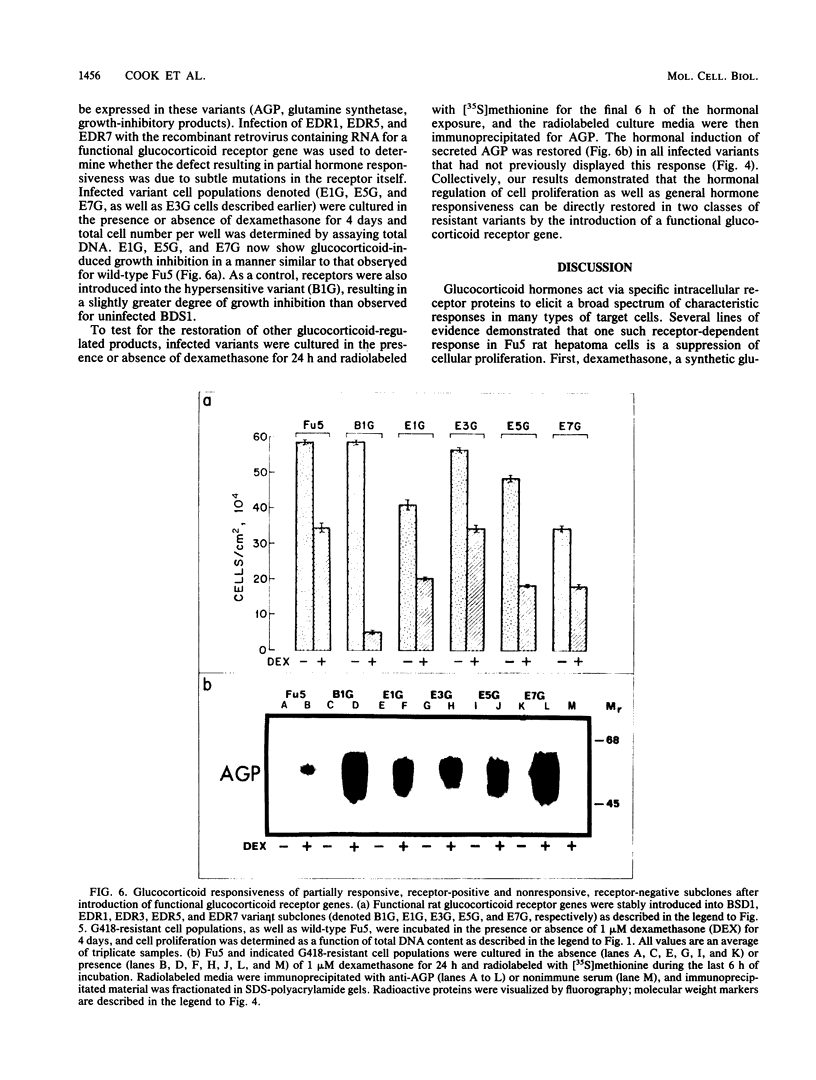
Abstract
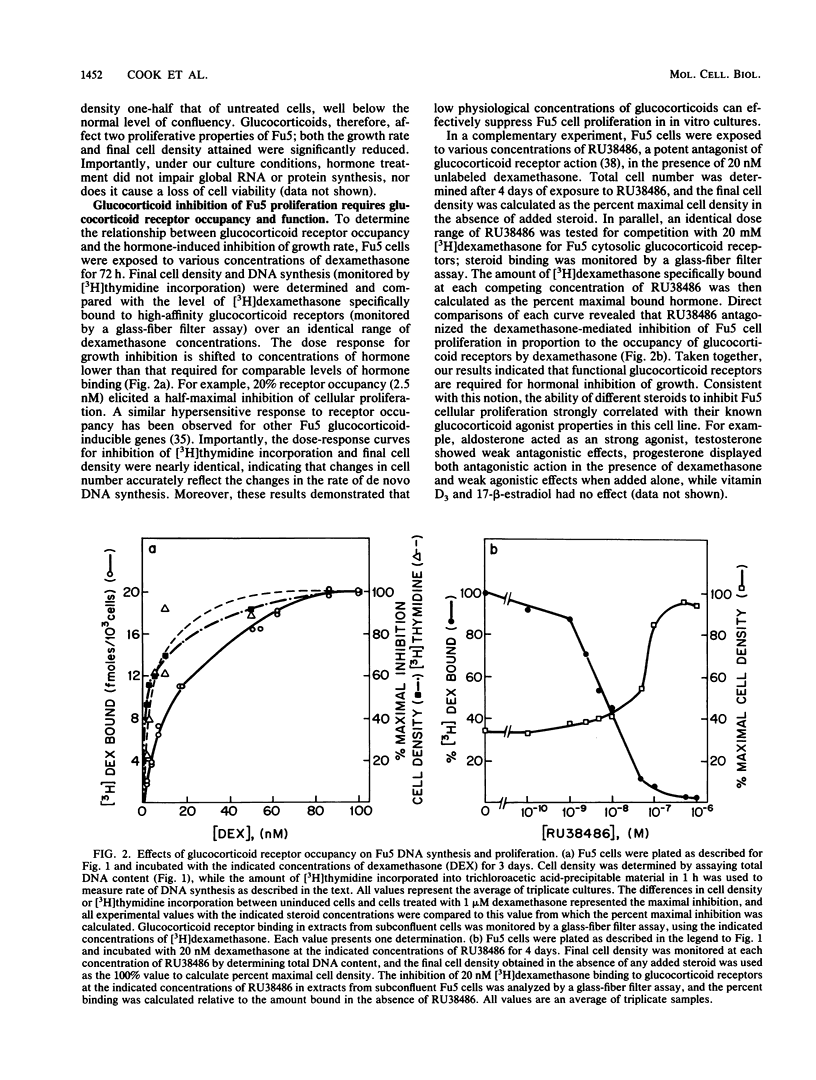
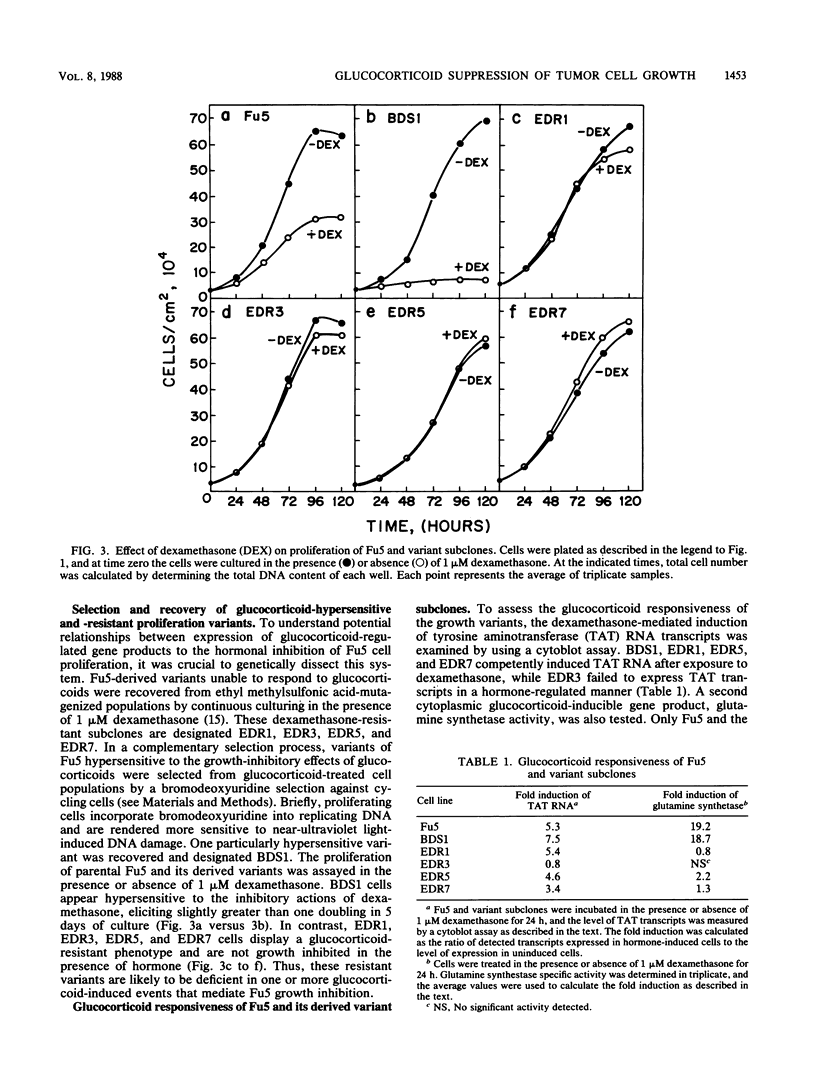
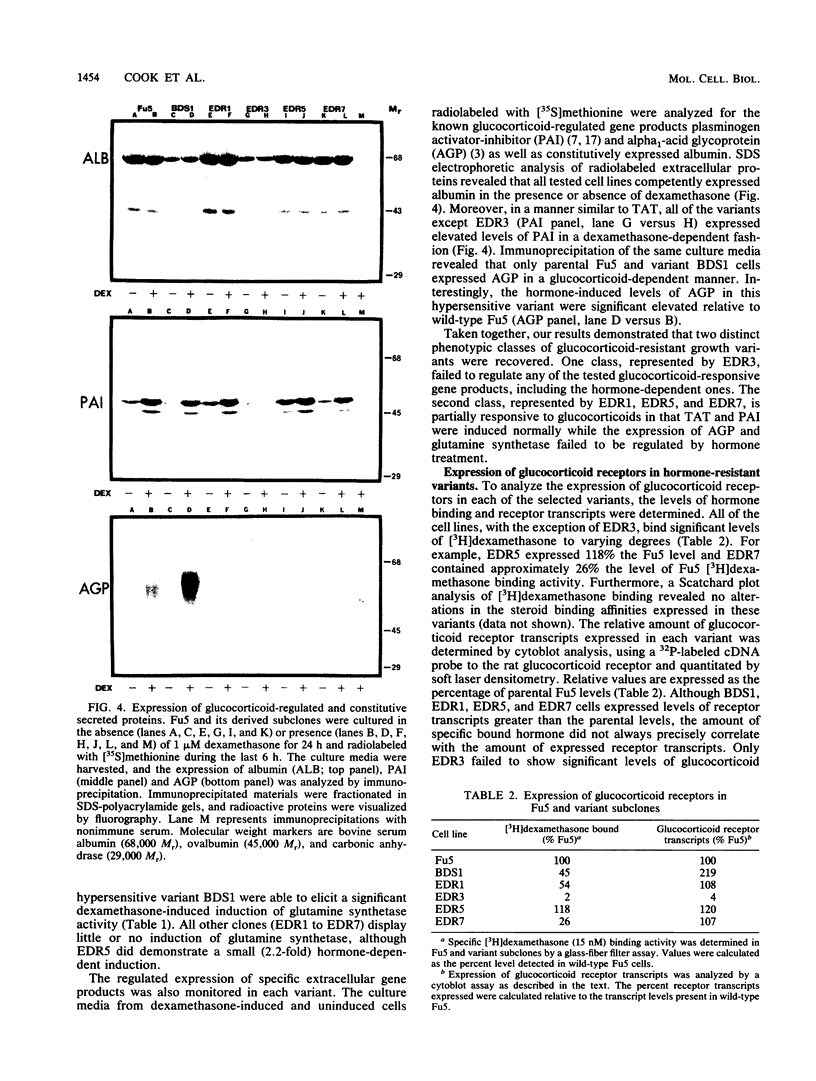
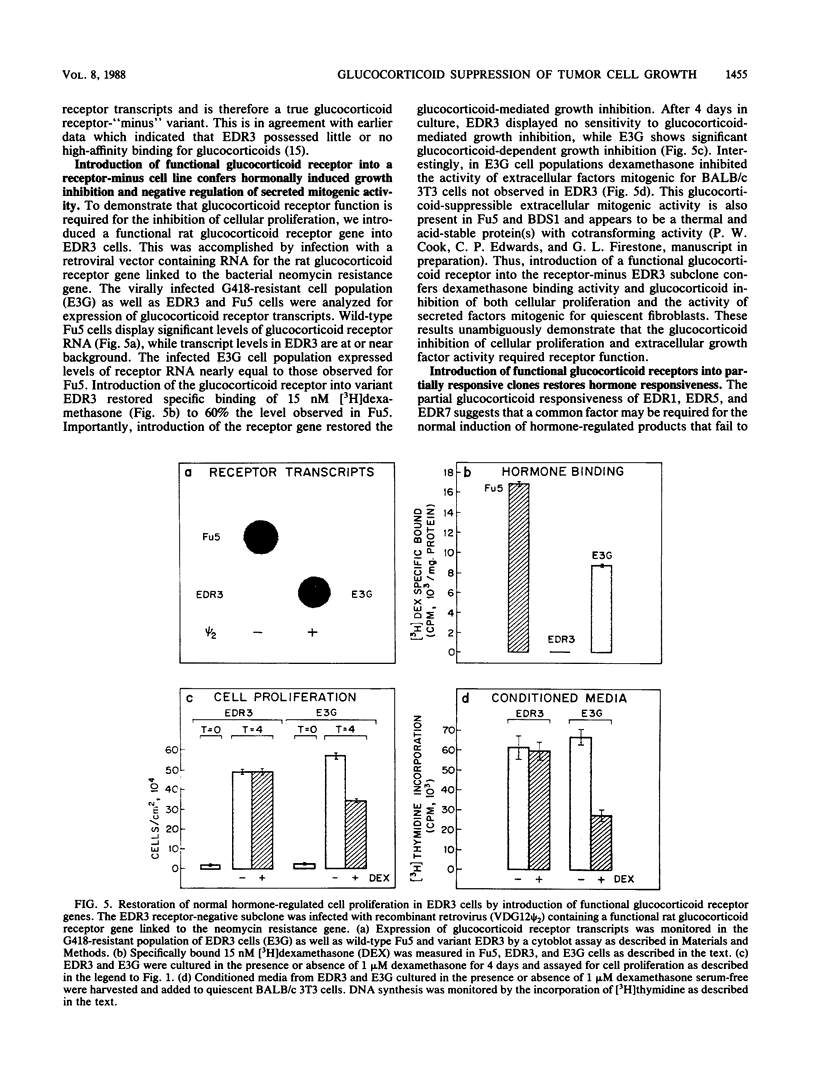
Exposure of the Fu5 rat hepatoma cell line to glucocorticoids, such as dexamethasone and hydrocortisone, suppressed the growth rate and final density of cells grown in the presence of serum. This hormonal effect was proportional to receptor occupancy and affinity and, in addition, the glucocorticoid antagonist RU38486 prevented this response. Two classes of dexamethasone-resistant variants that failed to be growth inhibited were recovered from ethyl methylsulfonate-mutagenized populations by continuous culture in the presence of 1 microM dexamethasone. The first class, represented by the EDR3 subclone, was completely glucocorticoid unresponsive and failed to express receptor transcripts. The second class, represented by the EDR1, EDR5, and EDR7 subclones, possessed significant levels of glucocorticoid receptor but were only partially glucocorticoid responsive when stimulated with saturating levels of hormone. Introduction of functional glucocorticoid receptor genes into both classes of dexamethasone-resistant variants by a recombinant retrovirus expression vector restored glucocorticoid responsiveness and suppression of cell growth. A hypersensitive variant (BDS1), recovered by bromodeoxyuridine selection, was fully glucocorticoid responsive, and its inhibition of proliferation was more acutely regulated by dexamethasone. Taken together, our results established that the inhibition of proliferation in Fu5 rat hepatoma cells represents a new glucocorticoid response that requires the expression of a functional glucocorticoid receptor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett C. A., Barnhorst M., Fooshee C. M., Saneto R. P. Selection of a dexamethasone-resistant H-4-IIE-C3 rat hepatoma tissue-culture line. In Vitro. 1979 Feb;15(2):128–137. doi: 10.1007/BF02618109. [DOI] [PubMed] [Google Scholar]
