Abstract
Objectives Patients undergoing complex hepatopancreatobiliary (HPB) operations are at high risk for surgical site infection (SSI). Factors such as biliary obstruction, operative time and pancreatic or biliary fistulae contribute to the high SSI rate. The purpose of this study was to analyse whether a multifactorial approach would reduce the incidence and cost of SSI after HPB surgery.
Methods From January 2007 to December 2009, 895 complex HPB operations were monitored for SSI through the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). In 2008, surgeon-specific SSI rates were provided to HPB surgeons, and guidelines for the management of perioperative factors were established. Observed SSI rates were monitored before and after these interventions. Hospital cost data were analysed and cost savings were calculated.
Results Observed SSI for hepatic, pancreatic and complex biliary operations decreased by 9.6% over a 2-year period (P < 0.03). The excess cost per SSI was US$11 462 and was driven by increased length of stay and hospital readmission for infection. Surgeons rated surgeon-specific feedback on SSI rate as the most important factor in improvement.
Conclusions High SSI rates following complex HPB operations can be improved by a multifactorial approach that features process improvements, individual surgeon feedback and reduced variation in patient management.
Introduction
Despite improvements in mortality following hepatic, pancreatic and complex biliary surgery, rates of overall morbidity and surgical site infection (SSI) subsequent to these procedures remain high.1 Reducing postoperative infection is important following hepatopancreatobiliary (HPB) operations because mortality has been associated with such complications.2–5 Historically, the rate of SSIs occurring within 30 days of HPB surgery has been high, reaching 20–40%.6–8 However, very few reports have addressed methods to reduce SSIs in these patients.
The factors that contribute to the occurrence of SSIs after HPB surgery are numerous and are more clearly subdivided into preoperative, intraoperative and postoperative categories. Preoperative factors include patient comorbid conditions (obesity, cardiopulmonary disease, bleeding disorder), malnutrition (weight loss, anorexia) and HPB pathology (bactibilia, biliary obstruction, malignancy).1,9,10 Intraoperative factors include long operative times,11,12 significant blood loss related to the division of major vascular structures,12,13 and complex biliary/pancreatic–enteric anastomoses.10 Postoperative factors include the development of pancreatic and biliary fistulae.14,15 The aims of this study were to determine whether a multifactorial approach to address known risk factors would decrease the incidence of SSI in HPB surgical patients and, if so, to document the cost savings.
Materials and methods
American College of Surgeons National Surgical Quality Improvement Program
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) is a national, validated, outcomes-based, risk-adjusted, peer-controlled programme for the measurement and enhancement of the quality of surgical care. The sampling strategy, data abstraction procedures, variables collected and structure have been published.16–19 A total of 136 preoperative (patient characteristics), intraoperative (processes of care) and postoperative (adverse outcomes) variables were prospectively collected by a trained, certified nurse reviewer (EMK). Surgical clinical nurse reviewers also ensure the validity of their data by assessing physician documentation and by contacting patients directly. Outcomes are assessed at 30 days after the index operation, and highly standardized and validated definitions are employed. During the study period, ACS-NSQIP used a systematic random sampling process that employed an 8-day cycle, whereby the first 40 cases to fulfil the inclusion and exclusion criteria were evaluated. This process therefore excluded an over-sampling of minor cases. Patients aged < 16 years were excluded. Quality was ensured by inter-rater reliability audits, as well as by online decision support, so that the level of disagreement within ACS-NSQIP was only 1.53% for all variables during the study period.
Patient population
The ACS-NSQIP database at Indiana University Hospital was queried to identify all patients who had undergone hepatic, pancreatic or complex biliary surgical procedures between 1 January 2007 and 31 December 2009. The current procedural codes for these operations can be found in the Appendix. These codes were used to identify all patients for analysis with complete 30-day outcomes for all variables. During the study period, more than 90% of HPB operations performed at Indiana University Hospital were captured in the ACS-NSQIP database. Institutional review board approval for the analysis of patients undergoing HPB surgery was obtained prior to initiation of this analysis.
Risk factors
During the first year of this analysis (2007), SSI rates were determined for hepatic, pancreatic and complex biliary surgery. In 2007 efforts to improve intraoperative glucose and temperature control, and to limit intraoperative blood transfusions were initiated. Once the observed SSI rates were reported in 2008, surgeon-specific rates were determined for hepatic, pancreatic and complex biliary operations, respectively, and fed back to the individual surgeons. Specific factors thought to be associated with high risk for the development of SSI were also identified (Table 1). These preoperative, intraoperative and postoperative factors were discussed by the HPB surgeons in 2008 when the 2007 data were available. Considerable consensus was reached with respect to preoperative nutrition, perioperative antibiotic management, blood transfusions, glycaemic control, temperature control, surgical technique and wound protection. Standardization of postoperative oxygenation, glucose control, and drain and wound management was also implemented. All of these changes were intended to streamline care provided to patients undergoing HPB surgery performed by eight HPB surgeons. Outcomes were then followed in 2008 and 2009 after the implementation of these changes. Thus, multiple potential risk factors were addressed simultaneously.
