastic drainage grid (test samples) and transferred to the test aerosolization chamber. An HU-25OG humidifier (Contronics, Sint-Oedenrode, Netherlands) was used to deliver aerosolized liquid. Prior to aerosolization, the HU-25OG was filled and drained with the control or test solution to be delivered and then refilled immediately before challenging inoculated coupons. The HU-25OG was set with the fan to maximum and relative humidity at 50% (delivering a droplet size of 1 to 3 μm). The aerosol was introduced to the chamber via 40-mm-diameter tubing. The chamber was positioned within an extracting fume hood, providing a constant air draw, and ventilated via a 12-mm-diameter hole on the sides of the chamber. Inoculated test sample coupons were exposed to test biocide aerosol for 20 min, followed by a 10-min settle time. All aerosolized biocides and a water control were independently tested according to the above-mentioned protocol a minimum of three times. Following drainage, the HU-25OG was filled and drained three times with deionized water to remove any residual biocide from within the humidifier.
Microbial sampling and recovery method.
Following treatment, test coupons were immediately transferred to 10 ml Letheen broth containing sterile glass beads, shaken for 20 min (200 rpm) to neutralize any remaining biocide, and vortexed for 5 s to ensure maximal recovery of microbial survivors from the test surfaces (neutralizer was validated before use). Viable counts were performed on neat and diluted recovery suspensions using a spiral plater (Don Whitley Scientific, Shipley, United Kingdom) onto TSA recovery plates, incubated at 37°C aerobically for 24 h, and counted to determine the numbers of CFU per coupon (CFU coupon−1). In addition, a 1-ml TSA pour plate of the neat recovery suspensions was performed to ensure an accurate minimum detection limit for the assay of 3 × 102 organisms and an absolute detection limit of 1 × 101 organisms.
Analysis of results.
To determine whether aerosolization treatment resulted in significant reductions in viable bacterial cells, an analysis of variance (ANOVA) was performed on the microbial recovery counts, followed by Dunnett's multiple-comparison test against the control (water) recovery counts and Tukey's test to compare different biocidal aerosol treatments, with a P value of <0.05 regarded as significant. A two-way ANOVA was also performed to elucidate any significant effects of both treatment type and material surface on antimicrobial efficacy. Graph construction and statistical analyses were conducted with the use of GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA).
RESULTS
Table 1 shows the recovery of viable cells from the surface of test materials immediately following preparation of surface-associated standard microbial challenges (i.e., after drying but before treatment). On stainless steel or plastic coupons, there was no significant reduction in the number of viable MSSA and MRSA cells and B. subtilis spores recovered compared to that in the inoculum; however, the study protocol resulted in a significant reduction in viable P. aeruginosa cells from the surface of both stainless steel (2.71-log reduction; P < 0.001) and plastic (1.37-log reduction; P < 0.001) coupons. When recovering from fabric, there was a significant reduction in the number of MSSA (0.33-log reduction; P < 0.001), MRSA (0.28-log reduction; P < 0.001), and P. aeruginosa (2.08-log reduction; P < 0.001) cells and B. subtilis (0.69-log reduction; P < 0.001) spores after drying compared to that in the inoculum. It was found that the microbial inoculum dried faster on stainless steel coupons (∼30 min) than on plastic coupons (∼60 min), and hence, a staggered inoculation protocol was required for subsequent experiments.
Table 1.
Recovery of viable methicillin-sensitive and methicillin-resistant S. aureus, P. aeruginosa, and B. subtilis spores compared to initial microbial surface loading after being dried onto various material test surfacesa
| Strain | Recovery onb: |
|||||
|---|---|---|---|---|---|---|
| Stainless steel |
Plastic |
Fabric |
||||
| % | Log CFU | % | Log CFU | % | Log CFU | |
| MSSA | 98 ± 6.2 | 7.5 ± 0.03 | 100 ± 8.6 | 7.5 ± 0.03 | 47 ± 4.6* | 7.2 ± 0.04* |
| MRSA | 78 ± 11 | 7.5 ± 0.06 | 81 ± 16 | 7.5 ± 0.09 | 56 ± 23* | 7.3 ± 0.18* |
| P. aeruginosa | 0.23 ± 0.04* | 5.0 ± 0.1* | 4.3 ± 1.1* | 6.3 ± 0.11* | 0.85 ± 0.27* | 5.6 ± 0.14* |
| B. subtilis | 100 ± 15 | 7.1 ± 0.06 | 100 ± 11 | 7.1 ± 0.05 | 20 ± 1.6* | 6.4 ± 0.03* |
n = 3. Values are reported to 2 significant figures.
Asterisks indicate significant reductions (P < 0.05) compared to the initial microbial loading.