- Baumann H., Firestone G. L., Burgess T. L., Gross K. W., Yamamoto K. R., Held W. A. Dexamethasone regulation of alpha 1-acid glycoprotein and other acute phase reactants in rat liver and hepatoma cells. J Biol Chem. 1983 Jan 10;258(1):563–570. [PubMed] [Google Scholar]
- Caro J. F., Amatruda J. M. Glucocorticoid-induced insulin resistance: the importance of postbinding events in the regulation of insulin binding, action, and degradation in freshly isolated and primary cultures of rat hepatocytes. J Clin Invest. 1982 Apr;69(4):866–875. doi: 10.1172/JCI110526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr B. I., Hayashi I., Branum E. L., Moses H. L. Inhibition of DNA synthesis in rat hepatocytes by platelet-derived type beta transforming growth factor. Cancer Res. 1986 May;46(5):2330–2334. [PubMed] [Google Scholar]
- Castellano T. J., Schiffman R. L., Jacob M. C., Loeb J. N. Suppression of liver cell proliferation by glucocorticoid hormone: a comparison of normally growing and regenerating tissue in the immature rat. Endocrinology. 1978 Apr;102(4):1107–1112. doi: 10.1210/endo-102-4-1107. [DOI] [PubMed] [Google Scholar]
- Coleman P. L., Patel P. D., Cwikel B. J., Rafferty U. M., Sznycer-Laszuk R., Gelehrter T. D. Characterization of the dexamethasone-induced inhibitor of plasminogen activator in HTC hepatoma cells. J Biol Chem. 1986 Mar 25;261(9):4352–4357. [PubMed] [Google Scholar]
- Cousens L., Eskin B. Filter assay method for the estrogen receptor protein: detection of both filled and unfilled estrogen binding sites. Anal Biochem. 1982 Mar 15;121(1):39–48. doi: 10.1016/0003-2697(82)90554-1. [DOI] [PubMed] [Google Scholar]