Table 1.
Risk factors for surgical site infection in hepatopancreatobiliary surgery
| Preoperative | Bactibilia |
| Biliary obstruction | |
| Diabetes | |
| Malignancy | |
| Nutrition | |
| Obesity | |
| Intraoperative | Antibiotic administration |
| Blood transfusions | |
| Glycaemic control | |
| Surgical technique | |
| Temperature control | |
| Wound protection | |
| Postoperative | Drain management |
| Fistula management | |
| Glycaemic control | |
| Oxygenation | |
| Wound management |
Outcomes
Outcomes collected by ACS-NSQIP were assessed at 30 days regardless of whether the patient had been discharged, remained hospitalized or had been admitted to a different institution. Outcomes including patient demographics, comorbidities, mortality (all-cause death within 30 days after the operation) and 30-day overall morbidity were recorded, but the primary endpoint assessed in this study was SSI. Surgical site infections were divided into two groups comprising superficial (superficial or deep incisional) infection and organ space infection (OSI), respectively. Superficial SSI was defined as an infection which occurred within 30 days after the index operative procedure and involved either skin/subcutaneous tissue (superficial) or the fascial/muscle layer (deep) of the incision, in a patient with at least one of the following:
purulent drainage from the incision but not from the organ/space component of the surgical site;
organisms isolated from an aseptically obtained culture of fluid or tissue from the superficial incision;
at least one of the signs or symptoms of infection, including pain or tenderness, localized swelling, redness and heat and a superficial incision deliberately opened by a surgeon and either found to be culture-positive or not cultured (a culture-negative finding did not meet this criterion);
a deep incision spontaneously dehisced or deliberately opened by a surgeon and either found to be culture-positive or not cultured (a culture-negative finding did not meet this criterion) and at least one of the signs or symptoms of fever (>38 °C), localized pain or tenderness, and
an abscess or other evidence of infection involving the deep incision found on direct examination, during reoperation, or by histopathologic or radiologic examination.
Organ space infection was defined as any infection that occurred within 30 days after the index operative procedure, appeared to be related to the operative procedure, involved any part of the body, excluding the skin incision, fascia or muscle layers, that had been opened or manipulated during the operative procedure in a patient with at least one of the following:
purulent drainage from a drain that was placed through a stab wound into the organ or space;
organisms isolated from an aseptically obtained culture of fluid or tissue in the organ or space, and
an abscess or other evidence of infection involving the organ or space found on direct examination, during reoperation, or by histopathologic or radiologic examination.
The observed SSI rate was defined as the percentage of SSIs observed in all patients undergoing HPB surgery at Indiana University Hospital during the study time period who were monitored via ACS-NSQIP. The expected SSI rate was defined as the observed SSI rate recorded by ACS-NSQIP for all participating centres matched for procedure type during the same period.
Secondary endpoints assessed in this analysis included: (i) length of stay (LoS); (ii) readmission rate, and (iii) variable direct cost. Length of stay included the hospital stay related to the index HPB operation as well as any additional days related to readmission(s). The 30-day all-cause readmission rate was used for this analysis. Variable direct costs for the index admission and readmissions were determined for each patient and then grouped by hepatic, pancreatic and complex biliary operations and averaged. Length of stay, readmissions and costs were also grouped by patients with: (i) no SSI; (ii) superficial SSI, and (iii) OSI.
HPB SSI questionnaire
In 2010, when 2009 SSI data became available, HPB attending surgeons and fellows were asked to rate 12 factors that may have contributed to the improvements in infection rates. The 12 factors in the HPB SSI Questionnaire were: (i) preoperative patient nutrition; (ii) intraoperative wound protection; (iii) intraoperative antibiotics; (iv) intraoperative glucose control; (v) intraoperative temperature control; (vi) intraoperative surgical technique; (vii) intraoperative blood loss/transfusion; (viii) postoperative oxygenation; (ix) postoperative glucose control; (x) postoperative drain management; (xi) postoperative wound management, and (xii) surgeon-specific SSI feedback. Each factor was graded on a 5-point Likert scale on which 1 = strongly disagree and 5 = strongly agree. Questionnaires were returned by seven HPB faculty members and two HPB fellows.
Statistical analyses
All statistical analyses were performed using sas Version 8.2 (SAS Institute, Inc., Cary, NC, USA). The Mann–Whitney U-test was used to compare means of continuous variables. The chi-squared test was used for non-parametric data. P-values of < 0.05 were considered to represent statistical significance for all comparisons.
