Abstract
Background Retrospective analysis of outcomes of R0 (negative margin) versus R1 (positive margin) liver resections for colorectal metastases (CLM) in the context of peri-operative chemotherapy.
Methods All CLM resections between 2000 and 2006 were reviewed. Exclusion criteria included: macroscopically incomplete (R2) resections, the use of local treatment modalities, the presence of extra-hepatic disease and no peri-operative chemotherapy. R0/R1 status was based on pathological examination.
Results Of 86 eligible patients, 63 (73%) had R0 and 23 (27%) had R1 resections. The two groups were comparable for the number, size of metastases and type of hepatectomy. The R1 group had more bilobar CLM (52% versus 24%, P = 0.018). The median follow-up was 3.1 years. Five-year overall and disease-free survival were 54% and 21% for the R0 group and 49% and 22% for the R1 group (P = 0.55 and P = 0.39, respectively). An intra-hepatic recurrence was more frequent in the R1 group (52% versus 27%, P = 0.02) and occurred more frequently at the surgical margin (22% versus 3%, P = 0.01).
Discussion R1 resections were associated with a higher risk of intra-hepatic and surgical margin recurrence but did not negatively impact survival suggesting that in the era of efficient chemotherapy, the risk of an R1 resection should not be considered as a contraindication to surgery.
Introduction
Resection of colorectal liver metastases (CLM) is the only potential curative treatment with 37–58% and 22–26% actuarial survival rates at 5- and 10-years, respectively.1–3 Surgical margin status has been reported to be a major prognostic factor after a CLM resection and a R0 resection remains the standard goal in this field.4–6 The advent of more effective chemotherapy regimens challenged the 1.0-cm dogma of a clear surgical margin leading to the concept of complete macroscopic removal of all lesions regardless of width.7,8 Furthermore, recent studies have suggested that even R1 resections were also associated with significant survival.9
In a series of 436 patients operated on for CLM, de Hass et al.9 reported similar overall-survival and recurrence-free survival rates after a margin-negative (R0) and margin-positive (R1) hepatectomy (so-called R1 resection by necessity).9 Although promising, these results remain debatable5,6,10,11 as the group of R1 resections received more frequently pre- and/or post-operative chemotherapy (P < 0.01). These results remained to be confirmed in a homogenous population of patients in terms of modern peri-operative chemotherapy.12–16
The aim of this retrospective study was to compare the oncological outcomes of R0 (margin ≥1 mm) versus R1 (margin <1 mm) resections for CLM where all patients received pre- and/or post-operative chemotherapy during a recent period.
Patients and methods
Patients
Between January 2000 and December 2006, 493 patients underwent a liver resection at a single institution (AP-HP, Henri Mondor University Hospital, Créteil, France). Among the 153 consecutive patients operated on for CLM, 67 (43.8%) were excluded from the present study for the following exclusion criteria: (i) incomplete macroscopic resection (R2), (ii) the presence of concomitant extra-hepatic disease, (iii) the use of local treatment modalities such as radiofrequency ablation in combination with surgery, (iv) no pre- and/or post-operative systemic chemotherapy. With the approval of the local Institutional Review Board, 86 patients were retrieved from a prospective database and were eligible for the study. All underwent a liver resection with curative intent, defined as an attempt to achieve a macroscopically complete resection of all hepatic tumour burdens with a clear macroscopic surgical margin.
Disease staging
All patients were staged upon physical examination, as well as the level of serum carcinoembryonic antigen (CEA), colonoscopy and multidetector computed tomography (CT) scans of the abdomen, pelvis and chest. When liver metastases were considered suitable for curative resection, hepatic magnetic resonance imaging (MRI) was performed. A response to chemotherapy was evaluated every four cycles by CT according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria.17 This was done during a multidisciplinary meeting including liver surgeons, oncologists, radiologists and pathologists.