- Deschatrette J., Weiss M. C. Characterization of differentiated and dedifferentiated clones from a rat hepatoma. Biochimie. 1974;56(11-12):1603–1611. doi: 10.1016/s0300-9084(75)80286-0. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Huff K. K., Spencer E. M., Lippman M. E. Induction of epidermal growth factor-related polypeptides by 17 beta-estradiol in MCF-7 human breast cancer cells. Endocrinology. 1986 Jan;118(1):138–142. doi: 10.1210/endo-118-1-138. [DOI] [PubMed] [Google Scholar]
- Durant S., Duval D., Homo-Delarche F. Factors involved in the control of fibroblast proliferation by glucocorticoids: a review. Endocr Rev. 1986 Aug;7(3):254–269. doi: 10.1210/edrv-7-3-254. [DOI] [PubMed] [Google Scholar]
- Firestone G. L., John N. J., Haffar O. K., Cook P. W. Genetic evidence that the steroid-regulated trafficking of cell surface glycoproteins in rat hepatoma cells is mediated by glucocorticoid-inducible cellular components. J Cell Biochem. 1987 Dec;35(4):271–284. doi: 10.1002/jcb.240350402. [DOI] [PubMed] [Google Scholar]
- Firestone G. L., John N. J., Yamamoto K. R. Glucocorticoid-regulated glycoprotein maturation in wild-type and mutant rat cell lines. J Cell Biol. 1986 Dec;103(6 Pt 1):2323–2331. doi: 10.1083/jcb.103.6.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone G. L. The role of protein glycosylation in the compartmentalization and processing of mouse mammary tumor virus glycoproteins in mouse mammary tumor virus-infected rat hepatoma cells. J Biol Chem. 1983 May 25;258(10):6155–6161. [PubMed] [Google Scholar]
- Firestone G. L., Yamamoto K. R. Two classes of mutant mammary tumor virus-infected HTC cell with defects in glucocorticoid-regulated gene expression. Mol Cell Biol. 1983 Feb;3(2):149–160. doi: 10.1128/mcb.3.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelehrter T. D., Sznycer-Laszuk R., Zeheb R., Cwikel B. J. Dexamethasone inhibition of tissue-type plasminogen activator (tPA) activity: paradoxical induction of both tPA antigen and plasminogen activator inhibitor. Mol Endocrinol. 1987 Jan;1(1):97–101. doi: 10.1210/mend-1-1-97. [DOI] [PubMed] [Google Scholar]
