Abstract
Background Microwave ablation (MWA) is increasingly used to achieve local control for liver tumours. This study sought to examine a monocentric experience with MWA, with a primary hypothesis that primary tumour histology was a significant predictor of early recurrence.
Methods Retrospective single-institution review identified consecutive patients with liver tumours treated by MWA. Cox proportional hazards models assessed significance of prognostic variables.
Results Seventy-two patients (43 female, 60%) underwent 83 MWA procedures for 157 tumours. Tumour histologies included hepatocellular cancer (10 operations), colorectal metastases (39), metastatic carcinoid (20) and other (14). The median tumour size was 2.0 cm. A concomitant liver resection was performed in 50 cases (60%). Crude peri-operative morbidity and mortality rates were 16% and 1%, respectively. The median follow-up was 16 months. Ablations were complete for 149 out of 157 tumours (95%). The median overall and recurrence-free survivals were 36 and 18 months, respectively. There was no difference in time to recurrence between the primary tumour types. In multivariable models, recurrence-free survival was independently associated with the use of neoadjuvant [hazard ratio (HR): 2.90, 95% confidence interval (CI): 1.09–7.76, P = 0.034] and adjuvant chemotherapy (HR: 0.36, 95% CI: 0.15–0.82, P = 0.016).
Conclusions MWA is a safe and feasible approach for local control of liver tumours. While chemotherapy administration was associated with time to recurrence after MWA, larger studies are needed to corroborate these findings.
Introduction
Surgical resection is generally considered the gold standard for potentially curative treatments of liver cancer.1,2 Unfortunately, many patients with hepatic tumours present with lesions that are deemed unresectable for a variety of technical, anatomic and patient-related reasons.3 For example, only 20–25% of patients with colorectal liver metastases (CLM)4 and 30–35% of patients with hepatocellular carcinoma (HCC)5 will ultimately undergo a resection. The available treatment options for unresectable patients include systemic chemotherapy, ablative techniques, hepatic artery-directed therapies, external beam radiation therapy and isolated liver perfusion.4,6–9
Experience with microwave ablation (MWA) for liver tumours has grown in the last several decades. Initially, the ablation zone size was limited by a single-antennae approach, and only tumours ≤2 cm were considered for treatment.10 Eventually, larger lesions could be treated by repositioning antennae11 or using multiple antennae simultaneously12 to create larger zones of coagulative necrosis. Recent reports suggest that tumours up to 6–9 cm in size may be managed with MWA.13,14 In comparison to radiofrequency ablation (RFA), MWA offers theoretical advantages of faster delivery, a wider zone of active heating, higher intratumoral temperatures and less susceptibility to heat sink when performed near large vessels.4,13–15 MWA has also been associated with lower median operating room costs ($13 389 for MWA compared with $25 687 for RFA).14
Based on a phase II trial13 and large single-centre experience14 from the USA, successful complete tumour ablation can be achieved for >90% of patients who undergo MWA. Tumour control at the site of ablation was excellent in these series, with only 2.7–6.0% of patients recurring locally. Unfortunately, nearly half the patients recurred at remote hepatic and extrahepatic sites. Factors that may predispose some patients to recur after MWA have not been well established. The objective of this study was to identify the demographic and clinical characteristics that may predispose patients to early local and/or distant recurrence after hepatic MWA. The primary hypothesis was that primary tumour histology was a significant predictor of early recurrence.
Methods
After Institutional Review Board approval, a single-institution retrospective review of all patients with hepatic malignancies who had been treated with MWA at Froedtert Hospital (Milwaukee, WI, USA) between July 2007 and June 2011 was performed. Treatment planning for patients with liver tumours is orchestrated through a weekly multidisciplinary conference of medical oncologists, radiation oncologists, interventional radiologists, hepatologists, transplant surgeons and surgical oncologists. This study encompasses all approaches to MWA (open, laparoscopic and percutaneous) in addition to including both disciplines of surgery and interventional radiology.