Results
Surgical site infections
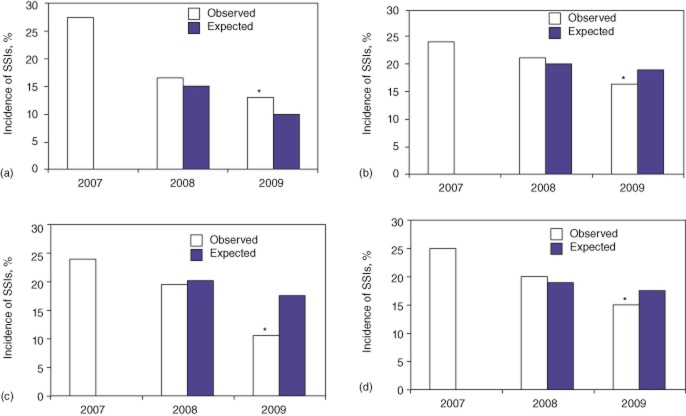
The 2007–2009 ACS-NSQIP dataset yielded 895 HPB procedures performed at Indiana University Hospital. Data on SSIs were initially subdivided according to whether they referred to hepatic (Fig. 1a), pancreatic (Fig. 1b) or complex biliary (Fig. 1c) procedures. Figure 1(a–c) compares the observed SSI rates in the initial period of the study (2007) and in the periods during (2008) and after (2009) the feedback of surgeon-specific SSI rates and the standardization of patient management. In addition, the expected SSI rates became available in 2008 and 2009 and provide a perspective of the trends for SSI in HPB surgery in the collective ACS-NSQIP database. Feedback and standardization decreased the incidence of observed SSI from 28.1% to 14.2% in hepatic operations, from 24.3% to 17.1% in pancreatic operations and from 24.0% to 11.4% in complex biliary operations. By 2009, the ratios of observed : expected (O : E ratios) SSI were 0.69, 0.79 and 0.47 for each of the three groups, respectively; thus each SSI rate was lower than the expected national mean. When the data were tabulated to examine all HPB operations (Fig. 1d), the SSI rate decreased from 24.8% to 15.2% to give an O : E ratio of 0.77 in 2009. This decrease in SSI rate of 9.6% from the initial observed 2007 rate to the final observed 2009 rate for hepatic, pancreatic and complex biliary operations was statistically significant (P < 0.03).
Figure 1.
Rates of surgical site infection (SSI) in (a) hepatic surgery, (b) pancreatic surgery, (c) complex biliary surgery and (d) composite hepatic, pancreatic and complex biliary surgery. *P < 0.03 versus 2007 observed SSI rate. Note that expected (national) SSI rates decreased in 2009 compared with 2008
Length of stay, readmissions and cost
Further analysis of the clinical and economic impact of SSIs in HPB surgery was performed for the entire study period. Groups were created to compare patients without SSI (80%), and those with either superficial SSI (10.1%) or OSI (9.9%). Mean LoS was 9 days, 12 days and 16 days after surgery in patients with no SSI, superficial SSI and OSI, respectively (Fig. 2), suggesting that the severity of infection prolonged hospital stay. Thirty-day readmission rates were 11.3%, 21.1% and 54.2% in patients with no SSI, superficial SSI and OSI, respectively (Fig. 3), which again suggests that the severity of infection increased the readmission rate following discharge from the index operation.
Figure 2.
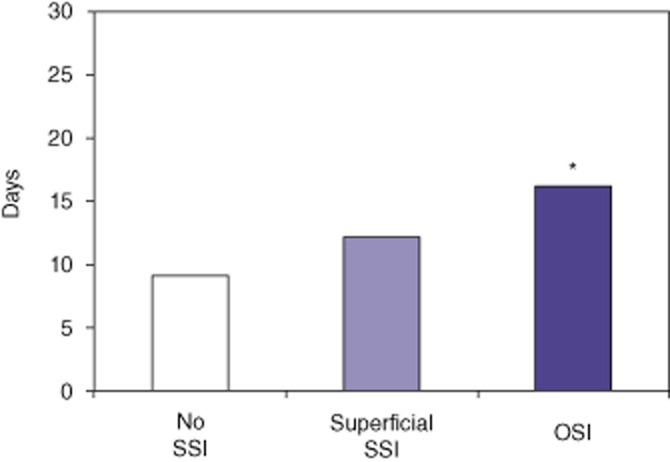
Length of stay in days following the operation. Patients were grouped according to whether they experienced no surgical site infection (SSI) (80.0%), superficial SSI (10.1%) or organ space infection (OSI; 9.9%). *P < 0.05 versus no SSI
Figure 3.
