Abstract
Background An early prediction of poor outcomes is essential in the management of patients after a liver resection. The aim of this study was to evaluate the role of selected biochemical parameters on post-operative day 1 (POD 1) in the prediction of morbidity and mortality after a liver resection for colorectal metastases.
Method This retrospective study was based on 236 major liver resections for colorectal metastases performed between 2006 and 2011. Results of biochemical tests of blood samples obtained on POD 1 were assessed as predictors of primary outcome measures (hepatic and overall morbidity, 90-day mortality) using multiple regression and receiver-operating characteristics (ROC).
Results Hepatic morbidity, overall morbidity and 90-day mortality rates were 18.6%, 28.0% and 4.7%, respectively. On the basis of multiple regression analysis and comparisons of the prediction models, serum bilirubin was selected for the prediction of hepatic (>2.05 mg/dl, sensitivity 69.2%, specificity 71.2%) and overall (>2.05 mg/dl, sensitivity 61.1% and specificity 71.2%) morbidity, and aspartate aminotransferase (AST) was selected for the prediction of 90-day mortality (>798 U/l, sensitivity 62.5% and specificity 90.4%).
Discussion Biochemical analyses of blood on POD1 enables stratification of patients into low- and high-risk groups for negative outcomes, with serum bilirubin associated with overall and hepatic morbidity and AST associated with mortality.
Introduction
A liver resection is the only potentially radical treatment modality in patients with metastases from colorectal cancer. Initially, it was offered only to selected patients according to the number and distribution of lesions.1 However, the criteria for choosing resection have significantly evolved in recent years, and currently, the possibility of oncologically radical removal of all lesions with special consideration of the future hepatic remnant volume determines a patient's eligibility for resective surgery.2 This aggressive approach in the treatment of patients with metastatic colorectal cancer is supported by recently reported 5-year overall and recurrence-free survival rates of 45.2%–58% and 15%–26.2%, respectively, similar to or higher than the rates in the period of stricter qualification.3–5
The previously reported post-operative mortality and morbidity rates associated with a liver resection vary widely from 0% to 8.2% and 14.4% to 47.0%, respectively.6–11 Pre- and operative factors leading to an increased risk of death in the immediate post-operative period have been extensively investigated and include the presence of selected comorbid conditions or a high Charlson comorbidity index, patient's age, pre-existing liver dysfunction, operative blood transfusions and extent of liver resection, among others.7,8,12,13 Given the relatively stable mortality rates and a tendency towards increasing morbidity rates,3 early prediction and the management of post-operative complications is crucial for the improvement of short-term outcomes. Considering pre-operative factors, significant predictors of morbidity include the American Society of Anesthesiologists score, increased body mass index, smoking and selected biochemical parameters, among others.7,11,14 Although pre-operative risk stratification based on these factors is useful in the evaluation of a patient's eligibility for a liver resection, the prediction of morbidity and mortality based on post-operative factors is potentially more accurate, as such factors reflect the combined influence of pre-operative status and intra-operative course. Therefore, several post-operative risk scores were established previously. These include definitions of post-hepatectomy liver failure: peak post-operative serum bilirubin >7 mg/dl;15 the ‘50–50 criteria’ (serum bilirubin concentration >50 μmol/l and prothrombin time <50%);16 and a score recently proposed by the International Study Group of Liver Surgery [ISGLS criteria, increased serum bilirubin concentration and INR (international normalized ratio)].17 Notably, both the ‘50-50 criteria’ and ISGLS definitions are based on evaluation of serum biochemical tests performed no earlier than post-operative day (POD) 5.
There are few up-to-date studies concerning normal fluctuations of biochemical blood parameters after a liver resection. According to Reissfelder et al., serum bilirubin, the (INR), and activity of aspartate (AST) and alanine (ALT) aminotransferase usually return to normal values during the first 5 to 7 post-operative days after an immediate post-operative increase.18 However, significant differences regarding some of these factors, particularly serum bilirubin, may be observed as early as POD 1 between patients with and without post-operative complications. The aim of this study was to evaluate the prognostic significance of the serum bilirubin concentration, INR, AST and ALT on POD 1 for mortality and morbidity after a liver resection, with a special focus on the occurrence of hepatic complications.
