Abstract
Monte Carlo simulation (MCS) of antimicrobial dosage regimens during drug development to derive predicted target attainment values is frequently used to choose the optimal dose for the treatment of patients in phase 2 and 3 studies. A criticism is that pharmacokinetic (PK) parameter estimates and variability in healthy volunteers are smaller than those in patients. In this study, the initial estimates of exposure from MCS were compared with actual exposure data in patients treated with ceftobiprole in a phase 3 nosocomial-pneumonia (NP) study (NTC00210964). Results of MCS using population PK data from ceftobiprole derived from 12 healthy volunteers were used (J. W. Mouton, A. Schmitt-Hoffmann, S. Shapiro, N. Nashed, N. C. Punt, Antimicrob. Agents Chemother. 48:1713–1718, 2004). Actual individual exposures in patients were derived after building a population pharmacokinetic model and were used to calculate the individual exposure to ceftobiprole (the percentage of time the unbound concentration exceeds the MIC [percent fT > MIC]) for a range of MIC values. For the ranges of percent fT > MIC used to determine the dosage schedule in the phase 3 NP study, the MCS using data from a single phase 1 study in healthy volunteers accurately predicted the actual clinical exposure to ceftobiprole. The difference at 50% fT > MIC at an MIC of 4 mg/liter was 3.5% for PK-sampled patients. For higher values of percent fT > MIC and MICs, the MCS slightly underestimated the target attainment, probably due to extreme values in the PK profile distribution used in the simulations. The probability of target attainment based on MCS in healthy volunteers adequately predicted the actual exposures in a patient population, including severely ill patients.
INTRODUCTION
A frequently used tool to choose the dosage regimen for clinical studies of new antimicrobial compounds is Monte Carlo simulation (MCS). These simulations are performed using the pharmacokinetic (PK) data for healthy volunteers by taking into account the wild-type MIC distribution of target microorganisms and the PK/pharmacodynamic (PD) target. The probability of target attainment (PTA) is determined for various dosage regimens based on these data for a range of MICs and PK/PD breakpoints (1, 2). However, it is often questioned whether the data for healthy volunteers that are used adequately reflect the pharmacokinetics in patients, not only for the average parameter values, but also for individual variability (3). Interindividual variability in healthy volunteers differs significantly from that in severely ill patients (3, 4). Using data from healthy volunteers, therefore, may result in inadequate dosage regimens for clinical patients or, from another point of view, inadequate breakpoints.
During the development of ceftobiprole (formerly BAL9141), a broad-spectrum cephalosporin active against most Gram-positive microorganisms, including methicillin-resistant Staphylococcus aureus (MRSA), dosage regimens were simulated based on PK data from 12 healthy volunteers, using MCS (5), to aid in the design of phase 2 and phase 3 trials, now almost a decade ago. The completion of a phase 3 study comparing ceftobiprole with a comparator in patients with nosocomial pneumonia (NP) and the pharmacokinetic sampling of ceftobiprole that was performed during that study offer the opportunity to determine the observed target attainment in clinical patients and to compare them with the probability of target attainment based on the earlier MCS. Here, we compare the PTA in healthy volunteers with clinical data derived from nosocomial-pneumonia patients to evaluate whether the use of MCS with data from healthy volunteers predicts the observed target attainment rate (OTA) in clinical patients.
MATERIALS AND METHODS
Data from nosocomial-pneumonia patients.
The microbiological, clinical, and demographic data used were from a clinical phase 3 nosocomial-pneumonia study (trial database, NTC00210964) conducted between 2005 and 2007. This is a retrospective cohort study of a clinical trial to compare the efficacy of ceftobiprole to a combination of ceftazidime and linezolid for treatment of NP, including ventilator-associated pneumonia (VAP). It was a randomized, double-blind, multicenter phase 3 study involving 781 subjects worldwide, for whom all data recorded in the trial database were provided to the authors. The main exclusion criteria were pregnancy, hypersensitivity to any related anti-infective, and any form of dialysis. The patients received ceftobiprole at 500 mg infused every 8 h (q8h) over 2 h. In patients with renal impairment, the dose was adjusted. The real dose administered was used in the analysis. Pharmacokinetic sampling was conducted at selected sites and consisted of a median number of 3 samples per patient (range, 1 to 7) taken at different time points, mostly during multiple-dosing intervals.