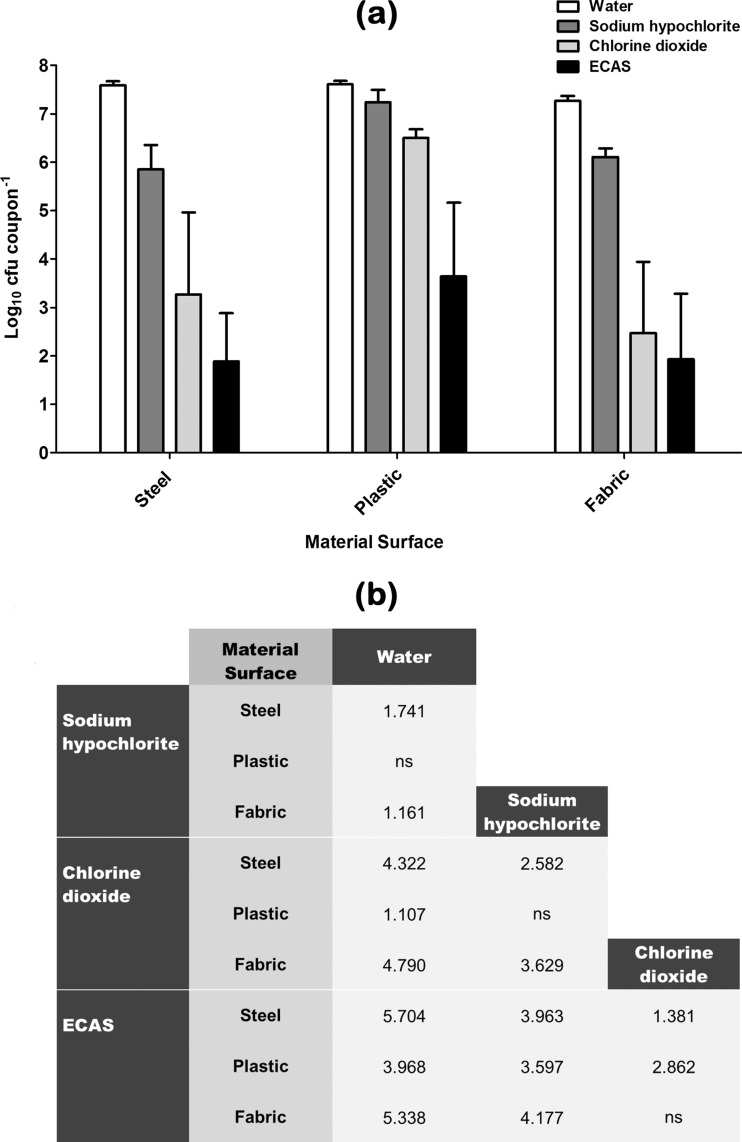
The effects of a 20-min aerosolization treatment regimen of water (control) or an active biocidal aerosol of sodium hypochlorite, chlorine dioxide, or ECAS (each prepared to 100 ppm free chlorine) against a standard steel-, plastic-, or fabric-surface-associated microbiological challenge are shown in Fig. 1, 2, 3, and 4. Recovery of a clinical methicillin-sensitive S. aureus (MSSA) type strain from material surfaces after being subjected to control or test aerosol treatments is shown in Fig. 1. When water alone was used to treat surfaces, there was no significant reduction in the number of viable recoverable survivors compared to that after drying (Table 1) for any of the test material surfaces. Sodium hypochlorite elicited a significant antimicrobial effect compared to water alone when used to treat steel- and fabric-surface-associated MSSA; however, it had no significant effect against plastic-surface-associated MSSA (Fig. 1b). Chlorine dioxide elicited a significant antimicrobial effect compared to water alone when used to treat all three material surfaces and was significantly more effective than sodium hypochlorite when used to treat steel and plastic material surfaces (Fig. 1b). ECAS elicited a significantly greater log reduction than water, sodium hypochlorite, and chlorine dioxide when used to treat steel- and plastic-surface-associated S. aureus and a significantly greater log reduction than water and sodium hypochlorite when used to treat fabric-associated MSSA (Fig. 1b). When comparing the log reductions achieved for a given biocide against a specific surface type, the greatest log reductions were seen when treating fabric and steel, compared to plastic-surface-associated MSSA.
Fig 1.
(a) Recovery of methicillin-sensitive S. aureus (MSSA) from various material surfaces after being subjected to a 20-min aerosolization treatment regimen with either sterile water (white bars) or one of three chlorine-containing biocides (sodium hypochlorite [dark grey bars], chlorine dioxide [light grey bars], ECAS [black bars]) prepared to a matched free-chlorine concentration of 100 ppm (n = 6) (means ± standard deviations [SD]). (b) Comparative efficacy matrix of the four aerosolization treatment regimens. A reported value indicates a significant difference (P < 0.05) in the number of microbes recovered (log10 CFU coupon−1) when comparing the two treatment regimens. ns, not significant.