In addition to standard demographic data, the clinical features of each patient were collected: histological type of tumour, tumour diameter, number of tumours, extent of liver tumour burden (unilateral versus bilateral), presence of background liver disease, presence of extrahepatic disease, previous treatments received and adjuvant treatments administered. All operations were performed with curative intent via an ultrasound-guided16 open or laparoscopic surgical approach (under general anaesthesia) or with a computed tomography (CT)-guided percutaneous approach (under light sedation). Evident™ MWA Surgical Antennae, Evident™ MWA Pump Tubing and Chambers, Valleylab™ MW Ablation Generators and Valleylab™ MW Ablation Pumps were used for all procedures (Covidien™, formerly Valleylab™; Boulder, CO, USA), with the generator set to 45 watts. Typically, a single antennae application would last 10 min, although the actual time of application was recorded for each operation. In some instances, concurrent hepatic and/or extrahepatic resections were performed, and in these instances the margin status was recorded.
The Clavien–Dindo17 system was used to classify adverse events that occurred within 90 days after MWA. Death within 90 days was considered peri-operative mortality. Recurrence-free survival (RFS) was the interval from the date of ablation until the date when either local or distant recurrence was noted, or follow-up ended. Although not the primary endpoint owing to heterogeneous tumour histologies, overall survival (OS) analyses were included and defined as the interval from the date of ablation until either the date of death or end of follow-up. All patients had previous cross-sectional imaging with either contrast-enhanced CT or magnetic resonance imaging (MRI). Ablations were considered incomplete if any amount of pre-procedure contrast enhancement patterns persisted on the immediate post-procedure CT or MRI, as determined by a dedicated hepatobiliary radiologist. Thereafter, patients were followed with contrast-enhanced cross-sectional imaging every 3 months for the first year, and then biannually. Recurrent disease within 1 cm of the ablation site was considered a local recurrence.13 Length of stay in the hospital was calculated by total days; outpatient procedures were considered a 1-day stay. Patients who had incomplete ablation or known residual disease were excluded from recurrence analysis. OS analysis included all patients.
Statistical analysis
With patients divided into histological groups, descriptive statistics were calculated for all variables. Between-group differences were tested with Fisher's exact (categorical variables) and Mann–Whitney U-tests (continuous variables). Survival curves were generated using the Kaplan–Meier method, and compared with the log-rank test for equality of survivor functions. Cox proportional hazards models were used to test all demographical and clinicopathological variables for independent prognostic significance with regards to timing of recurrence. Because tumour histology was the primary variable of interest, it was automatically included in the final model. Any prognostic factors showing P-values <0.2 on univariate analysis were included in a multivariable model. Possible variables associated with recurrence from previous reports of RFA (age, tumor size, and chemotherapy use) were also included in the final model. Significance was set at α = 0.05. Stata IC 12 (StataCorp LP, College Station, TX, USA) was used for all statistical analyses.
Results