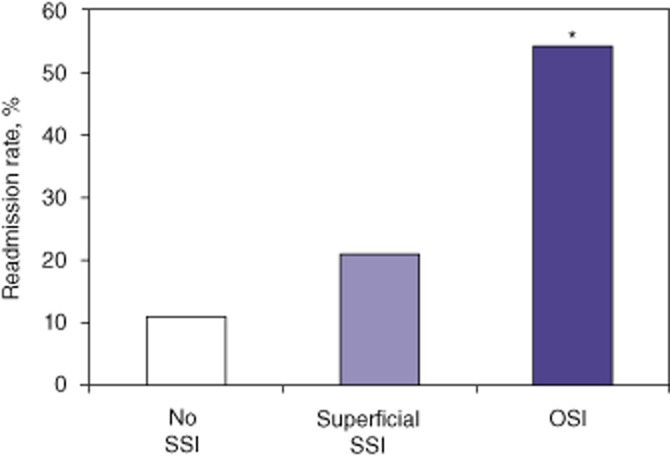
Readmission rates at 30 days in all patients. Patients were grouped according to whether they experienced no surgical site infection (SSI) (80.0%), superficial SSI (10.1%) or organ space infection (OSI; 9.9%). *P < 0.05 versus no SSI
Review of administrative databases revealed mean variable direct costs of US$12 255, US$18 142 and US$29 230 for patients with no SSI, superficial SSI and OSI, respectively (Fig. 4), which suggests that severe infections resulted in much higher medical costs. When the mean cost for patients with no SSI was compared with the mean cost for all SSIs (superficial plus organ space infections), a mean cost difference of US$11 462 per infection was observed. Compared with 2007, the overall HPB infection rate was reduced by 9.6%, which translated into 32.3 fewer infections per year. In 2009 342 HPB operations were performed. At a cost of US$11 462 extra per infection, the cost savings in 2009 amounted to US$370 223.
Figure 4.
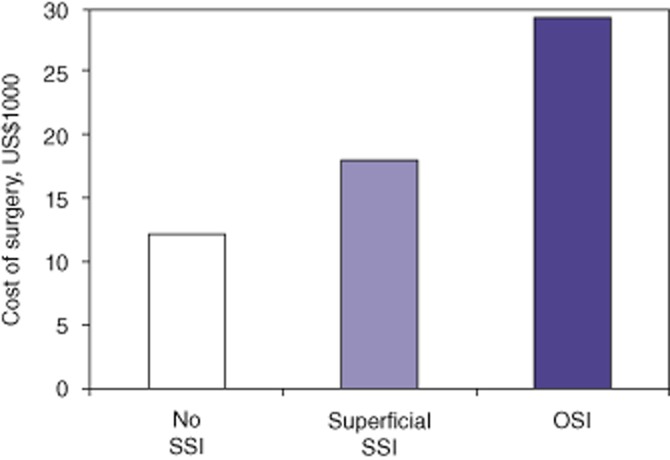
Mean direct variable hospital costs (US$1000). Patients were grouped according to whether they experienced no surgical site infection (SSI) (80.0%), superficial SSI (10.1%) or organ space infection (OSI; 9.9%). *P < 0.05 versus no SSI
Surgeon ratings of risk factors
The HPB surgeons and fellows thought that surgeon-specific SSI feedback was the most important factor in reducing the incidence of SSIs and awarded this factor a mean score of 4.75 (Table 2). Surgical technique and wound protection were believed to be the next most important factors; each of these achieved a mean score of 4.13. Antibiotic management, primarily re-dosing during long operations, was the fourth most important factor, achieving a mean score of 3.75. The next five factors obtained mean scores of 3.25 or 3.38, only slightly above a score designating ‘neutral’. Blood transfusions and wound management were rated as ‘neutral’, whereas postoperative oxygenation was not thought to play a role in reducing the occurrence of SSI.
Table 2.
Surgeon questionnaire ratings of factors influencing a decrease in the incidence of surgical site infection (SSI) in hepatopancreatobiliary surgery, scored on a scale of 1–5
| Mean score | |
|---|---|
| Surgeon SSI feedback | 4.75 |
| Surgical technique | 4.13 |
| Wound protection | 4.13 |
| Antibiotic management | 3.75 |
| Drain management | 3.38 |
| Glycaemic control, postoperative | 3.38 |
| Glycaemic control, intraoperative | 3.25 |
| Preoperative nutrition | 3.25 |
| Temperature control | 3.25 |
| Blood transfusions | 3.00 |
| Wound management | 3.00 |
| Postoperative oxygenation | 2.63 |
Discussion
Surgical site infection in HPB surgery remains an important morbidity that requires further attention from surgeons in order to improve patient outcomes. In the initial year of this study (2007), SSI rates were 28.1%, 24.3%, 24.0% and 24.9% for hepatic, pancreatic, complex biliary and all HPB operations, respectively. These data, provided by participation in ACS-NSQIP, stimulated the present group to strive for a lower SSI rate in HPB patients operated at this centre. As a result of surgeon-specific feedback and clinical management changes, SSI rates significantly decreased to 14.2%, 17.1%, 11.4% and 15.1% for hepatic, pancreatic, complex biliary and all HPB operations, respectively, in 2009. The clinical impact of SSI was evident in that patients with an infection had a longer LoS following surgery and required more readmissions. Approximately one half of infections were OSIs and these serious infections significantly (P < 0.05) increased LoS and readmissions. In turn, the economic impact of SSIs was fiscally relevant in that each SSI imposed an additional mean direct variable cost of US$11 462 on the health care system. The cost per SSI was primarily driven by the increased LoS and readmissions required by the necessity for more intensive medical therapy, including percutaneous drainage and reoperations for intra-abdominal abscesses.