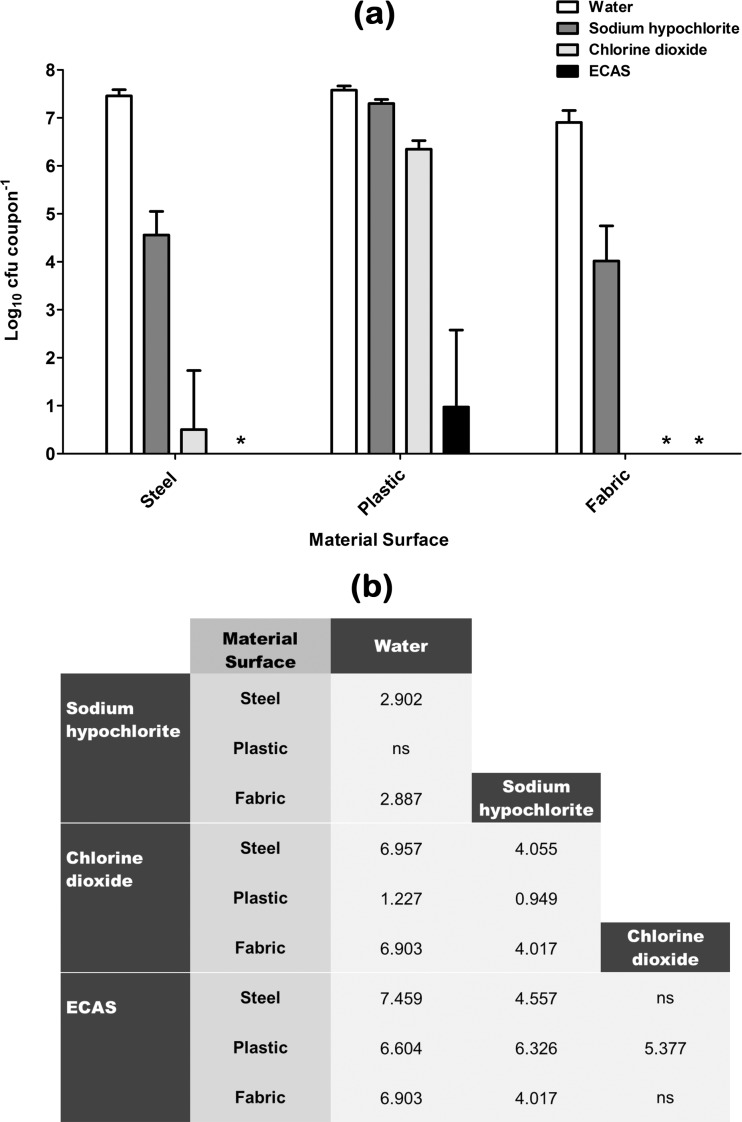
Fig 2.
(a) Recovery of methicillin-resistant S. aureus (MRSA) from various material surfaces after being subjected to a 20-min aerosolization treatment regimen with either sterile water (white bars) or one of three chlorine-containing biocides (sodium hypochlorite [dark grey bars], chlorine dioxide [light grey bars], ECAS [black bars]) prepared to a matched free-chlorine concentration of 100 ppm (n = 6) (means ± SD). *, no viable bacteria recovered. (b) Comparative efficacy matrix of the four aerosolization treatment regimens. A reported value indicates a significant difference (P < 0.05) in the number of microbes recovered (log10 CFU coupon−1) when comparing the two treatment regimens. ns, not significant.
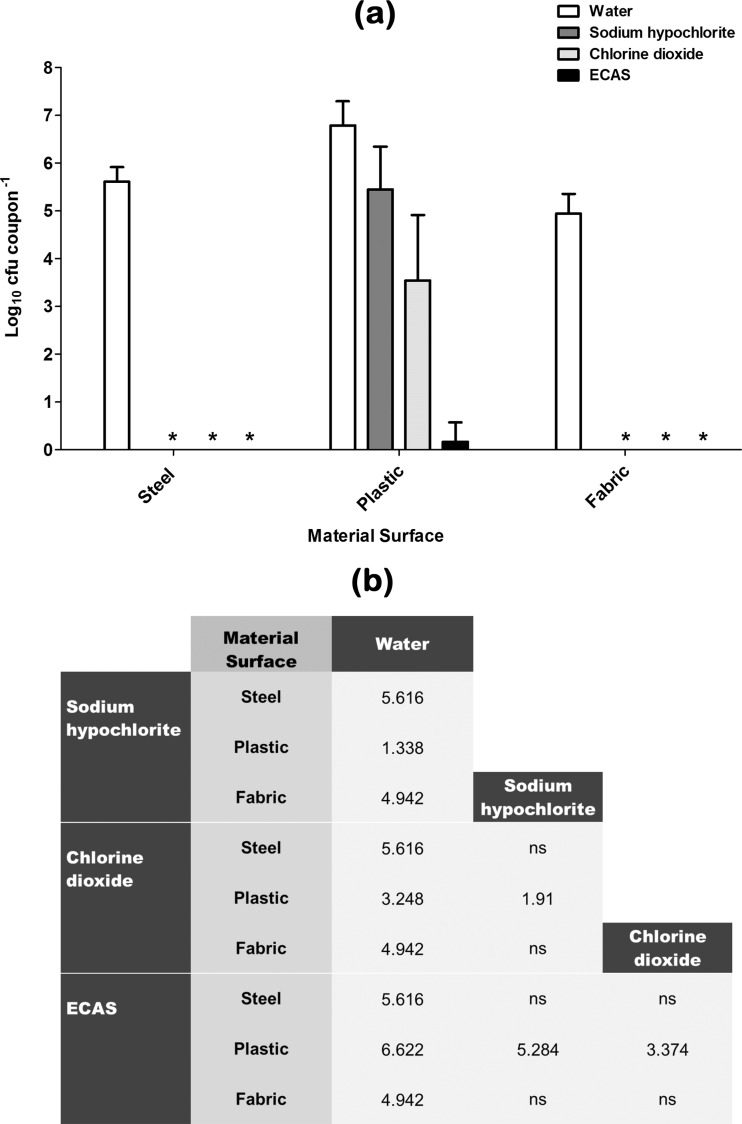
Fig 3.