Surgery
For all patients, timing of the surgery was determined by the multidisciplinary oncology committee. Segmental resections were classified as ‘anatomical’ whereas wedge excisions of less than a segment of liver were classified as ‘non-anatomical’. During the operation, a complete examination of the liver was performed by palpation and intra-operative ultrasonography to accurately determine the number, size and location of the lesions, their relationship to major vascular structures, and to rule out additional unknown lesions. A parenchymal transection was performed using the crush clamp technique or with a Cavi-Pulse Ultrasonic Surgical Aspirator (CUSATM, Model 200T; Valleylab, Boulder, CO, USA) under low central venous pressure anaesthesia with an intermittent Pringle manoeuvre if necessary. Although a 1-cm margin was the aim of the liver resection, a grossly negative macroscopic margin without tumour exposure was considered adequate.
Pathological assessment
All liver specimens were addressed fresh to the Department of Pathology and analysed before and after formalin fixation by a senior pathologist. All specimens were photographed before and after being sliced. The diameter of each liver metastasis was recorded. The surgical resection surface was painted with ink. The largest cut surface for each metastatic tumour was obtained from each liver specimen. Each metastatic tumour was embedded in paraffin, and examined on haematoxylin and eosin-stained slides of 3–5 μm. R0 and R1 resections were reviewed by a single pathologist (M.L.A.) and defined by the absence (tumour-free margin ≥1 mm for all resected lesions) or presence (tumour-free margin <1 mm for any resected lesion) of a microscopic tumour at the resection margins, respectively.
Radiological assessment of intra-hepatic recurrence
CT scans of all patients who developed intra-hepatic recurrence were reviewed by two senior radiologists (A.L. and L.B.) to assess whether recurrence occurred at the surgical margin or elsewhere in the liver.
Follow-up
Patients were followed by physical examination, CEA testing and CT scans of the abdomen, pelvis and chest every 4–6 months. No patient was lost to follow-up.
Study end-points
The primary end-point of the study was to compare long-term oncological outcomes of R0 (margin ≥1 mm) and R1 (margin <1 mm) resections for CLM. The secondary end points were to assess the pattern of recurrences with particular focus on intra-hepatic recurrences between the two groups.
Statistical analysis
Patients were identified from the institution's prospectively maintained database and analysed retrospectively. Results were expressed as median and first and third quartiles (Q1 to Q3) or counts and percentages. Patients with CLM resection margins of ≥1 mm constituted the R0 group and those with margins <1 mm the R1 group. Patient characteristics in the two groups were compared using Fisher's exact test for categorical variables and Wilcoxon's test for continuous variables. Overall survival, hepatic recurrence-free survival and recurrence-free survival were determined using the Kaplan–Meier method. Differences in survival between the two groups were estimated using the Cox proportional cause-specific hazards model and expressed as hazard ratios (HR) with 95% confidence intervals (CI). A two-sided P-value of 0.05 was considered significant. All analyses were performed using R 2.10.1 statistical software (The R foundation of Statistical Computing, Vienna, Austria).
Results
Study population
During the study period, 153 patients underwent resection CLM. Sixty-seven (43.8%) patients were excluded for R2 resection (n = 5), extrahepatic hepatic disease (localized resected carcinomatosis, n = 6, hepatic pedicle lymph node, n = 12, lung metastases, n = 8), or no chemotherapy (n = 36). The remaining 86 patients (56.2%) operated on for CLM fulfilled the inclusion criteria. Among them, 63 (73%) had R0 and 23 (27%) had R1 liver resections. Patient demographics and tumour characteristics are shown in Table 1.
Table 1.