- Goldfeld A. E., Firestone G. L., Shaw P. A., Gluecksohn-Waelsch S. Recessive lethal deletion on mouse chromosome 7 affects glucocorticoid receptor binding activities. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1431–1434. doi: 10.1073/pnas.80.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goustin A. S., Leof E. B., Shipley G. D., Moses H. L. Growth factors and cancer. Cancer Res. 1986 Mar;46(3):1015–1029. [PubMed] [Google Scholar]
- Grove J. R., Ringold G. M. Selection of rat hepatoma cells defective in hormone-regulated production of mouse mammary tumor virus RNA. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4349–4353. doi: 10.1073/pnas.78.7.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. C., Fischel R. E., Loeb J. N. Suppression of liver DNA synthesis by cortisone. Endocrinology. 1971 Jun;88(6):1471–1476. doi: 10.1210/endo-88-6-1471. [DOI] [PubMed] [Google Scholar]
- Henderson I. C., Loeb J. N. Hormone-induced changes in liver DNA synthesis: effects of glucocorticoids and growth hormone on liver growth and DNA polymerase activity. Endocrinology. 1974 Jun;94(6):1637–1643. doi: 10.1210/endo-94-6-1637. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Huang D. P., Schwartz C. E., Chiu J. F., Cook J. R. Dexamethasone inhibition of rat hepatoma growth and alpha-fetoprotein synthesis. Cancer Res. 1984 Jul;44(7):2976–2980. [PubMed] [Google Scholar]
- Knabbe C., Lippman M. E., Wakefield L. M., Flanders K. C., Kasid A., Derynck R., Dickson R. B. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987 Feb 13;48(3):417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Koontz J. W., Iwahashi M. Insulin as a potent, specific growth factor in a rat hepatoma cell line. Science. 1981 Feb 27;211(4485):947–949. doi: 10.1126/science.7008195. [DOI] [PubMed] [Google Scholar]
