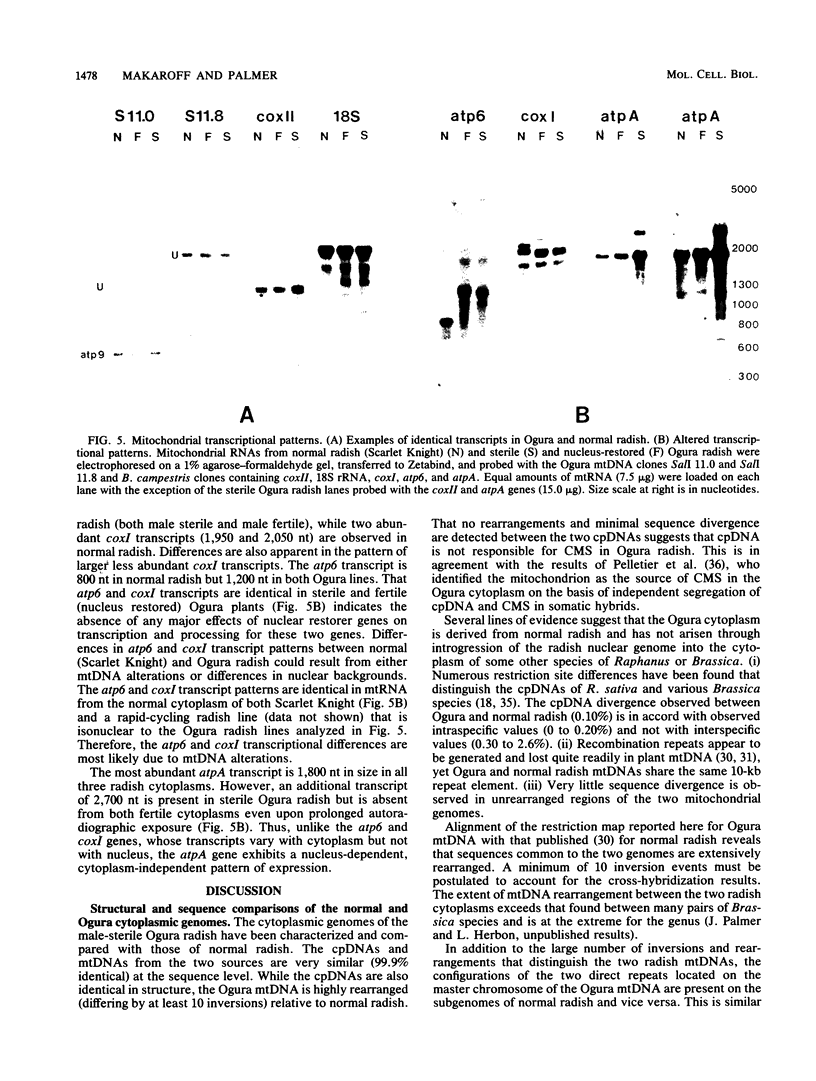
Abstract
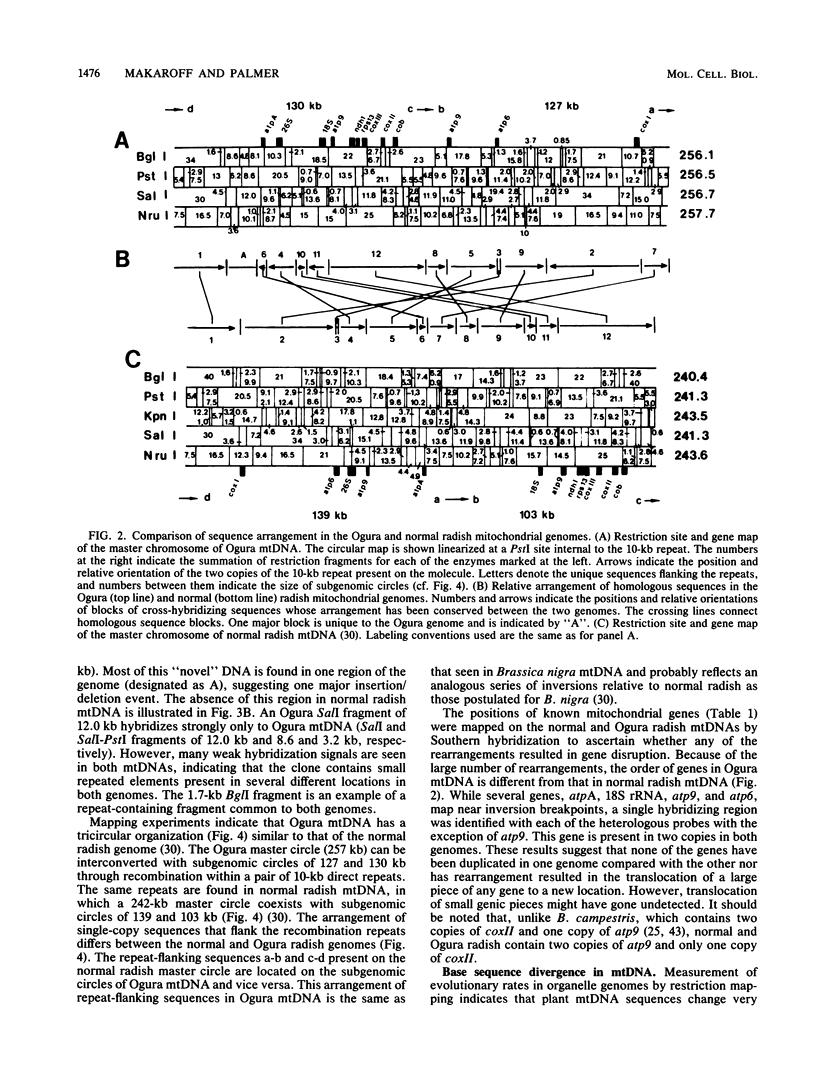
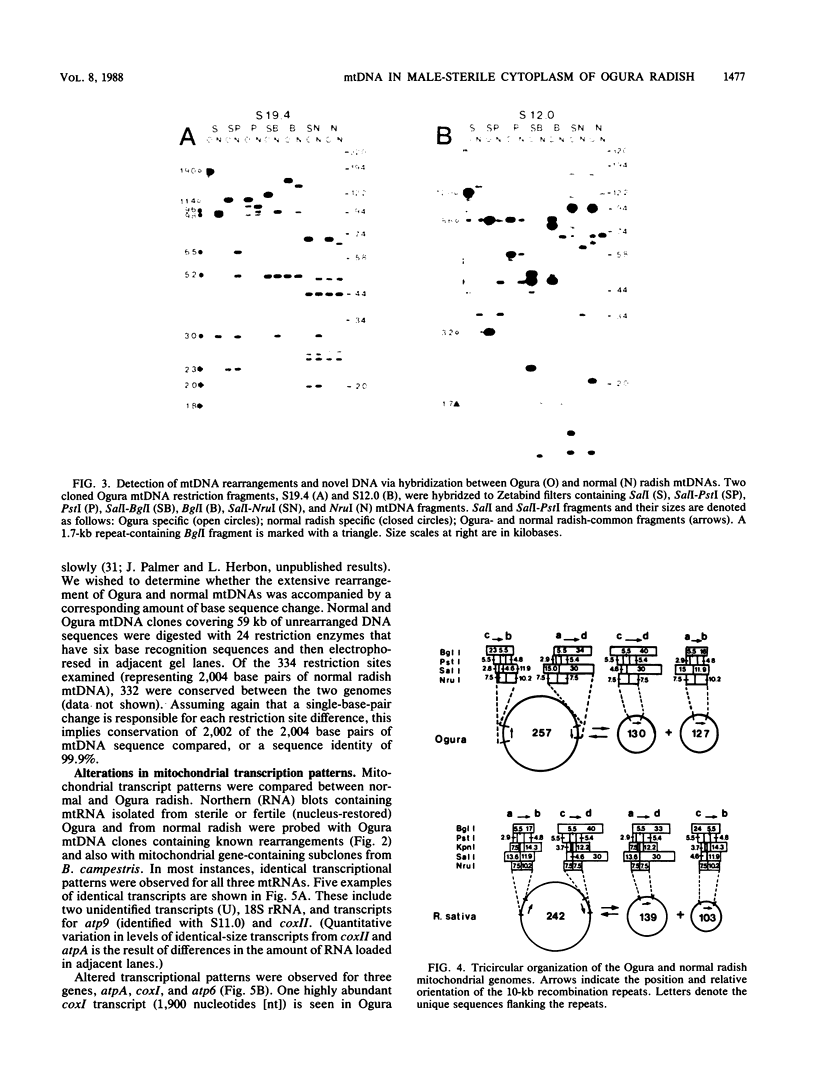
Maternally inherited mutations, such as cytoplasmic male sterility, provide useful systems in which to study the function of plant mitochondrial genomes and also their interaction with nuclear genes. We have studied the organization and expression of the organelle genomes of the male-sterile cytoplasm of Ogura radish and compared them with those of normal radish to identify alterations that might be involved in cytoplasmic male sterility. The chloroplast DNAs of Ogura and normal radish are virtually indistinguishable, whereas their mitochondrial DNAs are highly rearranged. Alignment of a restriction map constructed for the 257-kilobase Ogura mitochondrial genome with that published for the 242-kilobase genome of normal radish reveals that the two mitochondrial DNAs differ in arrangement by at least 10 inversions. The transcriptional patterns of several known mitochondrial genes and of rearranged mitochondrial sequences were examined in three nuclear backgrounds. Altered transcripts were observed for three mitochondrial genes, atpA, atp6, and coxI. Rearrangements map near each of these genes and therefore may be responsible for their transcriptional alterations. Radish nuclear genes that restore fertility to the Ogura cytoplasm have no effect on the atp6 and coxI transcripts, but do influence the atpA transcriptional pattern.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott A. G., Fauron C. M. Structural alterations in a transcribed region of the T type cytoplasmic male sterile maize mitochondrial genome. Curr Genet. 1986;10(10):777–783. doi: 10.1007/BF00405101. [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J., Hanson D. K., Fox T. D., Leaver C. J. Mitochondrial genome rearrangement leads to extension and relocation of the cytochrome c oxidase subunit I gene in sorghum. Cell. 1986 Nov 21;47(4):567–576. doi: 10.1016/0092-8674(86)90621-5. