Abstract
Background Post-hepatectomy liver failure (PHLF) has been defined by the International Study Group for Liver Surgery (ISGLS). The purpose of the present study was to examine the kinetics of conventional liver function tests (LFT) after a major liver resection and is the first to examine their utility in predicting PHLF in groups defined by the ISGLS.
Methods Consecutive patients undergoing a major liver resection for colorectal liver metastases were stratified into ISGLS groups and their LFT up to 1 year after surgery compared. Receiving-operating characteristic (ROC) analysis of LFT identified optimal thresholds in predicting category C liver failure.
Results In total, 32, 22 and 19 patients belonged to ISGLS groups A, B and C, respectively. The median international normalized ratio (INR) and bilirubin values on post-operative days 1, 3, 5 and 7 were significantly different among the groups (all P-values <0.05). ROC analysis of day 1 INR (AUC 0.813) and day 5 bilirubin (AUC 0.798) revealed thresholds of 1.35 and 52 μmol/l to have sensitivities of 85% and 81% and specificities of 63% and 73%, respectively, to predict group C liver failure.
Discussion Post-operative LFT after a major liver resection differs significantly among the three ISGLS groups. Thresholds of bilirubin and INR can be used to identify patients who are at a maximum risk of complications.
Introduction
Post-hepatectomy liver failure (PHLF) remains an important cause of morbidity and mortality after a major liver resection1–4 which is performed increasingly for curative resection of colorectal liver metastases (CRLM) in the western world.5–8 Monitoring liver function after surgery is essential to prevent, recognize, prognosticate and manage PHLF. While there are many excellent quantitative9–12 and volumetric studies13 that address this issue, conventional biochemical liver function tests (LFT) evaluated by routine blood test analysis remain widely used and form an indispensible part of most definitions of liver failure.14–16 The International Study Group for Liver Surgery (ISGLS) has recently proposed a definition for liver failure, grading patients into three groups based on the clinical severity of their post-operative hospital course.17 There are a few contemporary studies that have evaluated the long-term kinetics of post-operative biochemical LFT after a major liver resection18,19 and none to the authors knowledge in groups stratified as per the ISGLS definition. Most studies also include major as well as minor resection for primary hepatobiliary malignancies, metastases and benign conditions. Earlier studies20,21 do not provide a comparison of LFT in patients with and without surgery-related morbidity and may not be representative of the current population. Finally, background liver function and pre-existing liver disease will affect the post-operative course of LFT and likelihood of PHLF. Thresholds of LFT to predict PHLF using a common definition will vary between patients with different pathology. The objectives of this study were therefore twofold: (i) to examine the kinetics of conventional LFT for 1 year in patients who underwent a major liver resection for CRLM stratified into three groups as per the ISGLS guidelines for post-hepatectomy liver failure and (ii) to identify the utility of LFT in predicting category C PHLF in patients undergoing resection solely for CRLM.
Methodology
Patients
This study is a retrospective analysis of a prospective liver surgery database maintained at St James's University Hospital, Leeds, UK. Consecutive patients who underwent a major liver resection (defined as resection of three or more Couinaud's segments) by a single surgeon (J.P.A.L.) for CRLM from January 2005 to December 2010 were identified (n = 77). Patients who underwent resection for other diagnoses, e.g. hepatocellular carcinoma and cholangiocarcinoma were excluded. Patients who developed post-operative jaundice as a result of causes other than parenchymal insufficiency (e.g. drug induced/mechanical biliary obstruction) or whose notes were incomplete were excluded (n = 4). Patient management was discussed at a multidisciplinary team meeting to plan optimal pre-operative treatment, a resection strategy and post-operative care pathways.