Ambiru et al. documented incidences of SSI following HPB surgeries and reported SSI rates of 26%, 28% and 60% for hepatic, pancreatic and complex biliary procedures, respectively.10 In a univariate analysis, they identified significant risk factors for SSI to include preoperative obstructive jaundice, pancreatobiliary malignancies, number of enteric anastomoses, blood transfusion and postoperative glucose control.10 Further multivariate analysis found that biliary malignancies, three or more enteric anastomoses and poor postoperative glycaemic control were related to SSI.10 Factors such as an underlying malignancy or the number of anastomoses cannot easily be controlled. However, intra- and postoperative glucose control are most likely to be very important in HPB surgery. In the period referred to in the current study, tight postoperative glucose control was accomplished in 2007, but intraoperative glucose control was implemented in 2008.
A risk factor for SSI that is pertinent and has not been adequately studied is the presence of pancreatic or biliary fistulae.,14,15 The present group previously reported OSI rates in pancreatic surgery for 2006–2009 at all centres participating in ACS-NSQIP.20 This analysis found that OSI rates following pancreatic surgery were 10–11% and had not changed over a 4-year period. Organ space infections as documented in the ACS-NSQIP database represent a reasonable surrogate for Grade B and C fistulae as defined by the International Study Group on Pancreatic Fistula (ISGPS). Currently, efforts are underway at this institution to define the relationship between OSI and pancreatic fistula. In addition, a national Pancreatectomy Demonstration Project within ACS-NSQIP should further clarify this relationship and has the potential to improve outcomes.
A review of secondary endpoints in this study, including LoS, readmissions and cost, documented that they were driven primarily by the occurrence of SSIs. Furthermore, these endpoints were all found to increase progressively. These findings are not novel in that other surgical disciplines have reported that SSI increases LoS and readmission rates, and that OSI has a greater impact than superficial SSI in hip arthroplasty, colorectal, vascular and common general surgical procedures.21,24 Broex et al. published a meta-analysis reviewing studies that reported findings focusing on the absence or presence of SSI affecting LoS and cost.25 They found that health care-related costs were twice as high in patients with SSI and that this rise in costs was primarily dependent on an increased LoS. The findings in the present series are similar in that the 80.0% of HPB patients in whom SSI did not occur had the shortest LoS (9 days), the lowest readmission rate (11.0%) and incurred the lowest cost (US$12 225). The 10.1% of HPB patients in whom a superficial SSI occurred had an increased LoS (12 days) and a higher readmission rate (21.1%) and incurred higher costs (US$18 124). However, the 9.9% of HPB patients affected by an OSI had the longest LoS (16 days), the highest readmission rate (53.9%) and incurred the highest costs (US$29 230).
In this study, patients were found to have benefited from the changes implemented by this centre's HPB surgeons in terms of a lower incidence of SSI, as well as a shorter LoS and lower readmission rate. In addition, the health care system benefited from the lower medical costs. The calculated saving of >US$370 000 in a single year (2009) for a relatively small subset of patients is particularly valuable in the current era of health care cost containment. This example also confirms the paradigm that increased quality is associated with reduced cost. An additional subtle benefit of streamlining clinical management refers to the discourse that occurred among the eight HPB surgeons. A better understanding of each surgeon's preoperative approach, operative technique and postoperative management was open for discussion. In retrospect, each surgeon recalled learning about new aspects of care provided by his or her colleagues that had not previously been voiced or written. Although the study period concluded in 2009, nearly all of the clinical guidelines for the management of HPB operations have been maintained over time. A recent review of 2011 ACS-NSQIP data for SSI following pancreatectomy found an odds ratio of 0.84 in 2011, which is similar to the O : E ratio of 0.77 reported for 2009. Thus, surgeon feedback and reductions in variation in clinical management led to improvements in the rate of SSIs that persisted beyond the study period.
The changes implemented in this study may constitute a ‘bundle’ with which to reduce SSI in HPB surgery. The critical factors are listed in Table 2 and can be subcategorized by intra- and perioperative variables. Intraoperative variables include surgical technique, which primarily focuses on limiting the exposure of colonized bile and enteric contents to the surgical wound. Various steps are taken to minimize peritoneal and wound soilage, such as delaying bile duct division or placing bile duct clamps, as well as ‘isolating’ the gastrointestinal (GI) anastomosis with sterile towels. Once all GI anastomoses are complete, surgical gloves are changed, and contaminated towels and instruments, including the suction device and cautery, are removed from the sterile field or replaced. Upon completion of the procedure, the abdomen is irrigated with warm saline and meticulous haemostasis is achieved. Wound protection is also a part of surgical technique. Thus, an idophor-impregnated adhesive is routinely placed on the surgical field prior to the incision and a wound edge protector is used as a mechanical barrier to bacteria prior to formal exploration with open incisions. In laparoscopic procedures, a specimen retrieval bag is used routinely. Following fascial closure for open procedures, the wound is irrigated with warm saline and haemostasis is checked.