Seventy-two patients (38 female, 53%) underwent 83 MWA procedures. In total, 157 tumours were treated with MWA. Characteristics of the study populations are shown in Table 1 by tumour type. The cancer histologies in the other malignancies category were one gastrointestinal stromal tumour, one anal squamous cell cancer, five breast cancers, one cholangiocarcinoma, one clear cell carcinoma, one melanoma, two ovarian cancers, one renal cell carcinoma and one sarcoma. Ablations were relatively uncommon for tumours in segments 1–3 because of the largely resectable nature of this anatomic position (Fig. 1).
Table 1.
Characteristics of 83 microwave ablation procedures, by primary tumour type
| Variable | HCC | CLM | MC | Other cancer | Total | P-value |
|---|---|---|---|---|---|---|
| n = 10 | n = 39 | n = 20 | n = 14 | n = 83 | ||
| Median age, years (range) | 71 (56–88) | 62 (38–84) | 58 (25–82) | 58 (42–76) | 60 (25–88) | .050 |
| Gender, n (%) | .023 | |||||
| Female | 2 | 14 | 12 | 10 | 38 (46) | |
| Male | 8 | 25 | 8 | 4 | 45 (54) | |
| Descent, n (%) | .563 | |||||
| Caucasian | 7 | 33 | 18 | 13 | 71 (86) | |
| African-American | 3 | 4 | 2 | 1 | 10 (12) | |
| Hispanic | 2 | 2 (2) | ||||
| Synchronous, n (%) | <0.001 | |||||
| Yes | — | 30 | 12 | 1 | 43 (61) | |
| No | — | 9 | 7 | 12 | 28 (39) | |
| Route of ablation, n (%) | .001 | |||||
| Open | 4 | 34 | 20 | 8 | 66 (80) | |
| Laparoscopic | 3 | 3 | 4 | 10 (12) | ||
| Percutaneous | 3 | 2 | 2 | 7 (8) | ||
| Number of tumours ablated, n (%) | .004 | |||||
| 1 | 9 | 26 | 6 | 12 | 53 (64) | |
| 2 | 1 | 10 | 6 | 1 | 18 (22) | |
| ≥3 | 3 | 8 | 1 | 12 (14) | ||
| Median ablated tumour size, cm (range) | 2.0 (0.9–3.0) | 1.5 (1.0–5.5) | 1.6 (1.0–5.0) | 2.2 (0.9–3.0) | 2.0 (0.9–5.5) | .161 |
| Median no. probe applications (range) | 2 (1–4) | 2 (1–8) | 2.5 (1–18) | 2 (1–6) | 2 (1–18) | .748 |
| Median cumulative ablation time, min (range) | 18 (10–40) | 20 (5–70) | 20 (5–180) | 20 (10–60) | 20 (5–180) | .850 |
| Neoadjuvant chemotherapy, n (%) | <0.001 | |||||
| Yes | 33 | 7 | 5 | 45 (54) | ||
| No | 10 | 6 | 13 | 9 | 38 (46) | |
| Adjuvant chemotherapy, n (%) | .055 | |||||
| Yes | 15 | 9 | 4 | 28 (34) | ||
| No | 10 | 23 | 11 | 10 | 54 (66) | |
| Concomitant hepatectomy, n (%) | .227 | |||||
| None | 7 | 13 | 4 | 9 | 33 (40) | |
| Wedge resection/segmentectomy | 2 | 10 | 7 | 2 | 21 (25) | |
| Sectionectomy | 1 | 9 | 6 | 2 | 18 (22) | |
| Lobectomy | 7 | 2 | 1 | 10 (12) | ||
| Extended lobectomy | 1 | 1 (1) | ||||
| Extrahepatic disease, n (%) | .018 | |||||
| None present | 9 | 35 | 11 | 12 | 67 (81) | |
| Present and resected | 1 | 4 | 8 | 1 | 14 (17) | |
| Present, but unresectable | 1 | 1 | 2 (2) |
HCC, hepatocellular carcinoma; CLM, colorectal liver metastases; MC, metastatic carcinoid.
Figure 1.
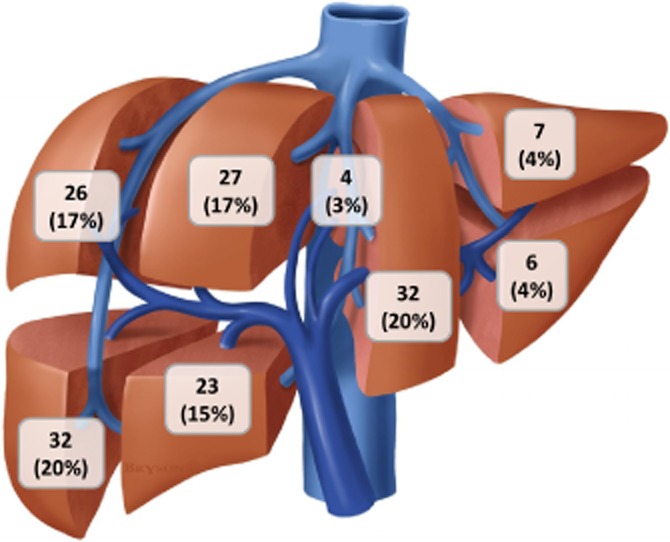
The number of tumours ablated by a liver segment during 83 microwave ablation procedures (n = 157). Published with permission from © brysonbiomed.com
The median hospital stay for an open ablation was significantly longer (6 days, range: 4–34, P < 0.001) than either laparoscopic (2 days, range: 1–7) or percutaneous ablations (1 day, range: 1–2). Even when patients who had undergone a concomitant surgical resection were excluded, these differences in length of stay remained significant (P < 0.001). All complications (n = 13, 16% morbidity rate) occurred after open ablations, and five complications were grade III or higher. One patient died from rapid progression of MC 52 days after a complication-free hemihepatectomy and MWA (overall 90-day mortality rate of 1%). The overall MWA success rate was 95% for curative-intent ablations, with 8 of 157 tumours incompletely ablated on initial postoperative imaging (all eight from open operations). An additional four patients with MC had unanticipated miliary disease making a complete ablation of all disease unfeasible. Between those with unresectable extrahepatic disease (2 patients), those with incomplete ablations (8 patients) and those with untargeted miliary disease (4 patients), there were 14 patients (17%) who had persistent disease after MWA.