Case records were reviewed to examine the clinical course of patients to classify them into the three groups as per the ISGLS proposal.17 Briefly, patients were classified into Group A if they had a normal clinical course or an inconsequential transient elevation of LFT. Group B patients included those who required a change in their clinical management, e.g. a longer stay in the high-dependency unit, the use of diuretics for ascites, non-invasive ventilation etc. Group C patients needed invasive management, e.g. the use of inotropes/invasive ventilation and percutaneous catheter drainage of ascites/pleural effusion. The electronic results server was searched to retrieve the values of bilirubin, alanine aminotransferase (ALT), alkaline phosphatase (ALP), albumin, international normalized ratio (INR), platelet count and C-reactive protein (CRP) pre-operatively, on the day of surgery and on post-operative days 1, 3, 5, 7 and 10. Also all these values (except CRP) were obtained routinely as per follow-up protocols at 3, 6 months and 1 year after surgery. The hospital reference range for liver function tests are as follows: bilirubin 1.7–17 μmoL/l, ALT 9–40 U/l, ALP 44–147 IU/l, albumin 35–50 g/l, INR 0.8–1.2, platelet count 150–400 000/μl and CRP 5–119 mg/l.
Data analysis
The median values of each investigation were plotted for each group at the above-mentioned time points to obtain graphs displaying their comparative kinetics. The course of LFT in the three ISGLS groups in the first post-operative week was compared. Statistical analysis was performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Categorical variables that might influence LFT or hospital course were compared among the three ISGLS groups using Pearson's chi-squared test. Age > 70 years, gender, the presence of co-morbidities (diabetes, hypertension and coronary artery disease), the use of Pringle's manoeuvre, the use of adjuvant chemotherapy (for primary colorectal cancer) prior to surgery, a pre-operative neutrophil–lymphocyte ratio and liver histology of the resected specimen (steatosis/fibrosis/sinusoidal obstructive syndrome) were the variables compared. Continuous variables were expressed as the median, range. LFT on post-operative days 1, 3, 5 and 7 were compared among the three ISGLF groups using the Kruskal–Wallis test. Receiver-operating characteristic (ROC) curves were plotted to identify optimal thresholds for LFT variables found to be significantly different between ISGLS groups. The area under the curve (AUC), sensitivity and specificity of various thresholds were computed. A P-value of less than 0.05 was considered significant.
Results
A total of 73 patients satisfied the selection criteria for this study. There were 47 males and 26 females. Thirty-two patients had an uncomplicated hospital course with only transient elevation of bilirubin and INR and belonged to group A. There were 22 and 19 patients in groups B and C, respectively. Table 1 gives a comparison of variables that might influence LFT and hospital course among the three groups. The rate of complications was the only parameter that was significantly different among the three groups (P = 0.011).
Table 1.
Comparison of variables by International Study Group for Liver Surgery groups after a major liver resection for colorectal liver metastases
| Variable | Group A | Group B | Group C | P-valuea |
|---|---|---|---|---|
| Number of patients, n | 32 | 22 | 19 | |
| Age>70 years, n (%) | 7 (21.9) | 9 (40.9) | 6 (31.6) | .322 |
| Gender (M : F) | 22:10 | 13:9 | 12:7 | .761 |
| Comorbidityb, n (%) | 12 (37.5) | 11 (50) | 6 (31.6) | .458 |
| Resection of 4 or more segments | 18 (56.3) | 12 (54.5) | 13 (68.4) | .614 |
| Resection of 5 or more segments | 9 (28.1) | 6 (27.3) | 4 (21.1) | .603 |
| Synchronous colonic resection | (0) | 2 (9.1) | 1 (5.3) | .264 |
| Pringle manoeuvre, n (%) | 20 (62.5) | 13 (59.1) | 15 (78.9) | .521 |
| Intra-operative transfusion | 1 (3.1) | 1 (4.5) | 1 (5.3) | .935 |
| Complications, n (%) | 3 (9.4) | 7 (31.8) | 8 (42.1) | .011 |
| Adjuvant chemotherapy for primary tumour, n (%) | 16 (50) | 9 (40.1) | 6 (31.6) | .186 |
| Pre-operative NLR > 5, n (%) | 6 (18.6) | 5 (22.6) | 5 (26.3) | .837 |
| Liver histologyc, n (%) | 22 (68.8) | 12 (54.5) | 10 (52.6) | .535 |
NLR, neutrophil to lymphocyte ratio.