Antibiotic administration follows the Surgical Care Improvement Project (SCIP) guidelines with respect to the timing of antibiotics, including re-dosing for longer operations. In terms of antibiotic selection, first-generation cephalosporins are employed in ‘clean’ cases such as distal pancreatectomy and enucleation. However, in patients in whom biliary stents have been placed for obstructive jaundice, antibiotics with a broader spectrum for biliary organisms are chosen. Intraoperative normothermia is achieved primarily with patient warming blankets, but as a part of this study, the use of warmed i.v. fluids was implemented. Intraoperative glucose control has two purposes, which include preoperative screening for undiagnosed diabetes and intraoperative infusion of i.v. insulin in appropriate patients. In addition, blood transfusion practices by individual surgeons and anaesthesiologists were monitored, and practice patterns were discussed, along with recommendations to limit perioperative transfusions.
Preoperative nutrition is one of the perioperative variables included in the HPB SSI package. Improving nutritional status was initially considered to fall within the remit of surgeons, but all HPB patients are currently seen by a dietician at 2–3 weeks preoperatively and provided with recommendations for liquid protein and vitamin and mineral supplementation. Postoperative variables include the annual provision of surgeon-specific SSI feedback, which is considered by the surgeons as the most important factor in reducing SSIs. Another area in which variation among surgeons has been reduced concerns drain management. During the study, the group began to check drain amylase levels earlier and to remove drains by postoperative day 3.26 In addition, one surgeon adopted a policy of no drain use. Postoperative glycaemic control was fully implemented at this institution prior to the initiation of this analysis. In addition, postoperative wound management focused on resident and physician extender education about distinguishing cellulitis from an SSI and the decision to open a wound by the attending surgeon. Postoperative oxygenation was also discussed, but conventional oxygenation was employed as the HPB surgeons did not believe this variable to be critical.
The limitations of the present study primarily centre on the use of ACS-NSQIP as the primary method of data acquisition. Although the vast majority of SSIs in HPB surgery patients occur within 30 days, the fact that this programme's data are restricted to a 30-day postoperative period represents a limitation. The grouping of all hepatic, pancreatic, complex biliary and all HPB operations in the assessment of SSI creates heterogeneity in each group. This grouping has the potential to dilute the SSI rate for the highest-risk procedures within these particular subgroups. One additional variable that might skew the data was the volume of cases performed laparoscopically. Over the study period, no significant increase in the number of laparoscopic procedures performed was observed between 2007 and 2009 as the increase in laparoscopic distal pancreatectomies and enucleations occurred prior to 2007.27
Perhaps the largest criticism of this analysis is that multiple processes were changed simultaneously, which makes it difficult to draw conclusions on cause and effect. The present authors consider that multiple process improvements were necessary to improve a disappointingly high SSI rate observed during this group's first full year in ACS-NSQIP. In the best interests of the patient, changes in multiple areas seemed appropriate, and surgeon-specific feedback on SSI rates also played a key role. In conclusion, the present authors believe that high SSI rates following complex HPB operations can be improved by taking a multifactorial approach that features process improvement, the provision of feedback to individual surgeons, and reduced variation in patient management.
Acknowledgments
Sandra K. Janitz, rn, Clinical Decision Support, Indiana University Medical Center, assisted with the cost analysis.