(a) Recovery of P. aeruginosa from various material surfaces after being subjected to a 20-min aerosolization treatment regimen with either sterile water (white bars) or one of three chlorine-containing biocides (sodium hypochlorite [dark grey bars], chlorine dioxide [light grey bars], ECAS [black bars]) prepared to a matched free-chlorine concentration of 100 ppm (n = 6) (means ± SD). *, no viable bacteria recovered. (b) Comparative efficacy matrix of the four aerosolization treatment regimens. A reported value indicates a significant difference (P < 0.05) in the number of microbes recovered (log10 CFU coupon−1) when comparing the two treatment regimens. ns, not significant.
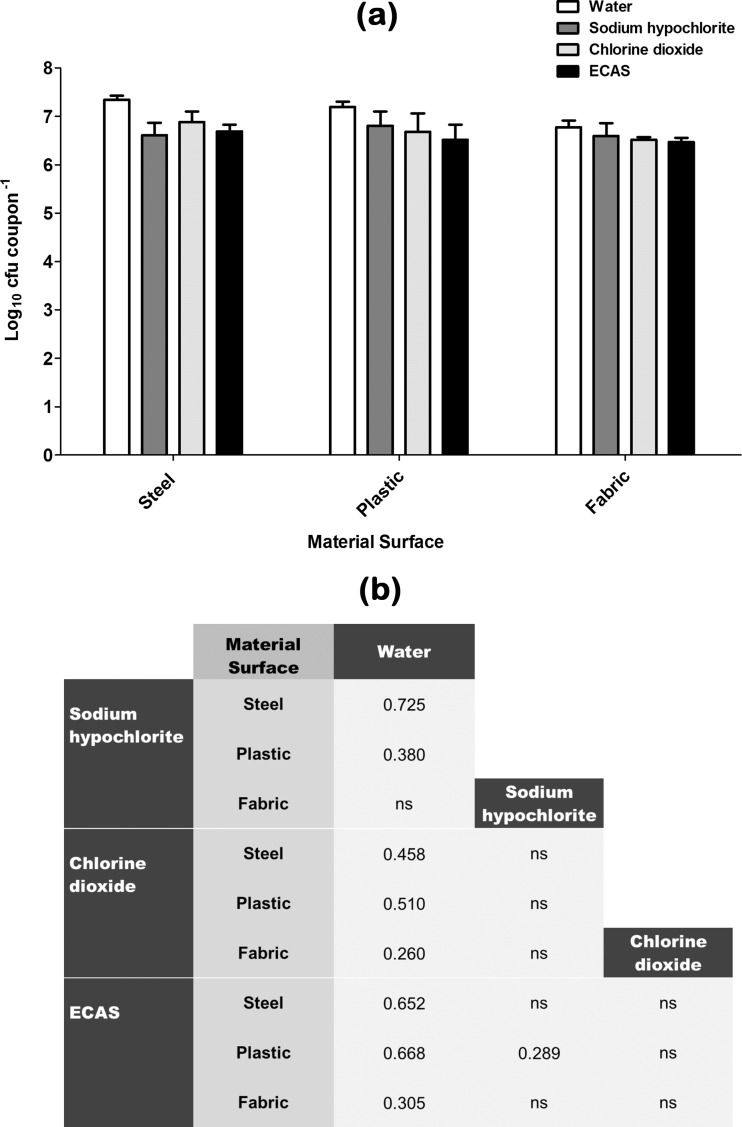
Fig 4.
(a) Recovery of B. subtilis spores from various material surfaces after being subjected to a 20-min aerosolization treatment regimen with either sterile water (white bars) or one of three chlorine-containing biocides (sodium hypochlorite [dark grey bars], chlorine dioxide [light grey bars], ECAS [black bars]) prepared to a matched free-chlorine concentration of 100 ppm (n = 6) (means ± SD). (b) Comparative efficacy matrix of the four aerosolization treatment regimens. A reported value indicates a significant difference (P < 0.05) in the number of microbes recovered (log10 CFU coupon−1) when comparing the two treatment regimens. ns, not significant.
Recovery of a clinical methicillin-resistant S. aureus (MRSA) strain from material surfaces after being subjected to control or test aerosol treatments is shown in Fig. 2, and trends similar to those observed for the MSSA type strain were seen (Fig. 1). When water alone was used to treat surfaces, there was no significant reduction in the number of viable recoverable survivors compared to that after drying (Table 1) for stainless steel and plastic coupons; however, a significant 0.38-log reduction (P < 0.01) was seen for fabric. Sodium hypochlorite elicited a significant antimicrobial effect compared to water alone when used to treat steel- and fabric-surface-associated MRSA (Fig. 2b); however, the reductions were significantly greater than that observed for the MSSA type strain (Fig. 1). Sodium hypochlorite elicited no significant antimicrobial effects compared to water alone when used to treat plastic-surface-associated MRSA (Fig. 2b). Chlorine dioxide elicited a significant antimicrobial effect compared to water alone when used to treat all three surface-associated MRSA challenge materials and is significantly more effective than sodium hypochlorite in all cases (Fig. 2b). In fact, complete kill (as determined by the limit of detection of the test) was observed when used to treat fabric-associated MRSA (Fig. 2a). ECAS elicited a significantly greater log reduction than water and sodium hypochlorite when used to treat all three surface-associated MRSA challenge types, whereby complete kill was observed against steel- and fabric-associated MRSA, and were significantly more effective than chlorine dioxide when used to treat plastic-associated MRSA (Fig. 2). Similarly to MSSA, far greater log reductions were achieved for a given biocide when used to treat fabric and steel, compared to plastic-surface-associated MRSA.