Pearson's chi-squared test.
Diabetes, hypertension or coronary artery disease.
Steatosis, fibrosis or sinusoidal obstructive syndrome.
A comparison of LFT, platelet count and CRP in the first week after surgery is given in Table 2. Only bilirubin and INR levels were repeatedly and significantly different between the three groups within the first week after a liver resection. The trends of bilirubin and INR levels after resection are depicted in Fig. 1a–b. The median bilirubin of group A remained mostly less than 20 μmol/l during the first week after surgery and this trend continued to 1 year of follow-up. Patients in group B had a bilirubin of 46 μmol/l on day 5 and a peak of 69 μmol/l on day 10 after which the levels dropped to the normal range at the first follow-up at 3 months. Group C patients had a steady climb of bilirubin with values of 92 μmol/l on day 5, a peak of 112 μmol/l on day 10 and a steep decline after 2 weeks to normal levels at 3 months (Fig. 1a).
Table 2.
Comparison of biochemical parameters in the first week in the three (International Study Group for Liver Surgery) groups after a major liver resection for colorectal liver metastases
| Post-operative parameter | ISGLS group | Day 1 | Day 3 | Day 5 | Day 7 |
|---|---|---|---|---|---|
| Bilirubin μmol/l | A | 23.5 (8–60) | 24.5 (12–75) | 22.0 (7–93) | 17.0 (7–123) |
| B | 30.0 (13–140) | 42.5 (13–82) | 43.0 (12–115) | 38.5 (15–156) | |
| C | 43.5 (13–104) | 56.0 (8–126) | 68.0 (10–206) | 109.0 (13–335) | |
| P = | 0.007 | 0.001 | 0.004 | 0.001 | |
| Alanine amino-transferase U/l | A | 185.0 (48–435) | 121.0 (29–284) | 86.0 (44–154) | 70.0 (25–173) |
| B | 238.5 (114–904) | 128.0 (47–516) | 93.5 (31–226) | 86.0 (26–155) | |
| C | 225.0 (37–1344) | 113.0 (69–1122) | 98.0 (52–610) | 83.0 (49–301) | |
| P = | .115 | .587 | .508 | .175 | |
| Alkaline phosphatase IU/l | A | 198.5 (84–386) | 208.5 (100–2472) | 389.5 (186–776) | 454.0 (240–1137) |
| B | 209.5 (90–748) | 222.0 (104–663) | 358.0 (106–985) | 833.0 (224–1870) | |
| C | 163.0 (106–693) | 198.0 (104–499) | 358.0 (115–874) | 631.0 (225–2740) | |
| P = | .646 | .879 | .542 | .326 | |
| Albumin g/l | A | 29.5 (24–43) | 35.0 (29–44) | 36.0 (31–48) | 36.0 (32–44) |
| B | 30.0 (20–43) | 32.0 (28–41) | 34.0 (25–42) | 33.5 (27–38) | |
| C | 29.5 (26–36) | 36.0 (22–39) | 34.5 (26–45) | 34.0 (27–40) | |
| P = | .899 | 0.041 | 0.025 | .129 | |
| International normalized ratio | A | 1.4 (1.0–1.8) | 1.4 (1.0–2.2) | 1.2 (1.0–1.5) | 1.1 (1.0–1.2) |
| B | 1.5 (1.0–2.2) | 1.8 (1.2–3.0) | 1.4 (1.0–2.4) | 1.2 (0.9–2.0) | |
| C | 1.8 (1.6–2.6) | 1.7 (1.4–2.8) | 1.6 (1.2–1.9) | 1.4 (1.0–1.6) | |
| P = | 0.000 | 0.017 | 0.001 | 0.031 | |
| Platelet count ×1000/μl | A | 219.0 (107–325) | 167.5 (95–227) | 224.0 (142–362) | 294.5 (186–473) |
| B | 191.0 (112–354) | 171.5 (100–333) | 204.0 (140–454) | 285.0 (155–551) | |
| C | 189.0 (72–349) | 155.0 (57–272) | 193.0 (93–332) | 202.5 (103–470) | |
| P = | .437 | .865 | .556 | 0.050 | |
| C-reactive protein mg/l | A | 29.0 (12–115) | 86.0 (19–194) | 51.0 (29–138) | 37.0 (20–44) |
| B | 29.0 (14–204) | 69.0 (22–158) | 55.0 (17–240) | 70.0 (24–196) | |
| C | 31.0 (11–96) | 57.0 (28–159) | 49.0 (6–224) | 63.0 (9–245) | |
| P = | .950 | .217 | .948 | .079 |
ISGLS, International Study Group for Liver Surgery. Values in cells represent median (range) and P-value by Kruskal–Wallis test.