Methods
This retrospective cohort study was based on data from 236 patients after a major liver resection for colorectal metastases performed between January 2006 and March 2011 in the Department of General, Transplant and Liver Surgery, Medical University of Warsaw (Poland). Major resections were defined as removal of three or more segments according to Couinaud's divisions. Patients undergoing a liver resection combined with radiofrequency or crioablation (n = 10) procedures were excluded. The exact details of the pre-operative assessment and operative technique were previously reported.19 Briefly, all patients underwent ultrasonography, computed tomography and/or magnetic resonance imaging for evaluation of liver pathology and volume of the remnant liver post-hepatectomy. The minimum volume of the remnant liver in patients considered eligible for a liver resection was approximately 30%, depending on the results of pre-operative biochemical tests and quality of liver parenchyma assessed intra-operatively. The Pringle manoeuvre was used selectively when faced with increased bleeding. The serum bilirubin concentration, INR and activity of AST and ALT were evaluated routinely on POD 1 (12–18 h post-operatively), and again in most patients, on PODs 2–3. At later post-operative times, these biochemical tests were performed at various time intervals, depending on the post-operative course.
Both in-hospital and 90-day mortality rates were calculated, with the second being one of the primary outcome measures. Others included occurrence of overall and hepatic complications, a group comprising post-operative liver failure, delayed recovery of liver function, biliary leak and subphrenic abscess. Patients with post-operative liver failure were defined as those with peak serum bilirubin levels exceeding 7 mg/dl, while delayed recovery of liver function was defined by occurrence of any of the clinical signs of liver insufficiency with a peak serum bilirubin level below 7 mg/dl.
Associations between serum bilirubin concentration, INR, and activity of AST and ALT on POD 1 with overall and hepatic morbidity, as well as with 90-day mortality, were evaluated in univariate and multiple analyses. Moreover, univariate analyses were performed using logistic regression with splines functions for quantitative variables. Multiple logistic regression analysis [Logistic Procedure of the SAS System (SAS Institute, Cary, NC, USA)] and generalized additive models (gam function of S-Plus) were used to assess independent biochemical predictors of overall/hepatic complications and death within the 90-day post-operative period. Receiver operating characteristics (ROC) curves were used to indicate approximate cut-off values for independent predictors. Sensitivity and specificity rates, as well as positive (PPV) and negative (NPV) predictive values, were estimated. Hazard ratios (HRs) and areas under the curves (AUCs) are presented with 95% confidence intervals. The Mann–Whitney U-test was used for AUC comparisons. SAS System v. 9.3 and S-Plus v. 6.1 were applied for computing statistical analyses. The level of statistical significance was set at 0.05.
Results
Baseline characteristics of the study cohort are shown in Table 1. The overall morbidity rate was 28.0% (66 of 236), including the 18.6% (44 of 236) rate of patients developing hepatic complications. More specifically, postoperative liver failure occurred in 8 (3.4%) patients, delayed recovery of liver function in 26 (11.0%) patients, biliary leak in 12 (5.1%) patients, and subphrenic abscess in 2 (0.8%) patients. In-hospital and 90-day mortality rates were 2.1% (5 of 236) and 4.7% (11 of 236), respectively. The exact causes of death were only known for patients who died during post-operative hospitalization and included the following: post-operative liver failure in two patients and sepsis and peri-operative bleeding in one patient each. The fifth patient died during implantation of a Y-graft due to peripheral emboli.
Table 1.