Patient and tumour characteristics
| Variable | Total (n = 86) | R0 group (n = 63) | R1 group (n = 23) | P |
|---|---|---|---|---|
| Patients | ||||
| Age, median (Q1–Q3), years | 62 (56–72) | 63 (56–71) | 58 (56–73) | .530 |
| Gender: male/female | 55/31 | 43/20 | 12/11 | .210 |
| Primary tumour (numbers) | ||||
| Colon ectum | 63/23 | 46/17 | 17/6 | >0.9 |
| T1-T2/T3-T4 | 6/74 | 4/55 | 2/19 | .542 |
| N0/N+ | 18/60 | 12/46 | 6/14 | .601 |
| Liver metastases | ||||
| Number of nodules, median (Q1–Q3) | 2 (1–3) | 2 (1–3) | 3 (1–3.5) | .130 |
| <3 nodules, n (%) | 53 (62%) | 42 (67%) | 11 | .140 |
| Bilobar, n (%) | 27 (31%) | 15 (24%) | 12 | .018 |
| Maximum diameter, mm, n (Q1–Q3) | 36 (25–52.5) | 36 (24–59) | 36.5 (30–50) | .770 |
| Maximum diameter < 50 mm, n (%) | 55 (65.5%) | 40 (64.5%) | 15 | .800 |
| Synchronous, n (%) | 55 (64%) | 38 (60%) | 17 | .310 |
| Pre-operative CEA > 200 (ng/ml), n (%) | 30 (35%) | 21 (33%) | 9 | .619 |
| Chemotherapy | ||||
| Pre- and post-operative, n (%) | 55 (63.9%) | 39 (61.9%) | 16 | .615 |
| Post-operative only, n (%) | 15 (17.4%) | 9 (14.2%) | 6 | .214 |
| Combined post-operative | 70 (81.4%) | 48 (76.2%) | 22 | .058 |
| Pre-operative only, n (%) | 16 (18.6%) | 15 (23.8%) | 1 | .058 |
| ≥6 pre-operative cycles, n (%) | 29 (34%) | 17 (27%) | 12 | .039 |
| Number of pre-operative lines, median (Q1–Q3) | 1 (1–2) | 1 (1–1) | 2 (1–3) | .562 |
CEA, carcinoembryonic antigen; Q1–Q3, first and third quartiles.
Pre- and/or post-operative chemotherapy
All included patients received both pre- and/or post-operative systemic chemotherapy (Table 1) with the following regimens: LV5FU2 (n = 5, 6%), FOLFOX (n = 53, 62%), FOLFIRI (n = 12, 14%) and FOLFOX or FOLFIRI in association with Bevacizumab (n = 16, 19%). Most patients received pre- and post-operative systemic chemotherapy (55/86, 63.9%). Although the median number of pre-operative lines of chemotherapy was similar between the two groups, patients in the R1 group received significantly more courses of pre-operative chemotherapy compared to those in the R0 group (P = 0.039).
Operative data
Among the 86 patients, 5 (6%) underwent a fully laparoscopic liver resection (all R0) and 6 (7%) additional patients underwent a laparoscopic hand-assisted hepatectomy (R0 n = 5, R1 n = 1). A major hepatectomy was carried out in 57 (66.3%) patients with no difference between the two groups (Table 2). Although a trend towards slightly higher rates of non- anatomical resections was observed in the R1 group [2 (8.7%) versus 4 (6.5%) patients in the R0 group], this difference was not statistically different.
Table 2.
Operative data
| Variable (n, %) | Total (n = 86) | R0 group (n = 63) | R1 group (n = 23) | P |
|---|---|---|---|---|
| Major hepatectomy | 57 (66.3%) | 41 (65.1%) | 16 | .800 |
| Combined anatomical and non-anatomical resection | 23 (26.7%) | 13 (21%) | 10 | .053 |
| Non-anatomical resection only | 6 (7.1%) | 4 (6.5%) | 2 | .094 |
| Combined hepatectomy and primary tumour resection | 18 (20.9%) | 13 (20.6%) | 5 | >0.9 |
Long-term outcomes
The median follow-up for the entire study population was 3.1 years (range: 2.0–4.3). Overall survival rates at 1-, 3- and 5-years were 98%, 74% and 54% in the R0 group and 96%, 81% and 49% in the R1 group, respectively [HR = 1.27 (0.58–2.76), P = 0.55] (Fig. 1). During the follow-up, 42 patients (66.7%) in the R0 group and 16 patients (69.6%) in the R1 group had recurrence (P = 0.600) (Table 3). Disease-free survival rates at 1-, 3-, and 5- years were 73%, 29% and 21% in the R0 group and 70%, 22% and 22% in the R1 group, respectively [HR = 0.28 (0.73–2.25), P = 0.39] (Fig. 2). Intra-hepatic recurrence was significantly (P = 0.029) more frequent in the R1 group (52%) compared with the R0 group (27%). More specifically, hepatic recurrence-free survival at 1-, 3-, and 5-years was 85%, 72% and 67% in the R0 group and 74%, 44% and 44% in the R1 group, respectively [HR = 2.39 (1.14–5.03), P = 0.021] (Fig. 3). According to the location of intra-hepatic recurrence, significantly more recurrences occurred at the surgical margin after R1 compared with a R0 resection (34.7% versus 6% respectively, P = 0.006).