Figure 1.
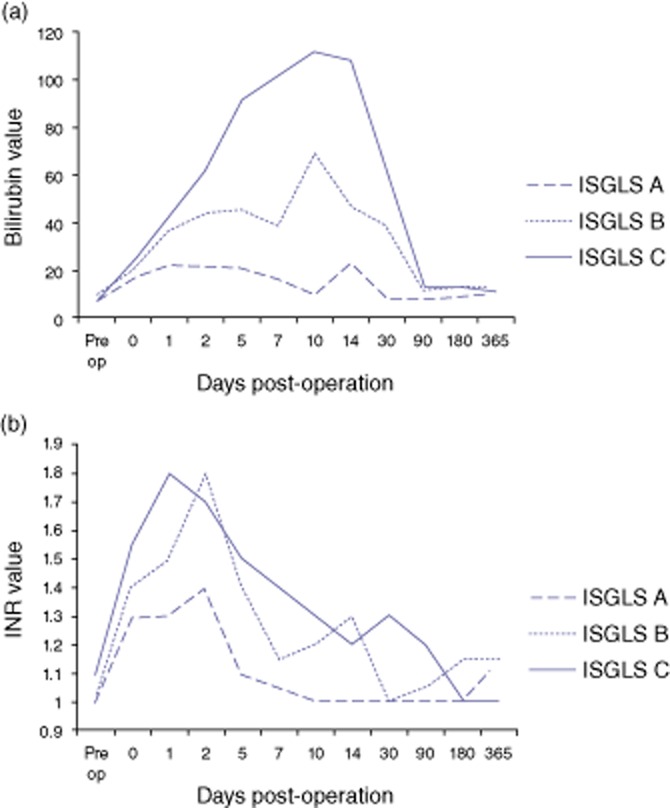
Post-operative kinetics of bilirubin (a) and the international normalized ratio (INR) (b) after a major liver resection for colorectal liver metastases. The median values are plotted over time for patients grouped as per the International Study Group for Liver Surgery
The median INR values peaked at 1.4 on day 3, 1.8 on day 3, 1.8 on day 1 for groups A, B and C, respectively. After day 5, the INR decreased steadily in all groups with values at 1, 1.2 and 1.3 on day 10 in groups A, B and C, respectively. Group C patients had normal values at the second follow-up at 6 months after surgery (Fig. 1b). There was no significant or sustained difference in the kinetics of other parameters measured between the three groups (data not presented).
A comparison of ROC curves for INR and bilirubin at post-operative days 1, 3, 5 and 7 is given in Table 3. The optimum times to identify patients with group C liver failure utilized INR values at post-operative day 1 and bilirubin values at day 5 (Fig. 2). An INR of 1.35 on day 1 had a sensitivity and specificity of 85% and 63%, respectively, in predicting group C liver failure. A bilirubin of 52 on day 5 had a sensitivity and specificity of 81% and 73%, respectively, in predicting group C liver failure.