The median follow-up was 16 months (range: 2–42). The 14 patients with persistent disease after MWA (who had a total of 28 tumors targeted for ablation) were excluded from further RFS analysis. Of the remaining 129 tumours (82%), there were 10 local recurrences (8%; all 10 recurrences were from tumors in segments 4–8). Other patterns of recurrence are shown in Table 2. At the end of follow-up, 22 out of 72 patients (31%) had died. Median, 1-, and 3-year RFS and OS rates for each primary cancer type are shown in Table 2. Neither RFS (P = 0.407) nor OS (0.862) curves significantly differed between cancer groups (Fig. 2). In multivariable models, the primary tumour type (P = 0.961, Table 3) and tumour size (P = 0.402) were not associated with time to recurrence. Younger age (P = 0.039) and treatment with neoadjuvant chemotherapy (P = 0.034) were independently associated with earlier cancer recurrence, whereas treatment with adjuvant chemotherapy (P = 0.016) was independently associated with a longer time to recurrence.
Table 2.
Outcomes of 83 microwave ablation procedures, by primary tumour type
| Variable | HCC | CLM | MC | Other cancer | Total | P-value |
|---|---|---|---|---|---|---|
| n = 10 | n = 39 | n = 20 | n = 14 | n = 83 | ||
| Complete ablation, n (%) | .006 | |||||
| Yes | 9 | 38 | 14 | 14 | 75 (90) | |
| No | 1 | 1 | 6 | 8 (10) | ||
| No. patients with complication, n (%) | .747 | |||||
| No complication | 8 | 34 | 16 | 12 | 70 (84) | |
| Grade 1–2 | 1 | 3 | 3 | 1 | 8 (10) | |
| Grade ≥3 | 1 | 2 | 1 | 1 | 5 (6) | |
| Median hospital stay, days (range) | 2 (1–11) | 6 (1–34) | 6 (5–24) | 5 (1–7) | 5 (1–34) | .003 |
| Recurrence, n (%) | .039 | |||||
| Persistent disease after ablation | 1 | 3 | 9 | 1 | 14 (17) | |
| No recurrence | 6 | 15 | 3 | 8 | 32 (39) | |
| Recurrence at ablation site only | 1 | 6 | 2 | 1 | 10 (12) | |
| Remote hepatic recurrence only | 1 | 7 | 6 | 3 | 17 (20) | |
| Extrahepatic recurrence only | 3 | 1 | 4 (5) | |||
| Intra- and extrahepatic recurrence | 1 | 5 | 6 (7) | |||
| Recurrence-free survival | .407 | |||||
| Median, months | NR | 10.9 | 24.4 | 17.9 | 18.1 | |
| 1-year, % | 59 | 47 | 76 | 58 | 59 | |
| 3-year, % | NR | 29 | 29 | 14 | ||
| Overall survival | .862 | |||||
| Median, months | NR | 36.1 | 33.0 | NR | 36.1 | |
| 1-year, % | 83 | 92 | 94 | 100 | 95 | |
| 3-year, % | NR | 35 | 43 | 75 | 41 |
HCC, hepatocellular carcinoma; CLM, colorectal liver metastases; MC, metastatic carcinoid; NR, not reached.
Figure 2.