Baseline characteristics of the study cohort
| Factors | All patients (n = 236) |
|---|---|
| Patient factors | |
| Gender | |
| Male | 126 (53.4) |
| Female | 110 (46.6) |
| Age | 60 (54–67) |
| Pre-operative biochemical parameters | |
| Serum bilirubin (mg/dl) | 0.6 (0.4–0.8) |
| INR | 1.01 (0.98–1.06) |
| AST (U/l) | 31 (24–39) |
| ALT (U/l) | 27 (20–39) |
| Comorbidity | |
| Diabetes mellitus | 29 (12.3) |
| Hypertension | 60 (25.4) |
| Cardiovascular disease | 16 (6.8) |
| COPD | 6 (2.5) |
| Hepatic steatosis | |
| Absent | 127 (53.8) |
| 30% or less | 90 (38.1) |
| 31–50% | 5 (2.1) |
| > 50% | 4 (1.7) |
| Missing | 10 (4.2) |
| Recurrence of liver metastases | 29 (12.3) |
| Operative factors | |
| Extent of resection | |
| Three segments | 16 (6.8) |
| Four segments | 139 (58.9) |
| Five segments | 77 (32.6) |
| Six segments | 4 (1.7) |
| Pringle manoeuvre | 74 (31.4) |
| Blood transfusions, yes | 50 (22.9)a |
| Plasma transfusions, yes | 43 (19.7)a |
| Total operative time (hours) | 4.3 (3.5–5.0) |
Data available for 218 patients.
Data are presented as n (%) or median (interquartile range); INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; COPD, chronic obstructive pulmonary disease.
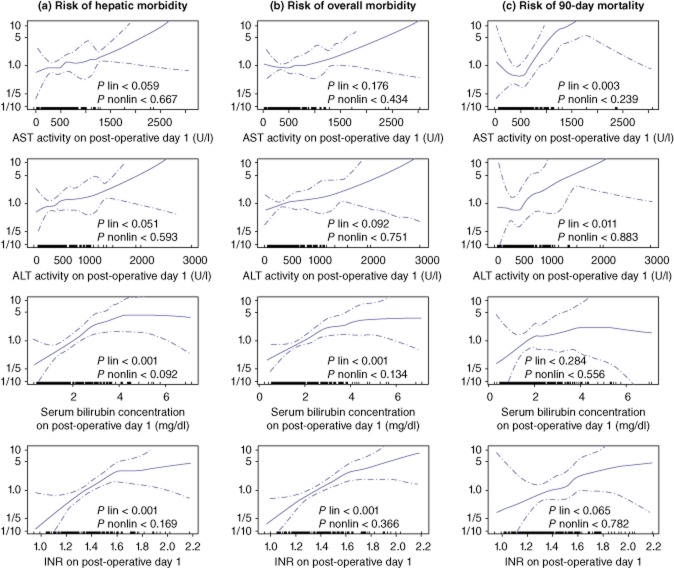
Univariate and multiple analyses of the associations between biochemical parameters on POD 1 and the 3 main outcome measures are presented in Table 2. According to the spline functions derived from generalized additive models, all of the observed associations were of a linear nature (all P for non-linear associations > 0.05; Fig. 1).
Table 2.
Associations between selected serum biochemical parameters on post-operative day 1 and hepatic morbidity, overall morbidity, and 90-day mortality in univariate and multiple analyses
| Outcome measure | Biochemical parameter | ORs calculated per: | Univariate | Multiple | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |||
| Hepatic morbidity | Bilirubin | 1.0 mg/dl | 1.75 | 1.31–2.34 | <0.001 | 1.57 | 1.14–2.17 | 0.006 |
| INR | 0.1 | 1.37 | 1.16–1.62 | <0.001 | 1.25 | 1.03–1.51 | 0.024 | |
| AST | 100 U/l | 1.09 | 1.01–1.19 | 0.039 | – | – | – | |
| ALT | 100 U/l | 1.10 | 1.01–1.21 | 0.034 | – | – | – | |
| Overall morbidity | Bilirubin | 1.0 mg/dl | 1.61 | 1.24–2.09 | <0.001 | 1.43 | 1.07–1.91 | 0.015 |
| INR | 0.1 | 1.40 | 1.21–1.63 | <0.001 | 1.31 | 1.11–1.54 | 0.001 | |
| AST | 100 U/l | 1.06 | 0.98–1.15 | 0.128 | – | – | – | |
| ALT | 100 U/l | 1.08 | 0.99–1.18 | 0.069 | – | – | – | |
| 90-day mortality | Bilirubin | 1.0 mg/dl | 1.33 | 0.83–2.12 | 0.242 | – | – | – |
| INR | 0.1 | 1.29 | 0.99–1.67 | 0.062 | – | – | – | |
| AST | 100 U/l | 1.16 | 1.05–1.29 | 0.005 | 1.16 | 1.05–1.29 | 0.005 | |
| ALT | 100 U/l | 1.17 | 1.04–1.32 | 0.011 | – | – | – | |
OR, odds ratio; 95% CI, 95% confidence intervals; INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Figure 1.