Table 3.
Comparison of receiver-operating curve (ROC) characteristics for the international normalized ration (INR) and bilirubin at post-operative days 1, 3, 5 and 7 to predict category C liver failure. The largest AUC for INR and bilirubin levels were observed on post operative days 1 and 5, respectively (in bold)
| Post-operative day | AUC | Standard error | P-value | 95% CI of AUC | |
|---|---|---|---|---|---|
| Bilirubin | 1 | .678 | .086 | .049 | .510 to 0.847 |
| 3 | .744 | .087 | .007 | .574 to 0.914 | |
| 5 | 0.798 | 0.076 | 0.001 | 0.649 to 0.946 | |
| 7 | .739 | .105 | .040 | .534 to 0.945 | |
| INR | 1 | 0.813 | 0.065 | 0.001 | 0.685 to 0.941 |
| 3 | .705 | .091 | .042 | .527 to 0.883 | |
| 5 | .744 | .016 | .016 | .586 to 0.901 | |
| 7 | .718 | .106 | .062 | .511 to 0.926 |
AUC, area under curve.
Figure 2.
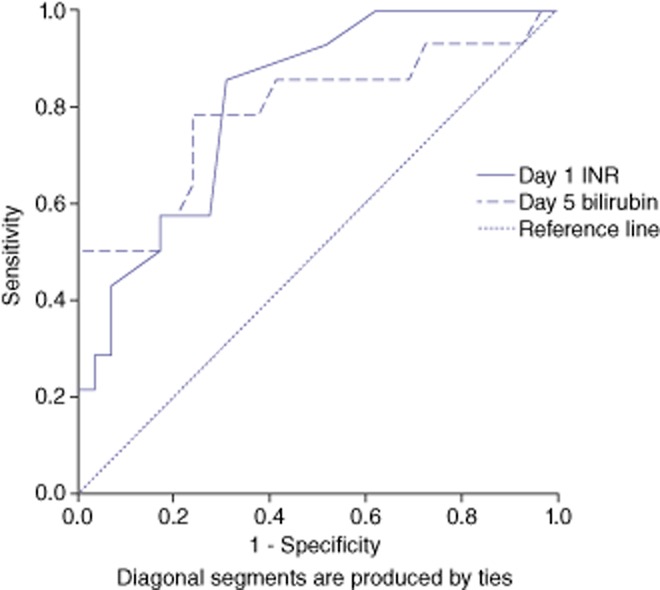
Receiver-operating characteristics (ROC) curves for post-operative day 1 international normalized ration (INR) and day 5 bilirubin demonstrating their utility in predicting Group C post-operative liver failure (as per the International Study Group for Liver Surgery) in patients undergoing a major liver resection for colorectal liver metastases
Discussion
This study was designed to evaluate post-operative LFT in groups stratified according to the ISGLS definition and assess their utility in predicting category C liver failure. Although it is a retrospective series with small numbers, there is a statistically significant difference in the kinetics of LFT in the three groups in the first post-operative week and this trend persists when followed over a period of 1 year. Previous studies have examined the course of LFT in patients undergoing a liver resection. These have included patients undergoing a major as well as minor resection and included patients with all diagnoses.20,21 In the majority of studies, the duration of follow-up has been for a short period of time. In a recent study19, the post-operative changes in LFT in a cohort of 835 patients undergoing liver resection identified that the serum bilirubin and INR differed based on the extent of resection. Furthermore, these were significantly affected by complications irrespective of the extent of resection. This study included 172 patients who underwent major liver resection for CRLM; however, detailed analysis of this subgroup was not provided. It was concluded that biochemical data may help recognize surgery-related complications early during the post-operative course and serve as the basis for the definition of complications after a hepatic resection. In the current series, a homogenous group of patients who underwent a major liver resection for CRLM were included. The number of patients who underwent a resection of four or five segments was not different among the three groups. The degree of hepatic steatosis, cirrhosis or sinusoidal obstruction syndrome was not different in the biopsy specimens among the three groups (Table 1). The patients in this study group had ‘normal livers’ pre-operatively. Patients undergoing a resection for non-CRLM (such as hilar cholangiocarcinoma or hepatocellular carcinoma) were excluded to avoid confounding factors related to pre-operatively deranged LFT and background liver dysfunction. The senior author follows a policy of upfront surgery when the metastases are resectable. So chemotherapy induced hepatotoxicity was not a problem. It was not possible to control for administration of adjuvant chemotherapy for the primary tumour but the proportion receiving this was not significantly different among the three ISGLS groups. Although volumetry and portal vein embolization were not used, this factor could not have differentially impacted the post-operative LFT in the three groups as the extent of resection was not different. Operative factors such as use the Pringle's manoeuvre, blood loss, etc. were not different among the three groups. All procedures were performed by the senior author (J.P.A.L.) and this also reduced variation as a result of surgeon expertise. Thus, the present study analysed LFT in patients stratified not according to the extent of resection or underlying disease severity but according to the severity of their post-operative clinical course as reflected by ISGLS categorization.