Population pharmacokinetic model in clinical patients.
Data from six studies in clinical patients and healthy volunteers were used in the population analysis: 3 in healthy volunteers, 1 in intensive care patients, 1 in patients with complicated skin and skin structure infections, and 1 in nosocomial-pneumonia patients. The population pharmacokinetic model was developed with the use of the Compaq Visual FORTRAN standard edition 6.6 (Compaq Computer Corp., Euston, TX) and the NONMEM software package (version VI, release 2; Icon Development Solutions, Ellicott City, MD). The model was implemented in the NONMEM ADVAN5 subroutine, and the analysis was performed using the FOCE method with INTERACTION (11). In general, model selection was based on a combination of the evaluation of the mean objective function value (MOFV), pharmacokinetic parameter estimates and their respective confidence intervals, and goodness-of-fit plots. To detect systematic deviations in the model fits, the goodness-of-fit plots were inspected visually.
To determine the basic structural pharmacokinetic parameters, various 2- and 3-compartment models were tested. To detect significant differences between two structural models, the MOFV with a prespecified level of significance (P < 0.001) was used (corresponding to a difference in MOFV of at least 10.8 points).
Interindividual variability on all structural parameters was tested using an exponential-distribution model. Possible correlations between interindividual variability coefficients on parameters were estimated and, if present, were accounted for in the between-subject variability model by inclusion of the respective covariance element(s) in the variance-covariance matrix. A significance level of 0.05 was selected (corresponding to a difference in MOFV of 3.84 points).
To further refine the model, covariate analysis was performed for the following variables: age, weight, body mass index (BMI), VAP (infection type, VAP subject versus non-VAP subject), creatinine value, creatinine clearance, gender, APACHE II score, and albumin level. Covariate analysis of parameters for which a random effect had been identified was performed by forward addition of each candidate covariate to the model structure until no further improvement of goodness of fit was observed. A significance level of 0.05 was selected (corresponding to a difference in MOFV of 3.84 points). A further criterion for acceptance of covariate effects was that the estimated 95% confidence interval of the covariate effect did not overlap with zero. The contribution of each covariate to the final model was confirmed by backward deletion of each covariate from the model to account for possible interaction between covariates. Residual intra- and interindividual variabilities were visually evaluated.
Three different residual-error models were tested during the procedure where applicable: additive, proportional, and combined-error models. A significance level of 0.05 was selected (corresponding to a difference in MOFV of 3.84 points).
The precision of the final population model for the entire population was established using the bootstrap option (100 times) in a S-plus interface consisting of repeated random sampling with replacement from the original data. The estimated parameters from the bootstrap analysis were compared to the estimates from the original data.
Target attainment in patients with nosocomial pneumonia.
The population model was subsequently used to determine the actual individual exposures by calculating the percent fT > MIC at various MIC values for each of 364 patients in the nosocomial-pneumonia study based on the individual pharmacokinetic parameter estimates. Unbound concentrations above a range of fixed MIC values (0.5 to 32 mg/liter) and as a percentage of the dosing interval (percent fT > MIC) were estimated for each patient by the use of KinFun (version 1.06; Medimatics, Maastricht, The Netherlands), which is based on an equation solver, using the population pharmacokinetic model based on either plasma concentrations or covariates where available, using NONMEM. A protein binding level of 16% was used in the analysis. To calculate the individual PK estimates based on covariates, regression analysis was performed based on the results from the population model. The observed target attainment was calculated for all patients (patients with estimates based on plasma samples and estimates based on covariates only; n = 364), as well as for the subgroup of patients with PK parameters derived by the population model directly, based on plasma concentrations from the individual patients (n = 52).