Associations between serum bilirubin concentration, international normalized ratio (INR), and activity of aspartate (AST) and alanine aminotransferases (ALT) on post-operative day 1 with hepatic morbidity (a), overall mortality (b) and 90-day mortality (c). Hazard curves (solid lines) are presented with 95% confidence intervals (dotted lines)
Regarding hepatic morbidity, areas under the ROC curves were 0.780 [95% confidence interval (CI), 0.698–0.862] for the predictive model based on both serum bilirubin concentration and INR, 0.737 (95% CI, 0.645–0.828) for the model based only on serum bilirubin concentration, and 0.714 (95% CI, 0.624–0.804) for the model based only on INR. Area under the ROC curve for the model predicting hepatic complications based solely on serum bilirubin concentration was statistically non-inferior to those based both on serum bilirubin concentration and INR (P < 0.162). Therefore, this model was chosen for the prediction of hepatic complications, with an approximate cut-off value of 2.05 mg/dl of serum bilirubin, providing a sensitivity of 69.2%, specificity of 71.2%, PPV of 39.1% and NPV of 89.7% (Fig. 2). Similarly, there were no significant differences between the prediction model based on both serum bilirubin concentration and INR (AUC, 0.749; 95% CI, 0.672–0.827) and the model limited to only serum bilirubin (AUC, 0.690; 95% CI, 0.604–0.776) with respect to overall morbidity (P < 0.099). The prediction of overall morbidity based on serum bilirubin concentration exhibited a sensitivity of 61.1%, specificity of 71.2%, PPV of 44.0% and NPV of 83.2% for values equal or over 2.05 mg/dl (Fig. 3). Finally, regarding the prediction of 90-day mortality, the area under the ROC curve for the model based on AST activity was 0.644 (95% CI, 0.353–0.936) with a sensitivity of 62.5%, specificity of 90.4%, PPV of 20.8% and NPV of 98.3% for values equal or over 798 U/l (Fig. 4). Notably, the prediction model for 90 day-mortality based on both AST and ALT was not associated with any significant benefits compared with the model based only on AST (P < 0.141).
Figure 2.
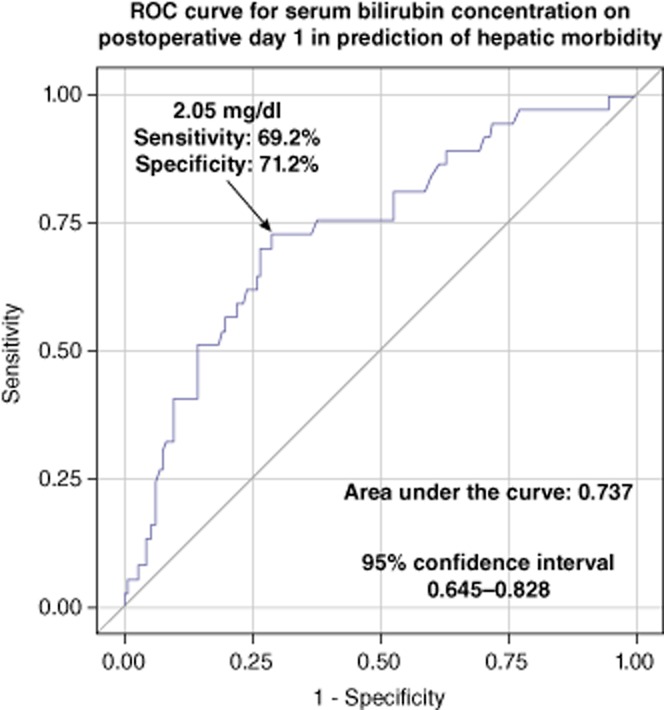
Receiver-operating characteristics (ROC) curve for the prediction model of hepatic morbidity based only on serum bilirubin on postoperative day 1
Figure 3.