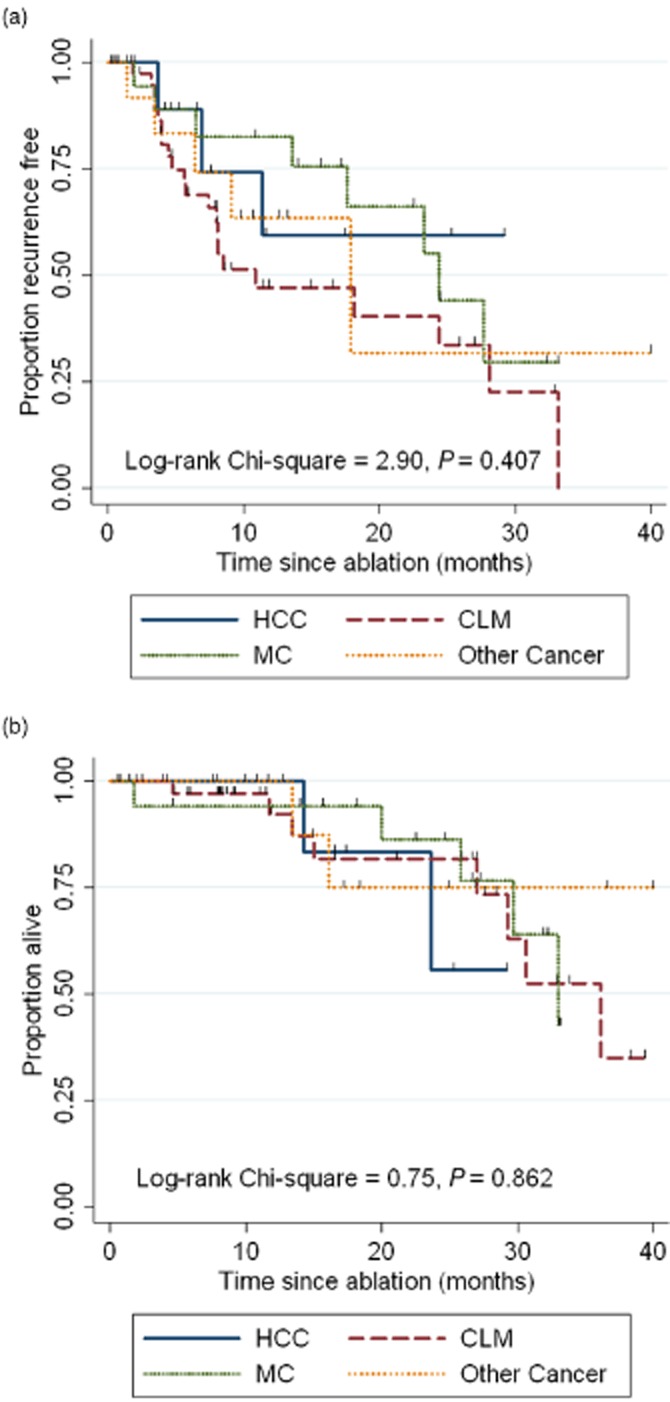
Recurrence-free survival (a) and overall survival (b) curves, by primary malignancy. HCC, hepatocellular carcinoma; CLM, colorectal liver metastatses; MC, metastatic carcinoid
Table 3.
Multivariable analysis of factors associated with early recurrence after microwave ablation
| Hazard ratio (95% confidence interval) | P-value | |
|---|---|---|
| Histology | .958 | |
| Hepatocellular carcinoma | 1.00 | |
| Colorectal cancer | .94 (0.21–4.12) | .932 |
| Metastatic carcinoid | .72 (0.15–3.36) | .675 |
| Other cancer | .89 (0.19–4.05) | .878 |
| Age, years | .97 (0.94–1.00) | .039 |
| Tumour size, cm | 1.16 (0.82–1.62) | .402 |
| Use of neoadjuvant chemotherapy | 2.90 (1.09–7.76) | .034 |
| Use of adjuvant chemotherapy | .36 (0.15–0.82) | .016 |
Discussion
Both technology and molecular understanding of hepatic tumour biology have improved in recent years. As a result, the number of treatment options for patients with unresectable tumours continues to expand. However, published evidence supporting systemic therapy compared with radiation or various liver-directed therapies for these patients is scarce. MWA is a relatively new thermoablative modality used to provide local control of liver tumours, and the history of its clinical utility has been thoughtfully outlined by other authors.13,14,18,19 Ablation has been proposed for several clinical scenarios: curative intent,2,20 reducing tumour burden,21,22 and bridging to transplantation.23,24 The factors associated with risk of early recurrence after MWA are not well described, and are important considerations when selecting patients for such treatment.
This study found that timing of recurrence after MWA was not significantly different between primary tumour types. Younger patients and those treated with neoadjuvant chemotherapy were at the highest risk for recurrence, whereas adjuvant chemotherapy given after MWA was independently associated with prolonged RFS. In similar studies of RFA for CLM, the size and number of tumours treated have been consistently shown to affect local recurrence rates;1 however, these associations were not found to be significant. As experience with MWA grows and larger patient populations can be studied, other factors associated with early recurrence may be identified.
In this study, most liver tumours were originally characterized by contrast-enhanced CT, and the same imaging modality was therefore used for surveillance. Two patients had liver tumours initially evident on MRI which were not clearly characterized on CT, and therefore MRI was used for surveillance. After MWA, all patients underwent contrast-enhanced cross-sectional imaging every 3 months for the first year, and biannually thereafter. Positron emission tomography was not used for surveillance in this study, and guidelines have not been established recommending its use after ablation.1 To our knowledge, no specific imaging has shown superior capability to detect recurrence after thermoablation.