MCS in volunteers.
MCSs were initially performed to propose a dosage regimen for future therapeutic studies with ceftobiprole in 2002–2003. A log-linear distribution was used in the MCS with MicLab 2.36 (Medimatics, Maastricht, The Netherlands). The results of the MCS using PK data from ceftobiprole (BAL9141) derived from 12 healthy volunteers were used (5): 6 subjects received 750 mg every 12 h, and 6 subjects received 500 mg every 12 h. All volunteers were healthy males aged 18 to 45 years with body mass indexes of 18 to 30 kg/m2, normal renal function (creatinine clearance > 90 ml/min), and normal hepatic function.
The exploratory protein binding of ceftobiprole at that time was 40% bound. PK analyses had been performed using the NPEM2 program (12), and a 2-compartment model best fit the data (r2, 0.98). The regimen of 750 mg every 12 h with a rate of infusion of 30 min was correlated with a high PTA, as well as the regimen using 500 mg every 8 h and a rate of infusion of 30 min (5). The results are shown in Table 1. The corresponding provisional susceptible breakpoint was therefore suggested to be ≤4 mg/liter if those regimens were to be applied.
Table 1.
Pharmacokinetic parameters for volunteers used in MCSa
| Parameter | Value |
|
|---|---|---|
| Mean | SD | |
| Clearance (liters/h) | 5.111 | 0.518 |
| Vol of distribution 1 (liters) | 10.386 | 2.013 |
| Vss (liters)b | 16.76 | |
| kcp (h−1)c | 0.542 | 0.237 |
| Kpc (h−1)c | 0.883 | 0.335 |
Based on our study as described in reference 5.
The volume of distribution at steady state (Vss) was calculated.
kcp and Kpc, equilibrium constants from the central (V1) to the peripheral (V2) compartment and from the peripheral to the central compartment, respectively.
The dosage regimen finally used in the clinical phase 3 study was slightly different from the original regimen used in the simulations—500 mg q8h infused over 2 h instead of over 0.5 h—and we therefore repeated the MCS using the original data set. In addition, the protein binding was corrected by the definitive value of 16% bound as opposed to 40% bound and was therefore used in the present study. To perform the MCS in the current study, a dosage regimen of 500 mg q8h infused over 2 h and a protein binding of 16% were used.
Comparison of the data.
Analysis using MCS of the data from healthy volunteers resulted in a PTA. From the NP study, percentages of target attainment in various groups of subjects were calculated. The PTA in volunteers was compared to the OTAs in several populations of nosocomial-pneumonia patients.
RESULTS
Population pharmacokinetic model.
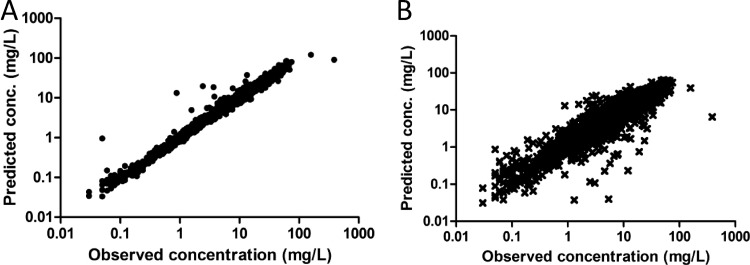
A total of 171 individuals from the six studies had information on dosing times, as well as concentrations, available and were included in the pharmacokinetic model. The demographic data for patients included in the analysis are shown in Table 2. A 3-compartment model with a coefficient of variability on clearance (CL) and the central volume of distribution (V1) best described the data. The coefficients were correlated with each other and best described by a combined-error model. The model was improved by including the creatinine clearance as a covariate [CL = 2.398 + (0.02884 × creatinine clearance)] on clearance and age as a covariate on the central volume of distribution [V1 = 9.998 + (0.1267 × age)]. The other covariates did not improve the model. Instead of age, BMI is often included as a covariate; however, the decrease in MOFV was only 3.0 points for BMI (not significant [NS]), and 44.3 points for age (significant). The parameter estimates of the final model are shown in Table 3. Plots of the individual and population predicted concentrations versus observed concentrations are shown in Fig. 1. The R2 values of the individual predicted and population predicted concentrations versus the observed concentrations were 0.959 and 0.823, respectively.