Recovery of a typed P. aeruginosa strain from material surfaces after being subjected to control or test aerosol treatments is shown in Fig. 3. When water alone was used to treat surfaces, there was no significant reduction in the number of viable recoverable survivors compared to that after drying for stainless steel and plastic coupons; however, a significant 0.50-log reduction (P < 0.01) was seen for fabric. Sodium hypochlorite elicited a significant antimicrobial effect when used to treat all three surface types, with no detectable survivors being recovered from the surface of steel or fabric (Fig. 3a). Chlorine dioxide elicited a significant antimicrobial effect compared to water alone when used to treat all three surface types and was significantly more effective than sodium hypochlorite when used to treat plastic-surface-associated P. aeruginosa (Fig. 3b). ECAS elicited a significantly greater log reduction than water and sodium hypochlorite when used to treat plastic-surface-associated P. aeruginosa (Fig. 3b) but were similarly effective as sodium hypochlorite and chlorine dioxide when used to treat steel and plastic since all three biocides elicited complete kill (Fig. 3a). Similarly to the results obtained for MSSA and MRSA surface-associated microbiological challenges, far greater log reductions of P. aeruginosa were achieved for a given biocidal aerosol when used to treat fabric and steel than when used to treat plastic.
Recovery of B. subtilis spores from material surfaces after being subjected to control or test aerosol treatments is shown in Fig. 4. When water alone was used to treat surfaces, there was no significant reduction in the number of viable recoverable survivors compared to that after drying (Table 1) for any test material surface. Compared to this water control, sodium hypochlorite elicited a significant sporicidal effect when used to treat steel- and plastic-associated B. subtilis spores; however, there was no significant reduction when used to treat fabric-associated B. subtilis spores (Fig. 4b). Chlorine dioxide elicited a significant sporicidal effect compared to water alone when used to treat all three surface types; however, the log reduction was not significantly different from that observed when treating with sodium hypochlorite (Fig. 4b). ECAS also elicited a significant sporicidal effect compared to water alone when used to treat all three surface types, with log reductions not significantly different from those with both sodium hypochlorite and chlorine dioxide, except on plastic, where it was found to be significantly more effective than sodium hypochlorite (Fig. 4b).
The data presented in this study were collectively analyzed by two-way analysis of variance, whereby biocidal treatment and material surface were the defining factors. Both biocidal treatment and material surface had a significant effect on the number of microorganisms recovered from various material surfaces after treatment exposure. Moreover, a greater proportion of the differential kill observed was attributable to biocidal treatment type than to surface material, indicating that this is the dominant factor.
DISCUSSION
Initial experiments focused on standardizing the surface-associated microbial challenge, including organisms currently used in European standard biocidal assays (EN ISO 13697) (31). It was evident that after inoculation and drying, there were significant differences in the recovery rates of different species from different test materials. MSSA, MRSA, and B. subtilis spores suffered no detrimental effect after drying and recovery from plastic and stainless steel surfaces, in contrast to P. aeruginosa, which was significantly affected by drying. This is not unexpected, since both Gram-positive organisms and spores are known to be more resistant to the effects of drying than are Gram-negative organisms (32). In contrast, there was a significant reduction in the number of all target microorganisms recovered from fabric after drying. Since the numbers of both recoverable spores and Gram-positive organisms were reduced, it is postulated that this is due to inefficient recovery from this material type rather than the effects of drying. These significant reductions in some microbial populations after drying and recovery were consistent and quantifiable. A viable population of surface-associated microbial cells always remained to enable a potential 4-log reduction in microbial numbers to be quantified, sufficient to define a compound as having surface bactericidal activity according to EN ISO 13697 (31). Moreover, all effects of test biocidal aerosol treatment were compared to those of a water treatment, which controls for any reductions after inoculation and drying as well as ensures that any significant reductions were due to active antimicrobial processes.
Preliminary aerosolization experiments were performed to standardize the experimental system, and initially, a portable electrostatic aerosol generator was used, similar to that in a previous study investigating the aerosol delivery of ECAS (28). This device delivers high volumes of liquid but was found to wet surfaces to an unacceptable level within the aerosolization test chamber and lead to the investigation and implementation of transducer-based fogging devices. These devices use a piezoelectric transducer (resonating frequency, ∼1.6 MHz) whereby ultrasonic waves are focused on water, generating a cold, dry-feeling fog with a median droplet size of 1 to 3 μm (10-fold lower than the portable electrostatic aerosol applicator). Exposure to test biocide aerosols for 20 min (followed by a 10-min settle time) using a standard fog output (50% with maximum fan speed, utilizing ∼0.5 liters of test aerosol solution) resulted in minimal wetting of surfaces and hence would be appropriate for a diverse range of applications. This was therefore chosen as the standard treatment for all biocide, microbiological, and material surface variables.