Figure 1.
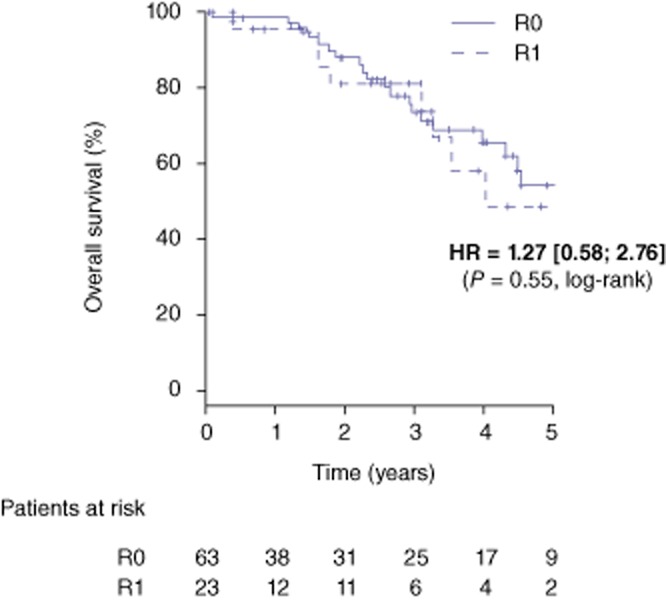
Overall survival after a R0 (n = 63) and R1 (n = 23) liver resection for colorectal metastases. HR; hazard ratio
Table 3.
Long-term outcome
| Variable | Total (n = 86) | R0 group (n = 63) | R1 group (n = 23) | P |
|---|---|---|---|---|
| Follow-up, median years (Q1–Q3) | 3.1 (2.0–4.3) | 3.1 (2–4.5) | 2.9 (1.9–4) | .584 |
| Recurrence | 58 (67.4%) | 42 (66.7%) | 16 | .600 |
| Extra-hepatic | 29 (33.7%) | 25 (39.7%) | 4 | .072 |
| Intra-hepatic | 13 (15.1%) | 6 (9.5%) | 7 | .036 |
| Both | 16 (18.6%) | 11 (17.5%) | 5 | .760 |
| Intra-hepatic recurrence location | ||||
| Overall intra-hepatic recurrence | 29 (33.7%) | 17 (27%) | 12 | .029 |
| At surgical margin | 7 (8.1%) | 2 (3.1%) | 5 | .013 |
| Curable | 16 (55.2%) | 9 (52.9%) | 7 | .770 |
| Repeat hepatectomy | 10 (11.6%) | 8 (47%) | 2 | .894 |
| Ablation | 6 (20.7%) | 1 (5.9%) | 5 | .005 |
Figure 2.