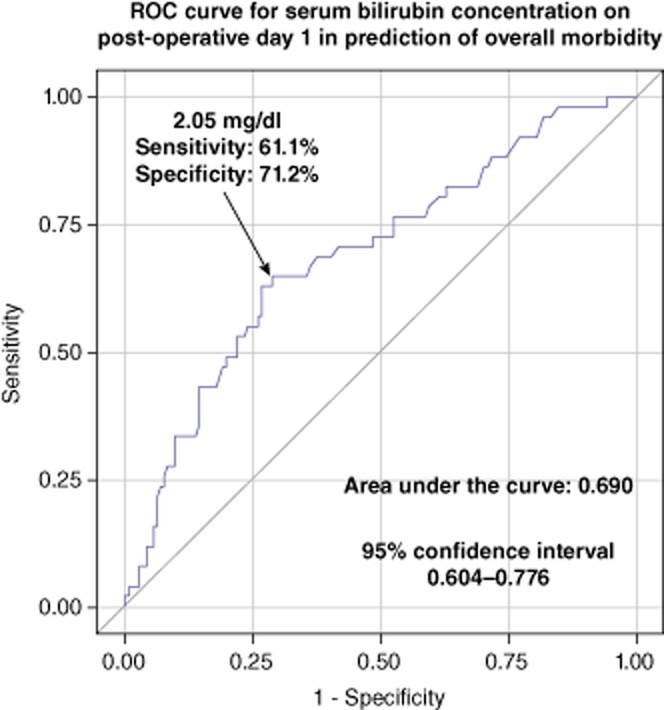
Receiver-operating characteristics (ROC) curve for the prediction model of overall morbidity based only on serum bilirubin on postoperative day 1
Figure 4.
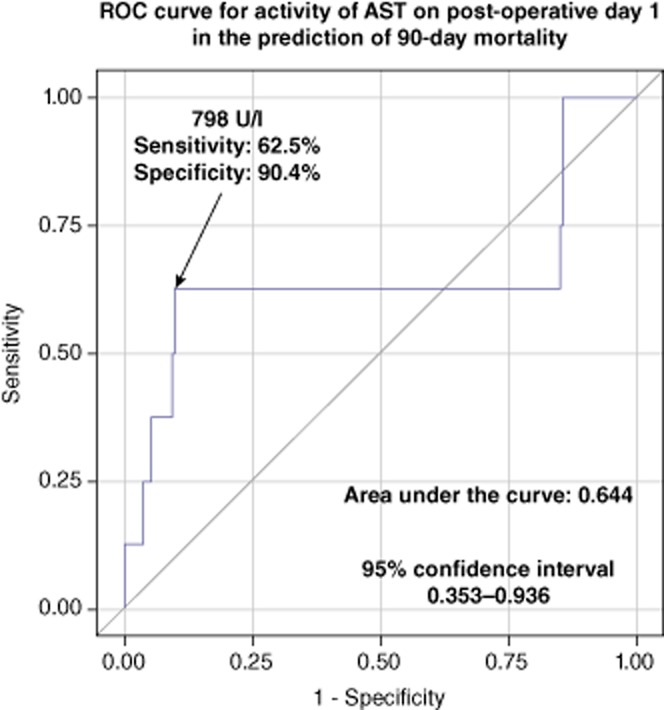
Receiver-operating characteristics (ROC) curve for the prediction model of 90-day mortality based on activity of aspartate aminotransferase (AST) on post-operative day 1
Eight patients fulfilled the criteria of post-operative liver failure according to the definition based on peak serum bilirubin concentration over 7 mg/dl, and two patients fulfilled the ‘50-50 criteria’ on POD 5. The sensitivity for the prediction of hepatic morbidity, overall morbidity and 90-day mortality was approximately three-fold higher for the criteria based on peak serum bilirubin concentration compared with the ‘50-50 criteria,’ with similar specificity. Details concerning comparison of these two different criteria are presented in Table 3.