Appendix
Procedures in hepatopancreatobiliary surgery by Current Procedural Terminology (CPT) code
| Group | CPT code | Procedure name |
|---|---|---|
| Biliary complex | 47460 | Transduodenal sphincterotomy or sphincteroplasty |
| Biliary complex | 47579 | Unlisted laparoscopy procedure, biliary tract |
| Biliary complex | 47711 | Excision of bile duct tumour, with or without primary repair of bile duct, extrahepatic |
| Biliary complex | 47712 | Excision of bile duct tumour, with or without primary repair of bile duct, intrahepatic |
| Biliary complex | 47715 | Excision of bile duct cyst |
| Biliary complex | 47720 | Cholecystoenterostomy, direct |
| Biliary complex | 47721 | Cholecystoenterostomy, with gastroenterostomy |
| Biliary complex | 47760 | Anastomosis, of extrahepatic biliary ducts and gastrointestinal tract |
| Biliary complex | 47765 | Anastomosis, of intrahepatic ducts and gastrointestinal tract |
| Biliary complex | 47780 | Anastomosis, Roux-en-Y, of extrahepatic biliary ducts and gastrointestinal tract |
| Biliary complex | 47785 | Anastomosis, Roux-en-Y, of intrahepatic biliary ducts and gastrointestinal tract |
| Liver | 47120 | Hepatectomy, resection of liver, partial lobectomy |
| Liver | 47122 | Hepatectomy, resection of liver, trisegmentectomy |
| Liver | 47125 | Hepatectomy, resection of liver, total left lobectomy |
| Liver | 47130 | Hepatectomy, resection of liver, total right lobectomy |
| Liver | 47379 | Unlisted laparoscopic procedure, liver |
| Liver | 47380 | Ablation, open, of one or more liver tumour(s), radiofrequency |
| Pancreas | 48120 | Excision of lesion of pancreas (e.g. cyst, adenoma) |
| Pancreas | 48140 | Pancreatectomy, distal subtotal, with or without splenectomy, without pancreaticojejunostomy |
| Pancreas | 48145 | Pancreatectomy, distal subtotal, with or without splenectomy, with pancreaticojejunostomy |
| Pancreas | 48146 | Pancreatectomy, distal, near-total with preservation of duodenum (Child-type procedure) |
| Pancreas | 48148 | Removal of pancreatic duct |
| Pancreas | 48150 | Pancreatectomy, proximal subtotal with total duodenectomy, partial gastrectomy, choledochoenterostomy and gastrojejunostomy, with pancreaticojejunostomy |
| Pancreas | 48152 | Pancreatectomy, proximal subtotal with total duodenectomy, partial gastrectomy, choledochoenterostomy and gastrojejunostomy, without pancreaticojejunostomy |
| Pancreas | 48153 | Pancreatectomy, proximal subtotal with near-total duodenectomy, choledochoenterostomy and duodenojejunostomy (pylorus-sparing), with pancreaticojejunostomy |
| Pancreas | 48154 | Pancreatectomy, proximal subtotal with near-total duodenectomy, choledochoenterostomy and duodenojejunostomy (pylorus-sparing), without pancreaticojejunostomy |
| Pancreas | 48155 | Pancreatectomy, total |
| Pancreas | 48510 | External drainage, pseudocyst of pancreas, open |
| Pancreas | 48520 | Internal anastomosis of pancreatic cyst to gastrointestinal tract, direct |
| Pancreas | 48540 | Internal anastomosis of pancreatic cyst to gastrointestinal tract, Roux-en-Y |
| Pancreas | 48548 | Pancreaticojejunostomy, side-to-side anastomosis (Puestow-type operation) |
| Pancreas | 48999 | Unlisted procedure, pancreas |
Conflicts of interest
None declared.
References
- Parikh P, Shiloach M, Cohen ME, Bilimoria KY, Ko CY, Hall BL, et al. Pancreatectomy risk calculator: an ACS-NSQIP resource. HPB. 2010;12:488–497. doi: 10.1111/j.1477-2574.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaga K, Kanematsu T, Takenaka K, Sugimachi K. Intraperitoneal septic complications after hepatectomy. Ann Surg. 1986;203:148–152. doi: 10.1097/00000658-198602000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson R, Saarela A, Tranberg KG, Bengmark S. Intra-abdominal abscess formation after major liver resection. Acta Chir Scand. 1990;156:707–710. [PubMed] [Google Scholar]
- Nagasue N, Kohno H, Tachibana M, Yamanoi A, Ohmori H, El-Assal ON. Prognostic factors after hepatic resection for hepatocellular carcinoma associated with Child–Turcotte class B and C cirrhosis. Ann Surg. 1999;229:84–90. doi: 10.1097/00000658-199901000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KJ, Greenblatt DY, Wan Y, Rettammel RJ, Winslow E, Cho CS, et al. Risk stratification for distal pancreatectomy utilizing ACS-NSQIP: preoperative factors predict morbidity and mortality. J Gastrointest Surg. 2011;15:250–259. doi: 10.1007/s11605-010-1390-9. [DOI] [PubMed] [Google Scholar]
- Wu CC, Yeh DC, Lin MC, Liu TJ, P'eng FK. Prospective randomized trial of systemic antibiotics in patients undergoing liver resection. Br J Surg. 1998;85:489–493. doi: 10.1046/j.1365-2168.1998.00606.x. [DOI] [PubMed] [Google Scholar]
- Arikawa T, Kurokawa T, Ohwa Y, Ito N, Kotake K, Nagata H, et al. Risk factors for surgical site infection after hepatectomy for hepatocellular carcinoma. Hepatogastroenterology. 2011;58:143–146. [PubMed] [Google Scholar]