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland M. M., Levings C. S., 3rd, Matzinger D. F. The tobacco mitochondrial ATPase subunit 9 gene is closely linked to an open reading frame for a ribosomal protein. Mol Gen Genet. 1986 Jul;204(1):8–16. doi: 10.1007/BF00330180. [DOI] [PubMed] [Google Scholar]
- Braun C. J., Levings C. S. Nucleotide Sequence of the F(1)-ATPase alpha Subunit Gene from Maize Mitochondria. Plant Physiol. 1985 Oct;79(2):571–577. doi: 10.1104/pp.79.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao S., Sederoff R., Levings C. S., 3rd Nucleotide sequence and evolution of the 18S ribosomal RNA gene in maize mitochondria. Nucleic Acids Res. 1984 Aug 24;12(16):6629–6644. doi: 10.1093/nar/12.16.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., Mendu N., Ginsburg H., Kridl J. C. Sequence analysis of the maize mitochondrial 26 S rRNA gene and flanking regions. Plasmid. 1984 Mar;11(2):141–150. doi: 10.1016/0147-619x(84)90019-2. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Levings C. S., 3rd, Timothy D. H. Novel recombinations in the maize mitochondrial genome produce a unique transcriptional unit in the Texas male-sterile cytoplasm. Cell. 1986 Feb 14;44(3):439–449. doi: 10.1016/0092-8674(86)90465-4. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Levings C. S., Timothy D. H. Nucleotide sequence of ATPase subunit 6 gene of maize mitochondria. Plant Physiol. 1985 Nov;79(3):914–919. doi: 10.1104/pp.79.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey R. E., Schuster A. M., Levings C. S., Timothy D. H. Nucleotide sequence of F(0)-ATPase proteolipid (subunit 9) gene of maize mitochondria. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1015–1019. doi: 10.1073/pnas.82.