- Koontz J. W. The role of the insulin receptor in mediating the insulin-stimulated growth response in Reuber H-35 cells. Mol Cell Biochem. 1984;58(1-2):139–146. doi: 10.1007/BF00240613. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Frantz J. D., Strominger J. L., Mulligan R. C. Expression of human class II major histocompatibility complex antigens using retrovirus vectors. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2150–2154. doi: 10.1073/pnas.84.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loeb J. N., Borek C., Yeung L. L. Suppression of DNA synthesis in hepatoma cells exposed to glucocorticoid hormone in vitro. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3852–3856. doi: 10.1073/pnas.70.12.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb J. N., Rosner W. Fall in hepatic cytosol glucocorticoid receptor induced by stress and partial hepatectomy: evidence for separate mechanisms. Endocrinology. 1979 Apr;104(4):1003–1006. doi: 10.1210/endo-104-4-1003. [DOI] [PubMed] [Google Scholar]
- Marx J. L. The yin and yang of cell growth control. Science. 1986 May 30;232(4754):1093–1095. doi: 10.1126/science.3458306. [DOI] [PubMed] [Google Scholar]
- Massagué J., Blinderman L. A., Czech M. P. The high affinity insulin receptor mediates growth stimulation in rat hepatoma cells. J Biol Chem. 1982 Dec 10;257(23):13958–13963. [PubMed] [Google Scholar]
- Mercier L., Thompson E. B., Simons S. S., Jr Dissociation of steroid binding to receptors and steroid induction of biological activity in a glucocorticoid-responsive cell. Endocrinology. 1983 Feb;112(2):601–609. doi: 10.1210/endo-112-2-601. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Godowski P. J., Maler B. A., Yamamoto K. R. Glucocorticoid receptor mutants that define a small region sufficient for enhancer activation. Science. 1987 Apr 24;236(4800):423–427. doi: 10.1126/science.3563519. [DOI] [PubMed] [Google Scholar]
- Mira-y-Lopez R., Reich E., Stolfi R. L., Martin D. S., Ossowski L. Coordinate inhibition of plasminogen activator and tumor growth by hydrocortisone in mouse mammary carcinoma. Cancer Res. 1985 May;45(5):2270–2276. [PubMed] [Google Scholar]