According to the European Standard quantitative nonporous surface test, bactericidal activity on a surface is defined as the “capability of a product to produce at least a 104 log reduction in the number of viable bacterial cells belonging to the reference strains” (31). This was used as the standard to which the antimicrobial activity of biocidal aerosols against surface-associated microorganisms was compared. When testing sodium hypochlorite against MSSA, it did not elicit a >4-log reduction on any test surface material, although significant reductions were seen on steel and fabric. Chlorine dioxide elicited a >4-log reduction against MSSA surface associated on steel and fabric but not against plastic, whereas ECAS elicited a >4-log reduction against MSSA associated on all three test surface materials. A similar trend was observed when treating surface-associated MRSA with biocidal aerosols, but greater reductions were seen in all cases. This is perhaps surprising given that previous studies have shown MRSA strains to be more resistant to certain biocides than MSSA (33), although little can be inferred from this single strain comparison since other studies have assessed the relative sensitivity of a broad range of MSSA and MRSA strains. This highlights the importance of including strains relevant to the in-use application of a given biocide when testing new biocides and delivery mechanisms. P. aeruginosa was comparatively more sensitive to treatment with all three biocides, with complete kill being observed with all treatment types against steel- and fabric-associated microorganisms (>4-log reduction), although plastic-coupon-associated P. aeruginosa strains were again more resistant to biocidal aerosol treatment. This may be due to the relative hydrophobicity of the test surfaces chosen since all test biocides are carried within water droplets as part of aerosol dispersion. The test plastic (polypropylene) material used within this study was found to be hydrophobic, as evidenced by small water droplets forming on the upward-facing surface of coupons after treatment, in contrast to the confluent liquid layer that formed on the surface of stainless steel. This again highlights the importance of integrating and testing not only application-specific species and strains but also relevant material types. As determined by two-way ANOVA, a greater proportion of the differential kill observed in this study was attributable to choice of biocidal aerosol treatment rather than surface material. To enable direct comparison of three test chlorine-containing biocides, they were free-chlorine matched before aerosol delivery. Under these conditions, the order of antimicrobial efficacy of biocidal aerosol treatment was as follows: ECAS > chlorine dioxide > sodium hypochlorite. However, it should be noted that there were instances in which no significant difference between biocidal aerosol treatments was observed. Overall, sodium hypochlorite had only low-level activity when used to treat surface-associated microorganisms, compared to ECAS and chlorine dioxide, which both produced significant microbial reductions, indicating that this would be an effective delivery mechanism for these biocide types. ECAS are generally referred to as hypochlorous acid, although they contain a variety of oxidants and free radicals known to possess antimicrobial properties in addition to free chlorine (25) and may explain the comparative efficacy observed in this study when free chlorine matched with chlorine dioxide. One of the main advantages of ECAS and chlorine dioxide is that they can be generated on site, although chlorine dioxide traditionally requires manual mixing of sachets whereas ECAS generation can be automated when an electrochemical cell, power, and salt are provided.
In general, when comparing the sensitivities of all target strains tested within this study, the order of resistance (irrespective of biocide type) was as follows: B. subtilis spores > MSSA (type strain) > MRSA (clinical strain) > P. aeruginosa (type strain). It is therefore evident that there was only limited activity of all three biocidal aerosols against spores and that it was well below the 4-log-fold reduction seen as the threshold of surface active biocidal action, although the European Standard quantitative nonporous surface test from which this value was derived was not developed to assess the sporicidal efficacy of biocides (31). Nonetheless, all biocides elicited small significant reductions (<1-log reduction), with the exception of sodium hypochlorite against fabric-associated spores. Spores are known to be more resistant to biocidal treatment than vegetative cells due to a diversity of physiological factors (34, 35). It is known that all three biocides are active against spores (25, 36); therefore, if biocidal aerosols were to be deployed for their decontamination, it is likely that an increased contact time or more-potent formulation would be required. For example, with regard to ECAS, the physicochemical parameters are dependent on the operating parameters of the cell. Therefore, since the redox potential (ORP) of ECAS is considered the most important factor when predicting their antimicrobial efficacy (25), a solution with >1,155 mV (that used within this study) can be used. Interestingly, another biocidal aerosol (hydrogen peroxide) required repeated cycles to be sporicidal (14), and this may also be required for chlorine-containing biocidal aerosols.
In terms of microbiological decontamination, aerosolized biocides (typical droplet size, >1 μm) will fall out of air (according to the sedimentation rate) and be active on material surfaces. The efficacy of a given aerosolized biocide when used within a specific application will be dependent on the design of the aerosolization device and, hence, standardization of output (fog rate and droplet size) as well as the volume and topography of the environment to be decontaminated. For example, the use of fans has (unsurprisingly) been shown to improve the effectiveness of aerosolized biocides by increasing the distribution of active fog and reducing the time from production to surface contact (6, 7). Aerosol technology facilitates continuous delivery of biocidal solutions, enabling constant replenishment of active agent on material, and has the potential to reach areas inaccessible to traditional “liquid” cleaning. Aerosol delivery of biocides is seen not as an alternative to thorough and appropriate physical cleaning (12) but as an adjunct to improve the microbial quality of a physical environment. Within health care environments, this may include integration into infection control programs through periodic decontamination of clinical wards, intensive care units, and/or operating theaters, since removal of surface-associated microorganisms is thought to reduce infection rates of patients (3). Moreover, if biocide dosing pumps or an ECAS generator was coupled to fogging devices and left in situ, this may occur through automated decontamination regimens similar to the systems in existence for delivery of hydrogen peroxide (37).