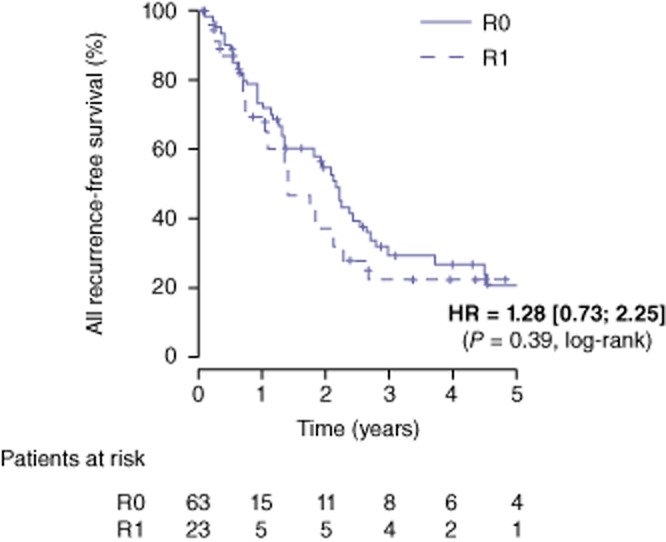
Recurrence-free survival after a R0 (n = 63) and R1 (n = 23) liver resection for colorectal metastases. HR; hazard ratio
Figure 3.
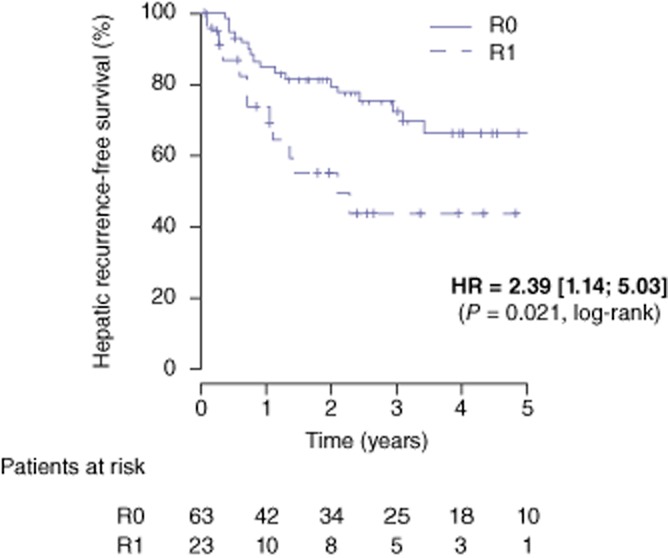
Hepatic recurrence-free survival after a R0 (n = 63) and R1 (n = 23) liver resection for colorectal metastases. HR; hazard ratio
Discussion
The present study is one of the first to compare the oncological outcomes of R0 and R1 resections of CLM in patients who received peri-operative systemic chemotherapy. The present study shows that R1 resections: (i) occur more frequently in patients with bilobar disease, (ii) are associated with a higher risk of intra-hepatic recurrence, (iii) are associated with more frequent surgical margin recurrences, and (iv) do not negatively impact disease-free and overall survival rates. These results suggest that in the era of efficient chemotherapy, the risk of an R1 resection should not be considered a contraindication to surgery with curative intent, as R1 patients had a satisfactory 5-years survival rate of 49% and half the patients did not have intra-hepatic recurrence.
In the present series, 27% of patients operated with a curative intent underwent an R1 hepatectomy for CLM, consistent with the reported range of 5% to 46%.8,12,18 In the current series, bilobar disease was associated with an increased risk of a R1 resection. The presence of multiple, large, bilateral tumours and tumours located centrally or close to a major vessel are the most common factors reported to be associated with an increased risk of R1 resection.8,18 Although some studies have suggested that intra-operative factors such as non-anatomic resections and extended resections may be associated with a higher likelihood of an R1 resection,9,18–20 other investigators have found this not to be the case.21 In the present series, anatomical resections were not superior to non-anatomic resections or combined anatomic and non-anatomic resections in terms of surgical margin clearance.