- Moguilewsky M., Philibert D. RU 38486: potent antiglucocorticoid activity correlated with strong binding to the cytosolic glucocorticoid receptor followed by an impaired activation. J Steroid Biochem. 1984 Jan;20(1):271–276. doi: 10.1016/0022-4731(84)90216-4. [DOI] [PubMed] [Google Scholar]
- Mottola C., Czech M. P. The type II insulin-like growth factor receptor does not mediate increased DNA synthesis in H-35 hepatoma cells. J Biol Chem. 1984 Oct 25;259(20):12705–12713. [PubMed] [Google Scholar]
- Otto A. M., Natoli C., Richmond K. M., Iacobelli S., Jimenez de Asua L. Glucocorticoids inhibit the stimulatory effect of epidermal growth factor on the initiation of DNA synthesis. J Cell Physiol. 1981 Apr;107(1):155–163. doi: 10.1002/jcp.1041070117. [DOI] [PubMed] [Google Scholar]
- Platt E. J., Karlsen K., Lopez-Valdivieso A., Cook P. W., Firestone G. L. Highly sensitive immunoadsorption procedure for detection of low-abundance proteins. Anal Biochem. 1986 Jul;156(1):126–135. doi: 10.1016/0003-2697(86)90163-6. [DOI] [PubMed] [Google Scholar]
- Ricca G. A., McLean J. W., Taylor J. M. Kinetics of induction of alpha 1-acid glycoprotein. Ann N Y Acad Sci. 1982;389:88–105. doi: 10.1111/j.1749-6632.1982.tb22127.x. [DOI] [PubMed] [Google Scholar]
- Richman R. A., Benedict M. R., Florini J. R., Toly B. A. Hormonal regulation of somatomedin secretion by fetal rat hepatocytes in primary culture. Endocrinology. 1985 Jan;116(1):180–188. doi: 10.1210/endo-116-1-180. [DOI] [PubMed] [Google Scholar]
- Richman R. A., Claus T. H., Pilkis S. J., Friedman D. L. Hormonal stimulation of DNA synthesis in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3589–3593. doi: 10.1073/pnas.73.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G., Schmid W., Strange C. M., Röwekamp W., Schütz G. Isolation of cDNA clones coding for rat tyrosine aminotransferase. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7205–7208. doi: 10.1073/pnas.79.23.