The experimental fogging system and assay protocol designed within this study have been shown capable of differentiating the comparative efficacies of multiple chlorine-matched biocidal aerosols against a spectrum of target organisms on a range of test surface materials (compared to a standard control treatment) and would be appropriate for testing other biocidal aerosol treatments or material surfaces. As stated by one author, there is need for “an appropriate evaluation framework” to compare decontamination processes (38), and this assay can be utilized as part of standard laboratory (phase 1) testing. Evidently, further application-specific investigations would be required in comparison to existing technology (e.g., hydrogen peroxide vapor) before any biocidal aerosol treatment regimen could be integrated into standard operating procedures, either within health care settings or for wider industrial use.
ACKNOWLEDGMENTS
We thank Pendred Humidification and Water Systems (Norman Pendred and Co., United Kingdom) for their loan of the HU-25OG Contronics humidifier.
Footnotes
Published ahead of print 4 March 2013
REFERENCES
- 1. Canton R, Morosini MI. 2011. Emergence and spread of antibiotic resistance following exposure to antibiotics. FEMS Microbiol. Rev. 35:977–991 [DOI] [PubMed] [Google Scholar]
- 2. HPA 2012. Healthcare-associated infection and antimicrobial resistance: 2010–2011. Health Protection Agency, London, United Kingdom [Google Scholar]
- 3. Dancer SJ. 2009. The role of environmental cleaning in the control of hospital-acquired infection. J. Hosp. Infect. 73:378–385 [DOI] [PubMed] [Google Scholar]
- 4. Fraise AP. 2004. Decontamination of the environment and medical equipment in hospitals, p 563–585 In Russell AD, Hugo WB, Ayliffe GAJ. (ed), Principles and practice of disinfection, preservation and sterilization, 3rd ed Blackwell, London, United Kingdom [Google Scholar]
- 5. Russell AD. 2004. Factors influencing the efficacy of antimicrobial agents, p 98–127 In Russell AD, Hugo WB, Ayliffe GAJ. (ed), Principles and practice of disinfection, preservation and sterilization, 3rd ed Blackwell, London, United Kingdom [Google Scholar]
- 6. Burfoot D, Hall K, Brown K, Xu Y. 1999. Fogging for the disinfection of food processing factories and equipment. Trends Food Sci. Technol. 10:205–210 [Google Scholar]
- 7. Pulvertaft RJ, Walker JW. 1939. The control of air-borne bacteria and fungus spores by means of aerosols. J. Hyg. (Lond.) 39:696–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Twort CC, Baker AH, Finn SR, Powell EO. 1940. The disinfection of closed atmospheres with germicidal aerosols. J. Hyg. (Lond.) 40:253–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Masterman AT. 1941. Air purification by hypochlorous acid gas. J. Hyg. (Lond.) 41:44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elford WJ, van den Ende J. 1945. Studies on the disinfecting action of hypochlorous acid gas and sprayed solution of hypochlorite against bacterial aerosols. J. Hyg. (Lond.) 44:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martyny JW, Harbeck RJ, Barker EA, Sills M, Silveira L, Arbuckle S, Newman L. 2005. Aerosolized sodium hypochlorite inhibits viability and allergenicity of mold on building materials. J. Allergy Clin. Immun. 116:630–635 [DOI] [PubMed] [Google Scholar]
- 12. Bagge-Ravn D, Gardshodn K, Gram L, Vogel BF. 2003. Comparison of sodium hypochlorite-based foam and peroxyacetic acid-based fog sanitizing procedures in a salmon smokehouse: survival of the general microflora and Listeria monocytogenes. J. Food Prot. 66:592–598 [DOI] [PubMed] [Google Scholar]
- 13. Fiser A. 1978. Disinfection of air and dust in fattening houses for chickens by lactic-acid aerosol. Acta Vet. Brno 47:173–183 [Google Scholar]
- 14. Andersen BM, Rasch M, Hochlin K, Jensen FH, Wismar P, Fredriksen JE. 2006. Decontamination of rooms, medical equipment and ambulances using an aerosol of hydrogen peroxide disinfectant. J. Hosp. Infect. 62:149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barbut F, Menuet D, Verachten M, Girou E. 2009. Comparison of the efficacy of a hydrogen peroxide dry-mist disinfection system and sodium hypochlorite solution for eradication of Clostridium difficile spores. Infect. Control Hosp. Epidemiol. 30:507–514 [DOI] [PubMed] [Google Scholar]
- 16. Shapey S, Machin K, Levi K, Boswell TC. 2008. Activity of a dry mist hydrogen peroxide system against environmental Clostridium difficile contamination in elderly care wards. J. Hosp. Infect. 70:136–141 [DOI] [PubMed] [Google Scholar]
- 17. Bartels MD, Kristoffersen K, Slotsbjerg T, Rohde SM, Lundgren B, Westh H. 2008. Environmental meticillin-resistant Staphylococcus aureus (MRSA) disinfection using dry-mist-generated hydrogen peroxide. J. Hosp. Infect. 70:35–41 [DOI] [PubMed] [Google Scholar]