Cumulative data suggest that a R1 resection is associated with an increased risk of hepatic recurrence, both distant to or at the surgical margin. Whereas recurrence in the liver was reported to occur in 14–38% of patients after a R0 resection, the rate of liver recurrence has been noted to be as high as 22–78% after R1 resections.8,12 Similarly, in the present series, intra-hepatic recurrence were significantly more frequent in the R1 group (52%) compared with the R0 group (27%, P = 0.029) with a 5-year hepatic recurrence-free survival of 67% in group R0 and 44% in group R1 (P = 0.021). As reported by others,6,10,11 when subdivided according to location of hepatic recurrence, significantly more recurrences occurred at the surgical margin after R1 compared with R0 resections in this study (21.7% versus 3.1% respectively, P = 0.01). This is probably the result of histological micrometastases found in the liver parenchyma surrounding CLM within 5 mm of the tumour border22,23 which have not been sterilized by chemotherapy24 or destroyed by surgical cautery. In contrast, de Hass et al. 9 reported similar recurrence rates at the surgical margin after a R1 resection compared with a R0 resection (9% versus 8%, P = 0.72). In the current study, the 3.1% rate of margin recurrence after a R0 resection is in the reported range of 3% to 8%.6,9–11
All but one previous study9 have demonstrated that R1 resections negatively impact long-term outcome with 5-year overall survival rates ranging from 0–20% compared with the 37–64% for R0 resections.6,12,18 In a series of 436 patients operated on for CLM, de Hass et al.9 reported that patients undergoing a R1 versus a R0 resection had similar 5-year survival rates (57% versus 61%, P = 0.27). In the latter series, R1 patients received significantly more chemotherapy before and after resection compared with their R0 counterparts. Within the present study, R1 patients received slightly more combined chemotherapy but the difference did not reach statistical significance. Therefore, in a homogeneous population regarding chemotherapy administration, it has been shown that 5-year overall survival rates were not different after a R0 and R1 hepatectomy (54% versus 49%). Tanaka et al.12 demonstrated in a series of 310 patients, that the R1 resection group had poorer overall survival than the R0 resection group. However, in this series, when patients were stratified according to their initial resectability, this negative impact of R1 resection was not observed in patients with initially unresectable or marginally resectable metastases, especially those with a favourable response to pre-hepatectomy chemotherapy.12 Together with the present results (17% of patients with initially unresectable disease in the R1 group) and those from de Hass et al.,9 the negative prognostic impact of R1 CLM resections should be reconsidered given the good survival rates in patients receiving effective peri-operative chemotherapy.8 The gap between overall- and disease-free survival rates within the current series confirms the major importance and efficacy of chemotherapy. The patients in the present study were treated by efficient chemotherapy regimens (FOLFOX or FOLFIRI) associated with targeted agents in 19% of them, corresponding to most recent patients in the series. These regimens have been demonstrated to improve both response rates and survival in the colorectal metastatic setting.25–28
The limitations of the present study were that a limited number of patients were analysed, the retrospective analysis of the data and a relatively limited follow-up that ended the first of January 2010. The few patients remaining at the end of the follow-up could be a limitation to the power analysis of the study.
In spite of the limited number of patient analysed, the present findings suggest a R0 resection should remain the gold standard recommendations for patients operated on for CLM. However, R1 resection margins should not be considered a contraindication to surgery provided complete macroscopic removal of all metastatic lesions and administration of effective peri-operative chemotherapy. In spite of a higher risk of intra-hepatic and surgical margin recurrences, R1 patients have a satisfactory 5-years survival rate of 49%. Not resecting them would lead to propose chemotherapy as the only alternative with a lower survival expectancy.
Conflicts of interest
None declared.