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid W., Müller G., Schütz G., Gluecksohn-Waelsch S. Deletions near the albino locus on chromosome 7 of the mouse affect the level of tyrosine aminotransferase mRNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2866–2869. doi: 10.1073/pnas.82.9.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segaloff A. Hormones and breast cancer. Recent Prog Horm Res. 1966;22:351–379. doi: 10.1016/b978-1-4831-9825-5.50012-9. [DOI] [PubMed] [Google Scholar]
- Shipley G. D., Childs C. B., Volkenant M. E., Moses H. L. Differential effects of epidermal growth factor, transforming growth factor, and insulin on DNA and protein synthesis and morphology in serum-free cultures of AKR-2B cells. Cancer Res. 1984 Feb;44(2):710–716. [PubMed] [Google Scholar]
- Takehara K., LeRoy E. C., Grotendorst G. R. TGF-beta inhibition of endothelial cell proliferation: alteration of EGF binding and EGF-induced growth-regulatory (competence) gene expression. Cell. 1987 May 8;49(3):415–422. doi: 10.1016/0092-8674(87)90294-7. [DOI] [PubMed] [Google Scholar]
- Thompson E. B., Aviv D., Lippman M. E. Variants of HTC cells with low tyrosine aminotransferinase inducibility and apparently normal glucorticoid receptors. Endocrinology. 1977 Feb;100(2):406–419. doi: 10.1210/endo-100-2-406. [DOI] [PubMed] [Google Scholar]
- Thompson E. B., Granner D. K., Gelehrter T., Erickson J., Hager G. L. Unlinked control of multiple glucocorticoid-induced processes in HTC cells. Mol Cell Endocrinol. 1979 Sep;15(3):135–150. doi: 10.1016/0303-7207(79)90034-0. [DOI] [PubMed] [Google Scholar]
- Thorndike J., Reif-Lehrer L. A sensitive assay for glutamyltransferase. Enzyme. 1971;12(2):235–241. doi: 10.1159/000459536. [DOI] [PubMed] [Google Scholar]
- WINTER C. A., SILBER R. H., STOERK H. C. Production of reversible hyperadrenocortinism in rats by prolonged administration of cortisone. Endocrinology. 1950 Jul;47(1):60–72. doi: 10.1210/endo-47-1-60. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]