Table 2.
Demographic data on patients
| Parameter | Value [mean (range)]a |
||
|---|---|---|---|
| Patients included in PK model | NP patients | NP patients with model estimates | |
| No. of patients | 171 | 364 | 52 |
| Age (yr) | 51.5 (17–92) | 60.9 (18–95) | 66.5 (19–92) |
| Sex (no. male/female) | 121/50 | 102/262 | 15/37 |
| Wt (kg) | 73.6 (40–115); n = 170 | 72.7 (25–130) | 69.8 (42.3–115) |
| Body mass index | 25.0 (15.6–41.4); n = 169 | 25.4 (11.3–42.9); n = 356) | 25.1 (16.1–41.4) |
| Creatinine clearance | 110.4 (11.8–612.1) | 102.6 (17.0–475.3); n = 360 | 93.8 (19.6–228.8); n = 48 |
| SIRSb (no. yes/no) | 49/122 | 270/94 | 31/21 |
| VAP (no. yes/no) | 67/104 | 91/273 | 0/52 |
| APACHE II score (range) | 14.5 (8–24); n = 79) | 13.7 (8–26) | 13.3 (8–24) |
The number of patients (n) is given only when it is different from that in the first row of the table.
SIRS, systemic inflammatory response syndrome.
Table 3.
Final results of the PK model in patients
| Parameter | Value |
||
|---|---|---|---|
| Mean | SE | Relative SE (SE%) | |
| Clearance (liters/h)a | 4.74 | 0.24 | 5.06 |
| Vol of distribution 1 (liters)a | 15.5 | 1.26 | 8.13 |
| Vol of distribution 2 (liters) | 1.93 | 0.34 | 17.6 |
| Vol of distribution 3 (liters) | 3.76 | 1.18 | 31.4 |
| Vss (liters)b | 21.2 | ||
| Intercompartmental clearance V1 and V2 (liter/h) | 0.372 | 0.108 | 29 |
| Intercompartmental clearance V1 and V3 (liters/h) | 3.05 | 1.83 | 60 |
| Covariate creatinine clearance on clearance | 0.00518 | 0.0011 | 21.2 |
| Covariate age on vol of distribution 1 | 0.0117 | 0.00153 | 31.1 |
| Variability on clearance | 0.423 | 0.233 | 55.1 |
| Variability on vol of distribution 1 | 0.478 | 0.179 | 37.4 |
| Residual error, proportional | 0.0288 | 0.00599 | 20.8 |
| Residual error, additive (mg/liter) | 2.31 × 10−4 | 4.09 × 10−4 | 177 |
A correlation between clearance and volume of distribution 1 was included in the final model.
The volume of distribution at steady state (Vss) was calculated.
Fig 1.
Individual (A) and population (B) predicted versus observed concentrations (conc.) of ceftobiprole in 171 patients.
Target attainment.
The individual exposures were calculated using the individual NONMEM parameter estimates for 364 NP patients in total. For 52 patients, NONMEM estimates were available based on pharmacokinetic sampling. For the remainder of the patients (n = 312), individual exposures were predicted based on the covariates clearance and age.