References
- Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–657. doi: 10.1097/01.sla.0000141198.92114.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlinger B, Van Cutsem E, Gruenberger T, Glimelius B, Poston G, Rougier P, et al. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol. 2009;20:985–992. doi: 10.1093/annonc/mdn735. [DOI] [PubMed] [Google Scholar]
- Karoui M, Vigano L, Goyer P, Ferrero A, Luciani A, Aglietta M, et al. Combined first-stage hepatectomy and colorectal resection in a two-stage hepatectomy strategy for bilobar synchronous liver metastases. Br J Surg. 2010;97:1354–1362. doi: 10.1002/bjs.7128. [DOI] [PubMed] [Google Scholar]
- Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir M, Lyden ER, Wang A, Smith LM, Ullrich F, Are C. Influence of margins on overall survival after hepatic resection for colorectal metastasis: a meta-analysis. Ann Surg. 2011;254:234–242. doi: 10.1097/SLA.0b013e318223c609. [DOI] [PubMed] [Google Scholar]
- Poultsides GA, Schulick RD, Pawlik TM. Hepatic resection for colorectal metastases: the impact of surgical margin status on outcome. HPB. 2010;12:43–49. doi: 10.1111/j.1477-2574.2009.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248:626–637. doi: 10.1097/SLA.0b013e31818a07f1. [DOI] [PubMed] [Google Scholar]
- Nuzzo G, Giuliante F, Ardito F, Vellone M, Giovannini I, Federico B, et al. Influence of surgical margin on type of recurrence after liver resection for colorectal metastases: a single-center experience. Surgery. 2008;143:384–393. doi: 10.1016/j.surg.2007.09.038. [DOI] [PubMed] [Google Scholar]
- Wakai T, Shirai Y, Sakata J, Valera VA, Korita PV, Akazawa K, et al. Appraisal of 1 cm hepatectomy margins for intrahepatic micrometastases in patients with colorectal carcinoma liver metastasis. Ann Surg Oncol. 2008;15:2472–2481. doi: 10.1245/s10434-008-0023-y. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nojiri K, Kumamoto T, Takeda K, Endo I. R1 resection for aggressive or advanced colorectal liver metastases is justified in combination with effective prehepatectomy chemotherapy. Eur J Surg Oncol. 2011;37:336–343. doi: 10.1016/j.ejso.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Adam R, Shimada H, Azoulay D, Lévi F, Bismuth H. Role of neoadjuvant chemotherapy in the treatment of multiple colorectal metastases to the liver. Br J Surg. 2003;90:963–969. doi: 10.1002/bjs.4160. [DOI] [PubMed] [Google Scholar]
- Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061. doi: 10.1097/01.sla.0000145964.08365.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976–4982. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Welsh FK, Tekkis PP, O'Rourke T, John TG, Rees M. Quantification of risk of a positive (R1) resection margin following hepatic resection for metastatic colorectal cancer: an aid to clinical decision-making. Surg Oncol. 2008;17:3–13. doi: 10.1016/j.suronc.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- DeMatteo RP, Palese C, Jarnagin WR, Sun RL, Blumgart LH, Fong Y. Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg. 2000;4:178–184. doi: 10.1016/s1091-255x(00)80054-2. [DOI] [PubMed] [Google Scholar]
- Zorzi D, Mullen JT, Abdalla EK, Pawlik TM, Andres A, Muratore A, et al. Comparison between hepatic wedge resection and anatomic resection for colorectal liver metastases. J Gastrointest Surg. 2006;10:86–94. doi: 10.1016/j.gassur.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Kokudo N, Miki Y, Sugai S, Yanagisawa A, Kato Y, Sakamoto Y, et al. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: minimum surgical margins for successful resection. Arch Surg. 2002;137:833–840. doi: 10.1001/archsurg.137.7.833. [DOI] [PubMed] [Google Scholar]
- Ng JK, Urbanski SJ, Mangat N, McKay A, Sutherland FR, Dixon E, et al. Colorectal liver metastases contract centripetally with a response to chemotherapy: a histomorphologic study. Cancer. 2008;112:362–371. doi: 10.1002/cncr.23184. [DOI] [PubMed] [Google Scholar]
- Benoist S, Brouquet A, Penna C, Julié C, El Hajjam M, Chagnon S, et al. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol. 2006;24:3939–3945. doi: 10.1200/JCO.2006.05.8727. [DOI] [PubMed] [Google Scholar]
- Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1107. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- Gruenberger B, Tamandl D, Schueller J, Scheithauer W, Zielinski C, Herbst F, et al. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol. 2008;26:1830–1835. doi: 10.1200/JCO.2007.13.7679. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]