The safety of MWA has been established in previous studies, with a peri-procedural mortality rate <0.01%.25,26 While one patient died within 90 days, this was attributed to a aggressive tumour biology and not a result of ablation. A systematic review of MWA outcomes suggest that aside from procedural pain and pyrexia, ascites and biliary complications are among the most common adverse events.18 Over half of the patients in this study underwent a concomitant hepatectomy, and as other authors have noted,14,19 attributing morbidity to ablation itself is challenging. Open operations accounted for all complications, suggesting that the laparotomy incision and/or transected liver surface represent the major source of morbidity after ablation. Percutaneous MWA avoids the need for general anaesthesia, allows for the shortest hospital stay and for instant feedback on the success of ablation. Small-volume disease, particularly on the liver capsule, may not be appreciated with a percutaneous approach. As a result of anatomic factors, some lesions are difficult to reach percutaneously. Laparoscopy and open surgery allow for gross hepatic inspection and the use of intra-operative ultrasound, which may find unanticipated lesions in up to 10–20% of operations.27
Some limitations of this study warrant attention. Methodologically, MWA was performed on heterogeneous tumour histologies for non-uniform indications. A concurrent hepatectomy may influence outcomes after thermoablation, and the interrelationship between such simultaneous treatments has not been well established. Some late recurrences may not have been appreciated because of a short follow-up interval. Although the low number of purely local recurrences is encouraging, it limits a rigorous statistical investigation. Therefore, future studies with larger numbers of tumours will be needed to better understand the clinical factors that may specifically predispose patients to local failure after MWA.
In conclusion, this study finds that MWA for liver malignancies offers a local recurrence rate of 8% and a median RFS of 18 months. The durability of local control did not vary significantly between primary tumour types. Younger patients and patients with larger tumours were found to be at an increased risk for early recurrence, which should be thoughtfully considered when MWA is proposed for the treatment of liver tumours. The safety profile for MWA itself is excellent, with open operations accounting for all morbidity in this series. Given that each route of ablation offers certain benefits and drawbacks, further study is needed to address the comparative long-term benefit of open, laparoscopic and percutaneous MWA.
Acknowledgments
Figure 1 designed by Bryson Biomedical Illustration, Inc. (Langhorne, PA, USA).
Conflicts of interest
None declared.
No sources of funding for this research.
No financial or commercial interests to disclose.
References
- Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD, 3rd, Dorfman GS, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28:493–508. doi: 10.1200/JCO.2009.23.4450. [DOI] [PubMed] [Google Scholar]
- Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006;141:460–466. doi: 10.1001/archsurg.141.5.460. discussion 6-7. [DOI] [PubMed] [Google Scholar]
- Kitisin K, Packiam V, Steel J, Humar A, Gamblin TC, Geller DA, et al. Presentation and outcomes of hepatocellular carcinoma patients at a western centre. HPB. 2011;13:712–722. doi: 10.1111/j.1477-2574.2011.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S, Pawlik TM. Outcomes of ablation versus resection for colorectal liver metastases: are we comparing apples with oranges? Ann Surg Oncol. 2009;16:2422–2428. doi: 10.1245/s10434-009-0491-8. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos GC, Lang H, Frilling A, Molmenti EP, Paul A, Nadalin S, et al. Resectability of hepatocellular carcinoma: evaluation of 333 consecutive cases at a single hepatobiliary specialty center and systematic review of the literature. Hepatogastroenterology. 2006;53:322–329. [PubMed] [Google Scholar]