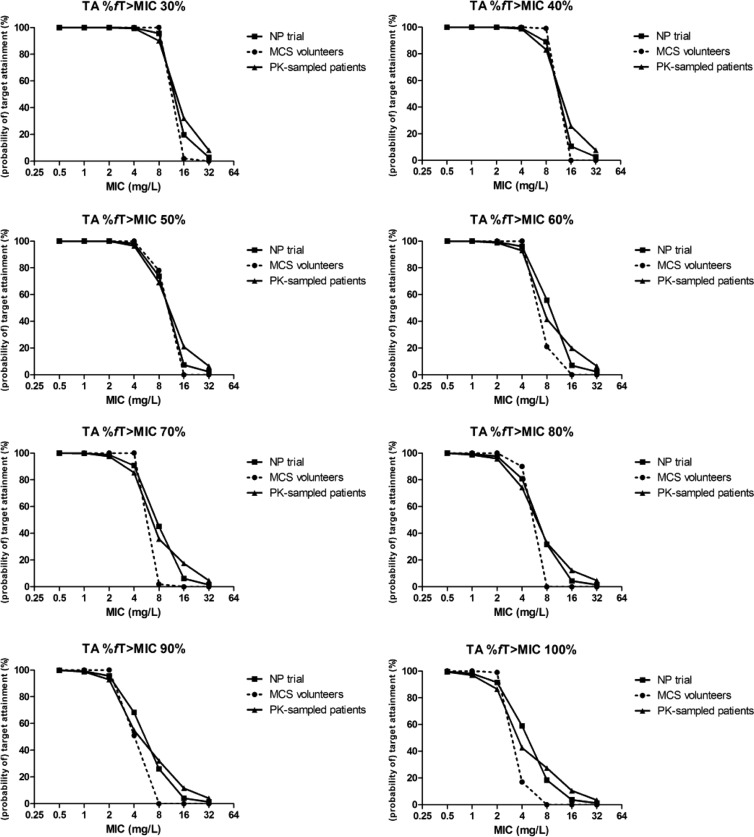
Figure 2 shows the OTA for three individual groups for targets of 30% fT > MIC up to 100% as a function of the MIC: the PK-sampled patients, the total group of NP patients (NP trial), and the healthy volunteers (MCS volunteers). The OTA for the PK-sampled patients (n = 52) compared well with the values for the total group of NP patients (Fig. 2 and 3) over the whole target range, although the curve was somewhat shallower, as a result of more variance in the PK-sampled group. The difference between the OTA in the NP patients and the PTA in healthy volunteers, although not substantial, was clearly present, in particular at the higher targets and MICs.
Fig 2.
Target attainment rates for volunteers (probability of target attainment), for NP patients (observed target attainment for all patients), and for the PK-sampled group (NONMEM patients; observed target attainment for patients with PK samples only) plotted per target percent fT > MIC.
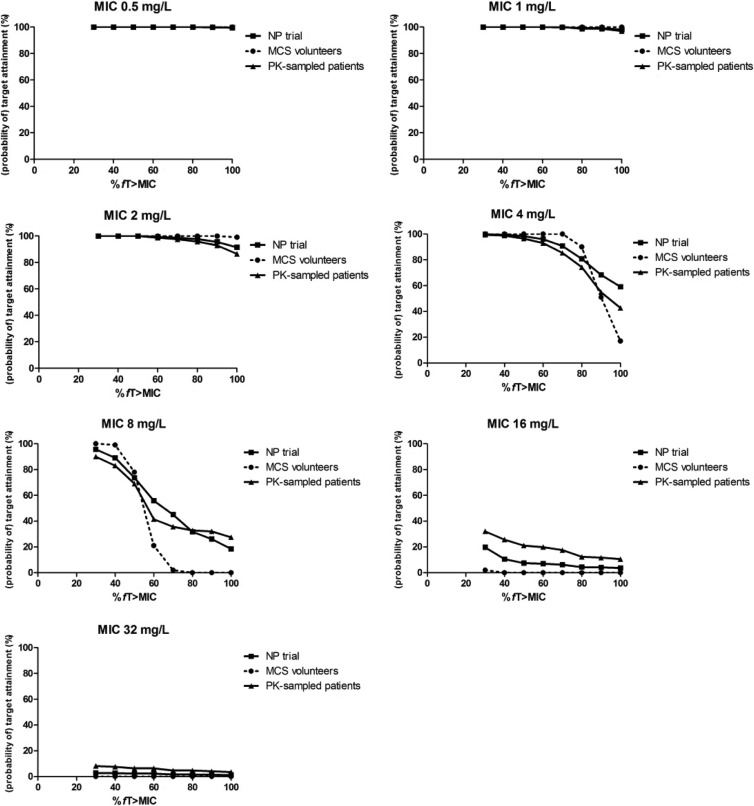
Fig 3.