Table 3.
Comparison of the criteria for post-operative liver failure based on peak post-operative serum bilirubin > 7 mg/dl and ‘50-50 criteria’ on post-operative day 5 in the prediction of hepatic morbidity, overall morbidity and 90-day mortality
| Outcome measure | ‘50-50 criteria’ on post-operative day 5 | Peak serum bilirubin concentration > 7 mg/dl | ||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | Sensitivity | Specificity | PPV | NPV | |
| Hepatic morbidity | 4.6% | 100% | 100% | 82.1% | 18.2% | 100% | 100% | 84.2% |
| Overall morbidity | 3.0% | 100% | 100% | 72.7% | 12.1% | 100% | 100% | 74.6% |
| 90-day mortality | 9.1% | 99.6% | 50.0% | 95.7% | 27.3% | 97.8% | 37.5% | 96.5% |
PPV, positive predictive value; NPV, negative predictive value.
Discussion
The results of this study highlight the previously underestimated role of biochemical parameters on POD 1 in the early prediction of poor outcomes after major resections for colorectal liver metastases. Notably, effective biochemical predictors were different for morbidity and mortality. While serum bilirubin and INR were associated with the occurrence of hepatic and overall complications, post-operative mortality was best predicted by AST activity, with the serum bilirubin concentration not reaching significance. This finding was rather unexpected, given that the activity of transaminases was not considered in post-operative mortality risk scores, such as those proposed by Mullen et al. on the basis of peak serum bilirubin levels,15 the ‘50-50 criteria’ by Balzan et al. 16 and in a definition of post-operative liver insufficiency published by the International Study Group for Liver Surgery.17
Considering the prediction of hepatic and overall complications, both the serum bilirubin concentration and INR on POD 1 retained significance in a multiple regression model. Given that INR values are potentially influenced by peri-operative plasma transfusions, prediction models based solely on serum bilirubin concentration were compared with models including both of these parameters. The results of this comparison indicated non-inferiority, and thus, the bilirubin-only prediction was considered in further analyses for both outcome measures.
Notably, the empirically established predictive cut-off value for serum bilirubin concentration on POD 1 >2.05 mg/dl regarding both hepatic and overall morbidity, and for serum AST activity >798 U/l regarding 90-day mortality did not allow precise discrimination between patients with poor and uneventful post-operative course. Nevertheless, these cut-off points exhibit moderate sensitivity and specificity and might be useful in stratifying patients into low- and high-risk groups as early as POD 1 for particular outcome measures. Because these results originate from a series of patients treated in a single centre and the number of end-points was limited, external validation is obviously essential to adequately assess their clinical significance in terms of prevention, early diagnosis and the management of particular events.
Short-term outcomes of patients in this series are similar to those reported by other authors.7–9,11–16,20 In one of the previous studies based on an unselected series of 1005 liver resections performed in the Department of General, Transplant and Liver Surgery at the Medical University of Warsaw, overall morbidity and mortality rates were 22.1% and 1.4%, respectively.19 Although several studies have reported zero or near-zero mortality after a liver resection, most of these report only in-hospital or 30-day rates.6,21–23 Of note, in-hospital mortality was more than two-fold lower compared with the 90-day rate in the present series. Therefore, all deaths occurring during the 90-day post-operative period should be considered in the evaluation of mortality rates, as this allows a more precise comparison between different studies and avoids underestimation of rates. Of note, this observation is in line with those of other reports focused on short-term outcomes after a liver resection.9,14,15,24
There are two main methodological advantages of the present study. First, it was performed on a rather homogenous group of non-cirrhotic patients only after major liver resections for colorectal metastases. Second, although the design was retrospective, biochemical parameters included in the analyses were performed routinely on POD 1 in all patients. This minimizes potential selection bias that could affect analyses of these parameters on later post-operative days.