- Ben-Josef E, Lawrence TS. Radiotherapy for unresectable hepatic malignancies. Semin Radiat Oncol. 2005;15:273–278. doi: 10.1016/j.semradonc.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Boone BA, Bartlett DL, Zureikat AH. Isolated hepatic perfusion for the treatment of liver metastases. Curr Probl Cancer. 2012;36:27–76. doi: 10.1016/j.currproblcancer.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Dubel GJ, Soares GM. Regional infusion-radioembolization. Surg Oncol Clin N Am. 2008;17:957–985. doi: 10.1016/j.soc.2008.04.008. xii. [DOI] [PubMed] [Google Scholar]
- Groeschl RT, Nachmany I, Steel JL, Reddy SK, Glazer ES, de Jong MC, et al. Hepatectomy for noncolorectal non-neuroendocrine metastatic cancer: a multi-institutional analysis. J Am Coll Surg. 2012;214:769–777. doi: 10.1016/j.jamcollsurg.2011.12.048. [DOI] [PubMed] [Google Scholar]
- Seki T, Wakabayashi M, Nakagawa T, Itho T, Shiro T, Kunieda K, et al. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994;74:817–825. doi: 10.1002/1097-0142(19940801)74:3<817::aid-cncr2820740306>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Ohmoto K, Miyake I, Tsuduki M, Shibata N, Takesue M, Kunieda T, et al. Percutaneous microwave coagulation therapy for unresectable hepatocellular carcinoma. Hepatogastroenterology. 1999;46:2894–2900. [PubMed] [Google Scholar]
- Wright AS, Lee FT, Jr, Mahvi DM. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol. 2003;10:275–283. doi: 10.1245/aso.2003.03.045. [DOI] [PubMed] [Google Scholar]
- Iannitti DA, Martin RC, Simon CJ, Hope WW, Newcomb WL, McMasters KM, et al. Hepatic tumor ablation with clustered microwave antennae: the US Phase II trial. HPB. 2007;9:120–124. doi: 10.1080/13651820701222677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17:171–178. doi: 10.1245/s10434-009-0686-z. [DOI] [PubMed] [Google Scholar]
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25(Suppl. 1):S69–S83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- Van Vledder MG, Boctor EM, Assumpcao LR, Rivaz H, Foroughi P, Hager GD, et al. Intra-operative ultrasound elasticity imaging for monitoring of hepatic tumour thermal ablation. HPB. 2010;12:717–723. doi: 10.1111/j.1477-2574.2010.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SL, Gravante G, Metcalfe MS, Strickland AD, Dennison AR, Lloyd DM. Efficacy and safety of microwave ablation for primary and secondary liver malignancies: a systematic review. Eur J Gastroenterol Hepatol. 2009;21:599–605. doi: 10.1097/MEG.0b013e328318ed04. [DOI] [PubMed] [Google Scholar]
- Jones C, Badger SA, Ellis G. The role of microwave ablation in the management of hepatic colorectal metastases. Surgeon. 9:33–37. doi: 10.1016/j.surge.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Liang P, Yu J, Yu XL, Wang XH, Wei Q, Yu SY, et al. Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: a multicentre analysis of 1363 treatment-naive lesions in 1007 patients in China. Gut. 2011;61:1100–1101. doi: 10.1136/gutjnl-2011-300975. [DOI] [PubMed] [Google Scholar]
- Gamblin TC, Christians K, Pappas SG. Radiofrequency ablation of neuroendocrine hepatic metastasis. Surg Oncol Clin N Am. 2011;20:273–279. doi: 10.1016/j.soc.2010.11.002. vii–viii. [DOI] [PubMed] [Google Scholar]
- Mayo SC, de Jong MC, Pulitano C, Clary BM, Reddy SK, Gamblin TC, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol. 2010;17:3129–3136. doi: 10.1245/s10434-010-1154-5. [DOI] [PubMed] [Google Scholar]
- Gamblin TC, Geller DA. Downstaging hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1466–1468. doi: 10.1002/lt.20528. [DOI] [PubMed] [Google Scholar]
- Heckman JT, Devera MB, Marsh JW, Fontes P, Amesur NB, Holloway SE, et al. Bridging locoregional therapy for hepatocellular carcinoma prior to liver transplantation. Ann Surg Oncol. 2008;15:3169–3177. doi: 10.1245/s10434-008-0071-3. [DOI] [PubMed] [Google Scholar]
- Bertot LC, Sato M, Tateishi R, Yoshida H, Koike K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol. 2011;21:2584–2596. doi: 10.1007/s00330-011-2222-3. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Meloni F, Solbiati L, Zanus G. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2011 doi: 10.1007/s00270-011-0241-8. in press. [DOI] [PubMed] [Google Scholar]
- van Vledder MG, Pawlik TM, Munireddy S, Hamper U, de Jong MC, Choti MA. Factors determining the sensitivity of intraoperative ultrasonography in detecting colorectal liver metastases in the modern era. Ann Surg Oncol. 2010;17:2756–2763. doi: 10.1245/s10434-010-1108-y. [DOI] [PubMed] [Google Scholar]