Target attainment rates for volunteers (probability of target attainment), for NP patients (observed target attainment for all patients), and for the PK-sampled group (NONMEM patients; observed target attainment for patients with PK samples only) plotted per MIC.
For a target percent fT > MIC of 30 to 50% and MICs of 4 mg/liter or lower, the PTA for healthy volunteers was similar to the OTA in pneumonia patients. For targets with a higher percent fT > MIC, the MCS performed in healthy volunteers clearly underestimated the OTA in NP patients for MIC values of 4 to 16 mg/liter. For both healthy volunteers and pneumonia patients, more than 90% of the individuals were exposed adequately to ceftobiprole for MIC values up to 4 mg/liter and percent fT > MIC up to 70%. These results are also summarized in Table 4. For coverage of both Gram-positive and Gram-negative microorganisms, i.e., those requiring a PTA of approximately 50% of the dosing interval, 99% of the patients were covered up to a MIC of 4 mg/liter. The differences between the PTAs (ΔPTAs) in healthy volunteers and NP trial patients, as well as PK-sampled patients, are shown in Table 5. For targets of 50% fT > MIC and 60% fT > MIC, the ΔPTAs in the treatment of microorganisms with a MIC of 4 mg/liter were 1.66% and 3.93%, respectively, when volunteers were compared with NP trial patients and 3.51% and 7.02% when volunteers were compared with PK-sampled patients.
Table 4.
Proportion (percent) of patients reaching a given percent fT > MIC for various target MIC values
| % fT > MIC | % of patients at MIC (mg/liter): |
||||||
|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | |
| 30 | 100 | 100 | 100 | 100 | 96.7 | 17.6 | 1.10 |
| 40 | 100 | 100 | 100 | 99.5 | 90.1 | 8.24 | 0.824 |
| 50 | 100 | 100 | 100 | 98.9 | 75.0 | 5.22 | 0.824 |
| 60 | 100 | 100 | 99.5 | 96.7 | 63.5 | 4.67 | 0.824 |
| 70 | 100 | 99.7 | 98.9 | 92.0 | 50.8 | 4.12 | 0.824 |
| 80 | 100 | 99.5 | 98.1 | 83.0 | 33.8 | 2.47 | 0.824 |
| 90 | 99.7 | 99.2 | 96.4 | 75.0 | 26.6 | 2.20 | 0.549 |
| 100 | 99.5 | 98.4 | 93.7 | 61.5 | 18.4 | 2.20 | 0.549 |
Table 5.
Differences in PTAs (ΔPTA) between volunteers and NP trial patients, as well as the subset of PK-sampled patients
| MIC | ΔPTA (%)a for target (% fT > MIC) of: |
|||||
|---|---|---|---|---|---|---|
| 40% |
50% |
60% |
||||
| NP trial patients | PK-sampled patients | NP trial patients | PK-sampled patients | NP trial patients | PK-sampled patients | |
| 0.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0.62 | 1.17 |
| 4 | 0.62 | 1.17 | 1.66 | 3.51 | 3.93 | 7.02 |
| 8 | 9.97 | 15.96 | 4.29 | 8.99 | −34.9 | −20.5 |
| 16 | −10.56 | −25.7 | −7.45 | −21.1 | −7.04 | −19.9 |
| 32 | −2.69 | −7.60 | −2.28 | −6.43 | −2.28 | −6.43 |
ΔPTA was calculated as follows: PTA of volunteers − PTA of NP trial patients or PTA of volunteers − PTA of PK-sampled patients.
In Fig. 3, the target attainment is depicted as a function of the target for MICs of 0.5 to 32 mg/liter. This alternative method of representing the data sustains the conclusions drawn above. Up to MICs of 2 mg/liter, there is hardly any difference in target attainment between the populations, whereas at 4 mg/liter, target attainment is above 90% up to 50% fT > MIC.