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey R. E., Timothy D. H., Levings C. S. A mitochondrial protein associated with cytoplasmic male sterility in the T cytoplasm of maize. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5374–5378. doi: 10.1073/pnas.84.15.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B. G., Leaver C. J. Nuclear and cytoplasmic genes controlling synthesis of variant mitochondrial polypeptides in male-sterile maize. Proc Natl Acad Sci U S A. 1980 Jan;77(1):418–422. doi: 10.1073/pnas.77.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., Leaver C. J. The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell. 1981 Nov;26(3 Pt 1):315–323. doi: 10.1016/0092-8674(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Hiesel R., Schobel W., Schuster W., Brennicke A. The cytochrome oxidase subunit I and subunit III genes in Oenothera mitochondria are transcribed from identical promoter sequences. EMBO J. 1987 Jan;6(1):29–34. doi: 10.1002/j.1460-2075.1987.tb04714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. Physicochemical characterization of mitochondrial DNA from pea leaves. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1830–1834. doi: 10.1073/pnas.69.7.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makaroff C. A., Palmer J. D. Extensive mitochondrial specific transcription of the Brassica campestris mitochondrial genome. Nucleic Acids Res. 1987 Jul 10;15(13):5141–5156. doi: 10.1093/nar/15.13.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. D., Herbon L. A. Tricircular mitochondrial genomes of Brassica and Raphanus: reversal of repeat configurations by inversion. Nucleic Acids Res. 1986 Dec 22;14(24):9755–9764. doi: 10.1093/nar/14.24.9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. D., Herbon L. A. Unicircular structure of the Brassica hirta mitochondrial genome. Curr Genet. 1987;11(6-7):565–570. doi: 10.1007/BF00384620. [DOI] [PubMed] [Google Scholar]