- Kondo K, Chijiiwa K, Ohuchida J, Kai M, Fujii Y, Otani K, et al. Selection of prophylactic antibiotics according to the microorganisms isolated from surgical site infections (SSIs) in a previous series of surgeries reduces SSI incidence after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2012 doi: 10.1007/s00534-012-0515-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Howard TJ, Yu J, Greene RB, George V, Wairiuko GM, Moore SA, et al. Influence of bactibilia after preoperative biliary stenting on postoperative infectious complications. J Gastrointest Surg. 2006;10:523–531. doi: 10.1016/j.gassur.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Ambiru S, Kato A, Kimura F, Shimizu H, Yoshidome H, Otsuka M, et al. Poor postoperative blood glucose control increases surgical site infections after surgery for hepato-biliary-pancreatic cancer: a prospective study in a high-volume institute in Japan. J Hosp Infect. 2008;68:230–233. doi: 10.1016/j.jhin.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Wang A, Zhou J, Ma XJ, Liao Q, Li GP, Zhao YP. Analysis of surgical site infection rate in pancreas operation and its related risk factors. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2007;29:562–565. [PubMed] [Google Scholar]
- Ball CG, Pitt HA, Kilbane ME, Dixon E, Sutherland FR, Lillemoe KD. Perioperative blood transfusions and operative time are quality indicators for pancreatoduodenectomy. HPB. 2010;12:465–471. doi: 10.1111/j.1477-2574.2010.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Gotohda N, Nakagohri T, Takahashi S, Konishi M, Kinoshita T. Risk factors of surgical site infection after hepatectomy for liver cancers. World J Surg. 2009;33:312–317. doi: 10.1007/s00268-008-9831-2. [DOI] [PubMed] [Google Scholar]
- Watanabe F, Noda H, Kamiyama H, Kato T, Kakizawa N, Ichida K, et al. Risk factors for intra-abdominal infection after pancreaticoduodenectomy: a retrospective analysis to evaluate the significance of preoperative biliary drainage and postoperative pancreatic fistula. Hepatogastroenterology. 2012;59:1270–1273. doi: 10.5754/hge12060. [DOI] [PubMed] [Google Scholar]
- Ali SA, Tahir SM, Memon AS, Shaikh NA. Pattern of pathogens and their sensitivity isolated from superficial site infections in a tertiary care hospital. J Ayub Med Coll Abbottabad. 2009;21:80–82. [PubMed] [Google Scholar]
- Daley J, Khuri SF, Henderson W, Hur K, Gibbs JO, Barbour G, et al. Risk adjustment of the postoperative morbidity rate for comparative assessment of the quality of surgical care. J Am Coll Surg. 1997;185:315–327. [PubMed] [Google Scholar]
- Khuri SF. The NSQIP: a new frontier in surgery. Surgery. 2005;138:837–843. doi: 10.1016/j.surg.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Fink AS, Campbell DA, Mentzer RM, Henderson WG, Daley J, Bannister J, et al. The National Surgical Quality Improvement Program in non-Veterans Administration hospitals: initial demonstration of feasibility. Ann Surg. 2002;236:344–354. doi: 10.1097/00000658-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuri SF, Henderson WG, Daley J, Jonasson O, Jones RS, Campbell DA, et al. The patient safety surgery study: background, study design, and patient populations. J Am Coll Surg. 2007;204:1089–1102. doi: 10.1016/j.jamcollsurg.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Leonardi MJ, Pitt HA, Kilbane ME, Howard TJ, Schmidt CM, Nakeeb A, et al. Organ space infection after pancreatic surgery: are we improving? HPB. 2011;13(Suppl. 1):39–40. [Google Scholar]
- Monge Jodra V, Sainz de Los Terreros Soler L, Diaz-Agero Perez C, Saa Requejo CM, Plana Farras N. Excess length of stay attributable to surgical site infection following hip replacement: a nested case–control study. Infect Control Hosp Epidemiol. 2006;27:1299–1303. doi: 10.1086/509828. [DOI] [PubMed] [Google Scholar]
- Mahmoud NN, Turpin RS, Yang G, Saunders WB. Impact of surgical site infections on length of stay and costs in selected colorectal procedures. Surg Infect. 2009;10:539–544. doi: 10.1089/sur.2009.006. [DOI] [PubMed] [Google Scholar]
- Tan TW, Kalish JA, Hamburg NM, Rybin D, Doros G, Eberhardt RT, et al. Shorter duration of femoral-popliteal bypass is associated with decreased surgical site infection and shorter hospital length of stay. J Am Coll Surg. 2012;215:512–518. doi: 10.1016/j.jamcollsurg.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725–730. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- Broex EC, van Asselt AD, Burggerman CA, van Tiel FH. Surgical site infections: how high are the costs? J Hosp Infect. 2009;72:193–201. doi: 10.1016/j.jhin.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Bassi C, Molinari E, Malleo G, Crippa S, Butturini G, Salvia R, et al. Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg. 2010;252:207–214. doi: 10.1097/SLA.0b013e3181e61e88. [DOI] [PubMed] [Google Scholar]
- Ziegler KM, Nakeeb A, Pitt HA, Schmidt CM, Bishop SN, Moreno J, et al. Pancreatic surgery: evolution at a high-volume centre. Surgery. 2010;148:702–709. doi: 10.1016/j.surg.2010.07.029. [DOI] [PubMed] [Google Scholar]