DISCUSSION
The ceftobiprole pharmacokinetics were best described by a 3-compartment model. Two covariates were included in the final model: creatinine clearance on the clearance, and age on the central volume of distribution. Although ceftobiprole is administered as a prodrug, we did not include a conversion model because of the rapid and quantitative conversion rate of prodrug to ceftobiprole. A similar approach was taken by Kimko et al. (6). The mean value for the clearance from the 3-compartment model in patients with complicated skin and skin structure infections as studied by Kimko et al. (6) was 5.36 liters/h. In contrast, Lodise et al. (2) did use a conversion model to analyze their data and calculated an overall clearance of 4.8 liters/h. Both values compare well to the clearance of 4.74 liters/h found in our analysis. Likewise, both Lodise and colleagues (2) and Kimko and colleagues (6) found creatinine clearance to be a significant covariate on ceftobiprole clearance. With respect to between-subject variability, Kimko and colleagues found values within the same range as ours. The Lodise analysis is based on BigNPOD, and the results are presented differently (2), making it difficult to compare them directly.
A difference between the initial pharmacokinetic analysis of the volunteer data and the data obtained from patients is that the data from the volunteers were described by a two-compartment model while a three-compartment model best fit the data from the six pooled trials. This might be due to the PK model of the volunteers having been based on a limited number of subjects, although the sampling of these subjects was extensive. The use of a two-compartment model in the MCS instead of a 3-compartment model may be an explanation for the target attainment in volunteers slightly underestimating the actual values found in patients. On the other hand, the mean terminal elimination half-lives in both groups were comparable, 2.37 h in volunteers and 2.26 h in the patient group (results not shown).
For the ranges of percent fT > MIC values used to evaluate dosage schedules in the phase 3 NP study, the MCS using data from a single phase 1 study in healthy volunteers accurately predicted the actual clinical exposure to ceftobiprole observed in these NP patients. For the higher values of percent fT > MIC and MICs, the MCS even slightly underestimated the target attainment, probably due to extreme values used in the simulations. The observed target attainment is based on individual estimates either directly derived from the PK model (PK-sampled group) or calculated using regression analysis on the covariates from the model. The comparison of the MCS of the volunteers with the OTA of the PK-sampled group did not lead to different conclusions, despite the increased variance (due to more precise estimates) in the PK-sampled group than in the data for all NP patients. The database used for population pharmacokinetic analysis also contained patients with other conditions than NP, such as complicated skin infections. When these patients were also included in the analyses (n = 171 patients with model estimates and 312 patients with calculated estimates), the conclusions from the results were similar (results not shown).
In vivo studies in animals have shown that the pharmacodynamics of ceftobiprole are similar to those of other cephalosporins (7). Therefore, it is expected that a near maximal killing effect at 24 h is achieved at 50% fT > MIC for staphylococci and 60% fT > MIC for Gram-negative microorganisms (7–9). The observed target attainment was high for a MIC of up to 4 mg/liter (OTA, 96.7%). For higher MIC values, the OTA was unacceptably low. Using a susceptibility breakpoint of 4 mg/liter, the studied dosage regimen of 500 mg q8h infused over 2 h resulted in a high probability of therapeutic success.
It is sometimes thought that MCS in healthy volunteers underestimates the actual exposures in clinically ill patients. The concept of inflated variance has been suggested to be one of the solutions to account for the increased variability in patients compared to volunteers (10). However, in this study, critically ill patients at the intensive care unit were also included in the NP patient population. The mean APACHE II score was 14.5 (from 79 patients), indicating that a significant number of severely ill patients were included in the analysis, as well as in the simulations, and there is no indication in the analysis that the severely ill patients are significantly underexposed, although the relationship between MIC and target attainment is slightly shallower in the patient population. Artificially increasing variability in healthy volunteers will even underestimate the exposure in these patients.