- Palmer J. D., Nugent J. M., Herbon L. A. Unusual structure of geranium chloroplast DNA: A triple-sized inverted repeat, extensive gene duplications, multiple inversions, and two repeat families. Proc Natl Acad Sci U S A. 1987 Feb;84(3):769–773. doi: 10.1073/pnas.84.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. D. Physical and gene mapping of chloroplast DNA from Atriplex triangularis and Cucumis sativa. Nucleic Acids Res. 1982 Mar 11;10(5):1593–1605. doi: 10.1093/nar/10.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottmann W. H., Brears T., Hodge T. P., Lonsdale D. M. A mitochondrial gene is lost via homologous recombination during reversion of CMS T maize to fertility. EMBO J. 1987 Jun;6(6):1541–1546. doi: 10.1002/j.1460-2075.1987.tb02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobieski J. M., Chen K. N., Filiatreau J. C., Pickett M. H., Fox G. E. 16S rRNA oligonucleotide catalog data base. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):141–148. doi: 10.1093/nar/12.1part1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. B., Bang A. G., Thompson W. F. The watermelon mitochondrial URF-1 gene: evidence for a complex structure. Curr Genet. 1986;10(11):857–869. doi: 10.1007/BF00418532. [DOI] [PubMed] [Google Scholar]
- Stern D. B., Newton K. J. Isolation of plant mitochondrial RNA. Methods Enzymol. 1986;118:488–496. doi: 10.1016/0076-6879(86)18095-5. [DOI] [PubMed] [Google Scholar]
- Wise R. P., Pring D. R., Gengenbach B. G. Mutation to male fertility and toxin insensitivity in Texas (T)-cytoplasm maize is associated with a frameshift in a mitochondrial open reading frame. Proc Natl Acad Sci U S A. 1987 May;84(9):2858–2862. doi: 10.1073/pnas.84.9.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. G., Hanson M. R. A fused mitochondrial gene associated with cytoplasmic male sterility is developmentally regulated. Cell. 1987 Jul 3;50(1):41–49. doi: 10.1016/0092-8674(87)90660-x. [DOI] [PubMed] [Google Scholar]