Bilirubin and INR have been well documented to predict PHLF and outcome after a liver resection.13–15,,22 What is novel is the fact that, to the authors' knowledge, this is the first study that documents that bilirubin and INR in the first week after a major liver resection are significantly different in the three categories of liver failure as defined by the ISGLS. This difference in kinetics of LFT persists well into the post-operative course. It is noteworthy that bilirubin levels in group B and C patients continued to be elevated in the second week and took up to 3 months to return to normal levels. This implies that it would be useful to mark out patients who are likely to have category C liver failure in the first week and direct resources towards them. The courses of ALT, ALP, platelets and albumin have less clinical importance in this context. CRP levels were not significantly different in the three groups in this study. Post-operative CRP levels on days 1 and 3 have been demonstrated to be significantly lower in patients with extended resections and in those with PHLF.23 CRP seems to be of value in early differentiation of those with and without liver failure but of limited value in further stratification of these patients into ISGLS groups probably because it reflects the synthetic function of the liver in addition to the regenerative process and also varies with the presence of complications.
This study has followed LFT for 1 year after surgery. It is noteworthy that bilirubin levels in group B and C patients continued to be elevated in the second week and took up to 3 months to return to normal levels. The presented study group included patients undergoing an extensive resection (five or more Couinaud's segments). The proportion of patients undergoing an extensive resection was not different between the ISGLS groups although the only two post-operative deaths occurred in patients with an extensive resection and within ISGLS group C.
The utility of bilirubin and INR in predicting PHLF is examined by this study. ROC curves identified that post-operative day 1 INR and day 5 bilirubin values presented the highest sensitivity and specificity in predicting group C liver failure. The bilirubin value of 52 μmol/l at post-operative day 5 identified in the present study matches that of Balzan et al. 14 In that study the conjunction of prothrombin time of <50% and bilirubin > 50 μmol/l on post-operative day 5 was a strong predictive factor of mortality which have since become popular as the ‘50-50’ criteria and have been prospectively validated.24 Mullen et al. 16 in a study of major liver resection in 1059 patients with non-cirrhotic livers showed by ROC analysis that a post-operative bilirubin of more than 7 mg/dl and INR of more than 2 were predictors of 90-day mortality. When these higher cut-off values were applied to the presented study population, it resulted in high specificities but low sensitivities for detection of group C liver failure. While the aforementioned studies used different thresholds of bilirubin and INR to define PHLF and predict mortality, the present study has used the ISGLS definition for liver failure and attempted to identify thresholds that identify the most critical subset of patients with liver failure (group C).