Out of three main definitions for post-hepatectomy liver failure, criteria based on peak bilirubin >7 mg/dl were applied. These were proposed by Mullen et al. based on a large series of non-cirrhotic patients after a major hepatectomy, a cohort similar to those included in the present study.15 Conversely, the ‘50-50 criteria’ were initially created in a slightly more heterogeneous group of patients.16 Although both were highly specific, the rate of sensitivity for predicting 90-day mortality was rather low. Nevertheless, comparison of these two criteria revealed that the definition used in this study was approximately three times more sensitive regarding mortality rate than the ‘50-50 criteria’ on POD 5. This may be partly related to the previously mentioned similarity between the cohorts of patients included in this study and those studied by Mullen et al.15 However, our results obviously do not rule out the potential superiority of the ‘50-50 criteria’ in more heterogeneous groups.
The limitations of this study are mainly related to its retrospective approach. Accordingly, to avoid both under- and overestimation, complications were not stratified according to Clavien–Dindo classification.25 Second, although the study cohort was relatively homogenous with respect to the extent of resection and histopathology of the non-tumoral liver, the small number of specific events precluded more complex prediction regarding these two important factors. Moreover, multiple logistic regression analyses used to evaluate independent predictors of 90-day mortality might have been biased by the low number of events. As this particular selection process was based only upon bivariate analyses, the absolute number of events per variable was 5.5 (11 deaths observed during the 90-day period). Considering the minimum of five events per variable reported in epidemiological studies,26 multiple logistic regression was in this case slightly above the verge of applicability. Therefore, models based on both AST and ALT (the second significant factor in univariate analyses) activity and solely on AST activity were compared. The lack of significant differences additionally supported the choice of AST as a predictor of 90-day mortality.
In summary, the serum bilirubin concentration and activity of AST on post-operative day 1 allow stratification of patients into low- and high-risk groups of morbidity and mortality after a liver resection, respectively. These results provide additional tools for early prediction and management of poor outcomes after a hepatectomy for colorectal metastases.
Conflicts of interest
None declared.
References
- Rees M, Plant G, Bygrave S. Late results justify resection for multiple hepatic metastases from colorectal cancer. Br J Surg. 1997;84:1136–1140. [PubMed] [Google Scholar]
- Misiakos EP, Karidis NP, Kouraklis G. Current treatment for colorectal liver metastases. World J Gastroenterol. 2011;17:4067–4075. doi: 10.3748/wjg.v17.i36.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haas RJ, Wicherts DA, Andreani P, Pascal G, Saliba F, Ichai P, et al. Impact of expanding criteria for resectability of colorectal metastases on short- and long-term outcomes after hepatic resection. Ann Surg. 2011;253:1069–1079. doi: 10.1097/SLA.0b013e318217e898. [DOI] [PubMed] [Google Scholar]
- Chan KM, Chiang JM, Lee CF, Yu MC, Lee WC, Chen JS, et al. Outcomes of resection for colorectal cancer hepatic metastases stratified by evolving eras of treatment. World J Surg Oncol. 2011;9:174. doi: 10.1186/1477-7819-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viganò L, Russolillo N, Ferrero A, Langella S, Sperti E, Capussotti L. Evolution of long-term outcome of liver resection for colorectal metastases: analysis of actual 5-year survival rates over two decades. Ann Surg Oncol. 2012;19:2035–2044. doi: 10.1245/s10434-011-2186-1. [DOI] [PubMed] [Google Scholar]