A high renal clearance, for example, in critically ill patients who are known to have augmented renal clearance, might also contribute to the lower percent fT > MIC in several patients. In our NP study, there were indeed several patients with a high renal clearance who had a relatively low exposure to ceftobiprole. For an MIC value of 2 mg/liter, for example, there were 4 patients with a relatively low percent fT > MIC (>50% and <70%). For those 4 patients, the mean creatinine clearance was 322 ml/min, while the mean value for the entire population was 103 ml/min. On the other hand, there were 24 patients in the analysis for MIC values of 2 mg/liter with a creatinine clearance of >200 ml/min. For these 24 patients, the mean percent fT > MIC value was 87.1%, with a median of 93.1%. This indicates that a high renal clearance contributes to the subpopulation with low exposure, but not all patients with high renal clearance have low exposure to ceftobiprole.
We conclude that the probability of target attainment based on the MCS in healthy volunteers adequately predicted the actual exposures in a patient population that included severely ill patients.
ACKNOWLEDGMENTS
This work was supported in part by an unrestricted grant from Basilea Pharmaceutica Ltd. and the EU project Saturn (grant Health-F3-2009-241796).
Footnotes
Published ahead of print 12 February 2013
REFERENCES
- 1. Mouton JW, Brown DF, Apfalter P, Canton R, Giske CG, Ivanova M, MacGowan AP, Rodloff A, Soussy CJ, Steinbakk M, Kahlmeter G. 2012. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: the EUCAST approach. Clin. Microbiol. Infect. 18:E37–E45 [DOI] [PubMed] [Google Scholar]
- 2. Lodise TP, Jr, Pypstra R, Kahn JB, Murthy BP, Kimko HC, Bush K, Noel GJ, Drusano GL. 2007. Probability of target attainment for ceftobiprole as derived from a population pharmacokinetic analysis of 150 subjects. Antimicrob. Agents Chemother. 51:2378–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roberts JA, Kirkpatrick CM, Lipman J. 2011. Monte Carlo simulations: maximizing antibiotic pharmacokinetic data to optimize clinical practice for critically ill patients. J. Antimicrob. Chemother. 66:227–231 [DOI] [PubMed] [Google Scholar]
- 4. Mouton JW, Punt N, Vinks AA. 2005. A retrospective analysis using Monte Carlo simulation to evaluate recommended ceftazidime dosing regimens in healthy volunteers, patients with cystic fibrosis, and patients in the intensive care unit. Clin. Ther. 27:762–772 [DOI] [PubMed] [Google Scholar]
- 5. Mouton JW, Schmitt-Hoffmann A, Shapiro S, Nashed N, Punt NC. 2004. Use of Monte Carlo simulations to select therapeutic doses and provisional breakpoints of BAL9141. Antimicrob. Agents Chemother. 48:1713–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kimko H, Murthy B, Xu X, Nandy P, Strauss R, Noel GJ. 2009. Population pharmacokinetic analysis of ceftobiprole for treatment of complicated skin and skin structure infections. Antimicrob. Agents Chemother. 53:1228–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andes D, Craig WA. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 50:1376–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–12 [DOI] [PubMed] [Google Scholar]
- 9. Drusano GL. 2004. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug.’ Nat. Rev. Microbiol. 2:289–300 [DOI] [PubMed] [Google Scholar]
- 10. Bhavnani SM, Dudley MN, Landersdorfer L, Drusano GL, Craig WA, Jones RN, Ambrose PG. 2010. Pharmacokinetic-pharmacodynamic basis for CLSI carbapenem susceptibility breakpoint changes, abstr A-1382. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother, Boston, MA [Google Scholar]
- 11. Beal SL, Sheiner LB, Boeckmann AJ. 2006. NONMEM users guides (1989–2006). Icon Development Solutions, Ellicott City, MD [Google Scholar]
- 12. Schumitzky A. 1991. Nonparametric EM algorithms for estimating prior distribution. Appl. Math. Comput. 45:141–157 [Google Scholar]