Whether the low specificity of thresholds of INR and bilirubin in this study on post-operative days 1 and 5 affect clinical utility, is a point that merits discussion. The conclusions of this paper are not practice changing and as has been the case with previous studies, biochemical tests alone cannot infallibly predict post-operative liver failure. Nevertheless, the data presented details the sensitivity and specificity of these two simple laboratory markers and thus their clinical utility in predicting ISGLS category C liver failure can be quantified. Day 1 INR > 1.35 would identify 85% of the people with category C PHLF, whereas day 5 bilirubin would do the same in 81% of the patients. Whereas, with the same thresholds for INR and bilirubin only 63% and 73% of patients without category C PHLF would be correctly identified as not having this condition. Thus, the false-positive rate (number of ‘normal people’ diagnosed to have PHLF) is high. An INR of > 1.35 on day 1 would falsely categorize 37% of the patients as at risk for category C PHLF; a day 5 bilirubin > 52 would have a false positivity of 27%. This does not negate their clinical utility as over diagnosing a serious problem (low specificity) is less of a problem than missing it (low sensitivity). The issue of low specificity and heterogeneity is also inherent in the ISGLS definition of PHLF. It is an inclusive definition designed to identify serious problems that affect the patient's hospital course and outcome. For instance, a patient with a minor bile leak from the transection surface can require image-guided percutaneous catheter placement. This may be a minor inconvenience clinically; however, it does place the patient in category C. In the present study, this definition has been utilized as it provides a standardized platform for reporting and comparison of outcomes and shown that it definitely stratifies patients into true groups as far the LFT are concerned.
If the study had incorporated greater numbers of patients multivariate analysis would have been feasible and possibly strengthened the results. However, even with the numbers of subjects in this study, the paper has clearly demonstrated what it set out to prove – the post-operative kinetics of LFT in patients undergoing major liver function are different in patients stratified into the three ISGLS groups. While no method is perfect in predicting patients who would develop PHLF, simple LFT are indispensible and this paper adds perspective to their clinical utility.
To conclude, this study examines the long-term post-operative kinetics of LFT and their utility in predicting PHLF in patients undergoing a liver resection. Its strengths are the inclusion of only patients who underwent a major liver resection (who are most vulnerable to develop this complication) for CRLM (the most common indication for liver resection in the western population). This study has also used a standard definition of PHLF recently proposed by the ISGLS and quantified the utility of simple laboratory tests (day 1 INR and day 5 bilirubin) in predicting patients who develop group C liver failure (the group of patients that need most intervention).
Conflicts of interest
None declared.
References
- Laurent C, Sa CA, Couderc P, Rullier E, Saric J. Influence of postoperative morbidity on long-term survival following liver resection for colorectal metastases. Br J Surg. 2003;90:1131–1136. doi: 10.1002/bjs.4202. [DOI] [PubMed] [Google Scholar]
- Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708. doi: 10.1097/01.sla.0000141195.66155.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider PD. Preoperative assessment of liver function. Surg Clin North Am. 2004;84:355–373. doi: 10.1016/S0039-6109(03)00224-X. [DOI] [PubMed] [Google Scholar]
- van den Broek MA, Olde Damink SW, Dejong CH, Lang H, Malago M, Jalan R, et al. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:767–780. doi: 10.1111/j.1478-3231.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- Chun YS, Vauthey JN, Ribero D, Donadon M, Mullen JT, Eng C, et al. Systemic chemotherapy and two-stage hepatectomy for extensive bilateral colorectal liver metastases: perioperative safety and survival. J Gastrointest Surg. 2007;11:1498–1504. doi: 10.1007/s11605-007-0272-2. [DOI] [PubMed] [Google Scholar]