- Itoh S, Shirabe K, Taketomi A, Morita K, Harimoto N, Tsujita E, et al. Zero mortality in more than 300 hepatic resections: validity of preoperative volumetric analysis. Surg Today. 2012;42:435–440. doi: 10.1007/s00595-011-0108-2. [DOI] [PubMed] [Google Scholar]
- Aloia TA, Fahy BN, Fischer CP, Jones SL, Duchini A, Galati J, et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB. 2009;11:510–515. doi: 10.1111/j.1477-2574.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir M, Smith LM, Ullrich F, Leiphrakpam PD, Ly QP, Sasson AR, et al. Pre-operative nomogram to predict risk of peri-operative mortality following liver resections for malignancy. J Gastrointest Surg. 2010;14:1770–1781. doi: 10.1007/s11605-010-1352-2. [DOI] [PubMed] [Google Scholar]
- Robertson DJ, Stukel TA, Gottlieb DJ, Sutherland JM, Fisher ES. Survival after hepatic resection of colorectal cancer metastases: a national experience. Cancer. 2009;115:752–759. doi: 10.1002/cncr.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZQ, Xu LN, Yang T, Zhang WZ, Huang XQ, Cai SW, et al. Hepatic resection: an analysis of the impact of operative and perioperative factors on morbidity and mortality rates in 2008 consecutive hepatectomy cases. Chin Med J (Engl) 2009;122:2268–2277. [PubMed] [Google Scholar]
- Breitenstein S, DeOliveira ML, Raptis DA, Slankamenac K, Kambakamba P, Nerl J, et al. Novel and simple preoperative score predicting complications after liver resection in noncirrhotic patients. Ann Surg. 2010;252:726–734. doi: 10.1097/SLA.0b013e3181fb8c1a. [DOI] [PubMed] [Google Scholar]
- Simons JP, Ng SC, Hill JS, Shah SA, Bodnari A, Zhou Z, et al. In-hospital mortality for liver resection for metastases: a simple risk score. J Surg Res. 2009;156:21–25. doi: 10.1016/j.jss.2009.03.073. [DOI] [PubMed] [Google Scholar]
- Cescon M, Vetrone G, Grazi GL, Ramacciato G, Ercolani G, Ravaioli M, et al. Trends in perioperative outcome after hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann Surg. 2009;249:995–1002. doi: 10.1097/SLA.0b013e3181a63c74. [DOI] [PubMed] [Google Scholar]
- Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540–548. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The ‘50-50 criteria’ on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Reissfelder C, Rahbari NN, Koch M, Kofler B, Sutedja N, Elbers H, et al. Postoperative course and clinical significance of biochemical blood tests following hepatic resection. Br J Surg. 2011;98:836–844. doi: 10.1002/bjs.7459. [DOI] [PubMed] [Google Scholar]
- Grat M, Grzegorczyk K, Lewandowski Z, Sujecki D, Szwedowski D, Boltuc A, et al. Intraoperative injuries during liver resection: analysis of 1,005 procedures. Hepatol Int. 2012;6:498–504. doi: 10.1007/s12072-011-9281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong T, Welsh FK, Wells J, Chandrakumaran K, John TG, Rees M. The impact of pre-operative serum creatinine on short-term outcomes after liver resection. HPB. 2009;11:622–628. doi: 10.1111/j.1477-2574.2009.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamashita K, et al. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443–449. doi: 10.1016/j.jamcollsurg.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Tamandl D, Gruenberger B, Herberger B, Schoppmann S, Bodingbauer M, Schindl M, et al. Selective resection of colorectal liver metastases. Eur J Surg Oncol. 2007;33:174–182. doi: 10.1016/j.ejso.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Delis SG, Bakoyiannis A, Karakaxas D, Athanassiou K, Tassopoulos N, Manesis E, et al. Hepatic parenchyma resection using stapling devices: peri-operative and long-term outcome. HPB. 2009;11:38–44. doi: 10.1111/j.1477-2574.2008.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TP, Virgo KS, Li MJ, Callander PW, Longo WE, Johnson FE. Outcomes after detection of metastatic carcinoma of the colon and rectum in a national hospital system. J Am Coll Surg. 1996;182:353–361. [PubMed] [Google Scholar]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]