- 18. Oh SW, Gray PM, Dougherty RH, Kang DH. 2005. Aerosolization as novel sanitizer delivery system to reduce food-borne pathogens. Lett. Appl. Microbiol. 41:56–60 [DOI] [PubMed] [Google Scholar]
- 19. Karabulut OA, Ilhan K, Arslan U, Vardar C. 2009. Evaluation of the use of chlorine dioxide by fogging for decreasing postharvest decay of fig. Postharvest Biol. Technol. 52:313–315 [Google Scholar]
- 20. Lambert PA. 2004. Mechanisms of action of biocides, p 139–153 In Russell AD, Hugo WB, Ayliffe GAJ. (ed), Principles and practice of disinfection, preservation and sterilization, 3rd ed Blackwell, London, United Kingdom [Google Scholar]
- 21. Tzanavaras PD, Themelis DG, Kika FS. 2007. Review of analytical methods for the determination of chlorine dioxide. Cent. Eur. J. Chem. 5:1–12 [Google Scholar]
- 22. Gomez-Lopez VM, Rajkovic A, Ragaert P, Smigic N, Devlieghere F. 2009. Chlorine dioxide for minimally processed produce preservation: a review. Trends Food Sci. Technol. 20:17–26 [Google Scholar]
- 23. Kraft A. 2008. Electrochemical water disinfection: a short review. Electrodes using platinum group metal oxides. Platin. Met. Rev. 52:177–185 [Google Scholar]
- 24. Huang YR, Hung YC, Hsu SY, Huang YW, Hwang DF. 2008. Application of electrolyzed water in the food industry. Food Control 19:329–345 [Google Scholar]
- 25. Thorn RMS, Lee SWH, Robinson GM, Greenman J, Reynolds DM. 2012. Electrochemically activated solutions: evidence for antimicrobial efficacy and applications in healthcare environments. Eur. J. Clin. Microbiol. 31:641–653 [DOI] [PubMed] [Google Scholar]
- 26. Robinson GM, Lee SW, Greenman J, Salisbury VC, Reynolds DM. 2010. Evaluation of the efficacy of electrochemically activated solutions against nosocomial pathogens and bacterial endospores. Lett. Appl. Microbiol. 50:289–294 [DOI] [PubMed] [Google Scholar]
- 27. Robinson GM, Tonks KM, Thorn RM, Reynolds DM. 2011. Application of bacterial bioluminescence to assess the efficacy of fast-acting biocides. Antimicrob. Agents Chemother. 55:5214–5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clark J, Barrett SP, Rogers M, Stapleton R. 2006. Efficacy of super-oxidized water fogging in environmental decontamination. J. Hosp. Infect. 64:386–390 [DOI] [PubMed] [Google Scholar]
- 29. Park GW, Boston DM, Kase JA, Sampson MN, Sobsey MD. 2007. Evaluation of liquid- and fog-based application of Sterilox hypochlorous acid solution for surface inactivation of human norovirus. Appl. Environ. Microbiol. 73:4463–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. British Standards Institution 2005. Chemical disinfectants and antiseptics. Basic sporicidal activity. Test method and requirements (phase 1, step 1). BS EN ISO 14347. European Committee for Standardization, Brussels, Belgium [Google Scholar]
- 31. British Standards Institution 2001. Chemical disinfectants and antiseptics. Quantitative non-porous surface test for the evaluation of bactericidal and/or fungicidal activity of chemical disinfectants used in food, industrial, domestic and institutional areas. Test method and requirements without mechanical action (phase 2/step 2). BS EN ISO 13697. European Committee for Standardization, Brussels, Belgium [Google Scholar]
- 32. Hirai Y. 1991. Survival of bacteria under dry conditions; from a viewpoint of nosocomial infection. J. Hosp. Infect. 19:191–200 [DOI] [PubMed] [Google Scholar]
- 33. Lambert RJW. 2004. Comparative analysis of antibiotic and antimicrobial biocide susceptibility data in clinical isolates of methicillin-sensitive Staphylococcus aureus, methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa between 1989 and 2000. J. Appl. Microbiol. 97:699–711 [DOI] [PubMed] [Google Scholar]
- 34. Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Setlow P. 2000. Resistance of bacterial spores, p 217–230 In Stortz G, Hengge Aronis R. (ed), Bacterial stress responses. ASM Press, Washington, DC [Google Scholar]
- 36. Young SB, Setlow P. 2003. Mechanisms of killing of Bacillus subtilis spores by hypochlorite and chlorine dioxide. J. Appl. Microbiol. 95:54–67 [DOI] [PubMed] [Google Scholar]
- 37. Fu TY, Gent P, Kumar V. 2012. Efficacy, efficiency and safety aspects of hydrogen peroxide vapour and aerosolized hydrogen peroxide room disinfection systems. J. Hosp. Infect. 80:199–205 [DOI] [PubMed] [Google Scholar]
- 38. Fu TY, Gent P, Kumar V. 2012. Reply to Destrez: efficacy, efficiency and safety aspects of hydrogen peroxide vapour and aerosolized hydrogen peroxide room disinfection systems. J. Hosp. Infect. 82:300. [DOI] [PubMed] [Google Scholar]