- Khatri VP, Petrelli NJ, Belghiti J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol. 2005;23:8490–8499. doi: 10.1200/JCO.2004.00.6155. [DOI] [PubMed] [Google Scholar]
- Lodge JP, Menon KV, Fenwick SW, Prasad KR, Toogood GJ. In-contiguity and non-anatomical extension of right hepatic trisectionectomy for liver metastases. Br J Surg. 2005;92:340–347. doi: 10.1002/bjs.4830. [DOI] [PubMed] [Google Scholar]
- Wicherts DA, de Haas RJ, Andreani P, Ariche A, Salloum C, Pascal G, et al. Short- and long-term results of extended left hepatectomy for colorectal metastases. HPB. 2011;13:536–543. doi: 10.1111/j.1477-2574.2011.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du ZG, Li B, Wei YG, Yin J, Feng X, Chen X. A new scoring system for assessment of liver function after successful hepatectomy in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2011;10:265–269. doi: 10.1016/s1499-3872(11)60044-1. [DOI] [PubMed] [Google Scholar]
- Redaelli CA, Dufour JF, Wagner M, Schilling M, Husler J, Krahenbuhl L, et al. Preoperative galactose elimination capacity predicts complications and survival after hepatic resection. Ann Surg. 2002;235:77–85. doi: 10.1097/00000658-200201000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmann M, Lock JF, Riecke B, Heyne K, Martus P, Fricke M, et al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann Surg. 2009;250:119–125. doi: 10.1097/SLA.0b013e3181ad85b5. [DOI] [PubMed] [Google Scholar]
- Sugimoto H, Okochi O, Hirota M, Kanazumi N, Nomoto S, Inoue S, et al. Early detection of liver failure after hepatectomy by indocyanine green elimination rate measured by pulse dye-densitometry. J Hepatobiliary Pancreat Surg. 2006;13:543–548. doi: 10.1007/s00534-006-1114-4. [DOI] [PubMed] [Google Scholar]
- Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540–548. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The ‘50-50 criteria’ on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon KV, Al Mukhtar A, Aldouri A, Prasad RK, Lodge PA, Toogood GJ. Outcomes after major hepatectomy in elderly patients. J Am Coll Surg. 2006;203:677–683. doi: 10.1016/j.jamcollsurg.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Ibrahim S, Chen CL, Wang CC, Wang SH, Lin CC, Liu YW, et al. Liver regeneration and splenic enlargement in donors after living-donor liver transplantation. World J Surg. 2005;29:1658–1666. doi: 10.1007/s00268-005-0101-2. [DOI] [PubMed] [Google Scholar]
- Reissfelder C, Rahbari NN, Koch M, Kofler B, Sutedja N, Elbers H, et al. Postoperative course and clinical significance of biochemical blood tests following hepatic resection. Br J Surg. 2011;98:836–844. doi: 10.1002/bjs.7459. [DOI] [PubMed] [Google Scholar]
- Ezaki T, Koyanagi N, Toyomasu T, Ikeda Y, Sugimachi K. Natural history of hepatectomy regarding liver function: a study of both normal livers and livers with chronic hepatitis and cirrhosis. Hepatogastroenterology. 1998;45:1795–1801. [PubMed] [Google Scholar]
- Pelton JJ, Hoffman JP, Eisenberg BL. Comparison of liver function tests after hepatic lobectomy and hepatic wedge resection. Am Surg. 1998;64:408–414. [PubMed] [Google Scholar]
- Bismuth H, Houssin D, Mazmanian G. Postoperative liver insufficiency: prevention and management. World J Surg. 1983;7:505–510. doi: 10.1007/BF01655941. [DOI] [PubMed] [Google Scholar]
- Rahman SH, Evans J, Toogood GJ, Lodge PA, Prasad KR. Prognostic utility of postoperative C-reactive protein for posthepatectomy liver failure. Arch Surg. 143:247–253. doi: 10.1001/archsurg.2007.75. [DOI] [PubMed] [Google Scholar]
- Paugam-Burtz C, Janny S, Delefosse D, Dahmani S, Dondero F, Mantz J, et al. Prospective validation of the ‘fifty-fifty’ criteria as an early and accurate predictor of death after liver resection in intensive care unit patients. Ann Surg. 2008;249:124–128. doi: 10.1097/SLA.0b013e31819279cd. [DOI] [PubMed] [Google Scholar]


