Abstract
This pilot study adapted a well-established drug self-administration paradigm to examine the effects of mood induction on the ability to resist high-calorie foods and subsequent food consumption differently in 15 obese individuals (40.0% women, BMI: 35.1±3.70) and 15 non-obese individuals (46.7% women, BMI: 23.0±1.96). Participants completed two laboratory sessions (positive vs. negative mood conditions) consisting of 3-hour food deprivation, followed by mood induction, and a 3-hour ad-lib eating period, where they were asked to choose between favorite high-calorie snacks and monetary reinforcement. Obese individuals were less able to resist eating and increased high-calorie food consumption during the positive mood condition than the negative condition. Non-obese individuals were less able to resist eating during the negative mood condition than the positive condition, but their total consumption was not affected by the mood conditions. In obese individuals, food craving was associated with less ability to resist eating and greater calorie consumption during the negative mood condition. This is the first study to experimentally demonstrate that mood state may increase vulnerability to food consumption by reducing the ability to resist eating. The ability to resist eating may be a novel dimension of eating behaviors that has a significant contribution to understanding mood-eating relationships.
Keywords: Obesity, mood, eating behaviors, high-calorie foods, food craving
1. Introduction
Obesity is a leading health risk for chronic diseases and conditions in the United States, such as cardiovascular diseases, type-II diabetes, and certain cancers (Ogden, Yanovski, Carroll, & Flegal, 2007). In 2007-2008, the prevalence of obesity was 33.8% (Flegal, Carroll, Ogden, & Curtin, 2010), with annual medical expenditure attributable to obesity estimated at $147 billion (Flegal, et al., 2010). The modest efficacy of nutrition- and exercise-related interventions highlights the need for new approaches to control body weight. An abundance of food, particularly high-calorie palatable foods, and overeating have been argued to be partly responsible for the current obesity epidemic (Pandit, de Jong, Vanderschuren, & Adan, 2011). A recent epidemiology study has indeed found increasing trends in frequency of snacking, energy density of snacks, and the contribution of snacks to total calorie consumption (Piernas & Popkin, 2010). It is therefore critical to develop effective obesity intervention strategies that focus on reduction of high-calorie food consumption.
While multiple factors contribute to obesity, overeating due to loss of control over food intake is important. Eating behaviors are highly cue-dependent, and mood states are thought to influence overeating behaviors. When queried about relapse situations, overweight dieters frequently reported both temptations to overeat and overeating when experiencing positive and negative mood states (Grilo, Shiffman, & Wing, 1989). In human laboratory studies, negative mood states, including stress, have been shown to alter food preference to highly-palatable foods (Oliver, Wardle, & Gibson, 2000) and to have disinhibiting effects on eating, particularly in individuals with tendencies to engage in restrained (Habhab, Sheldon, & Loeb, 2009; Lattimore & Caswell, 2004; Schotte, Cools, & McNally, 1990; Wallis & Hetherington, 2004) or emotional eating (Oliver, et al., 2000; Wallis & Hetherington, 2004, 2009). Experimental studies have also demonstrated that increased positive mood enhanced consumption of palatable food in normal weight individuals (Cools, Schotte, & McNally, 1992; Yeomans & Coughlan, 2009), although the effects of negative mood appeared to be more potent (Yeomans & Coughlan, 2009). Collectively, these studies across diverse subject groups and weight categories suggest that mood, regardless of valence, can trigger overeating and loss of control over eating. Importantly, Grilo et al. (1989) found that coping attempts may prevent overeating or overcome temptations to overeat in response to such emotional cues; however, those findings are weakened by reliance on retrospective self-report data and require experimental manipulation in a laboratory setting to arrive at firmer conclusions. We are unaware of existing human laboratory studies that have modeled the ability to resist eating high-calorie palatable foods, which could be a crucial component in promoting weight loss efforts and healthy weight maintenance to counteract the current obesity epidemic.
For the current study, we adapted a well-established human drug self-administration paradigm that we had previously developed, which assesses the effects of positive and negative mood induction on the ability to resist smoking (McKee, et al., 2011). In this paradigm, following mood induction, a lighter, cigarette, and ashtray are presented, and participants are asked to resist smoking and are offered an increasing amount of monetary compensation the longer they can resist smoking. The inclusion of money as an alternative reinforcement was a critical part of this model to provide incentive for not smoking, and to enhance the likelihood that the effects of stress on the reinforcing value of smoking would be detected (McKee, 2009). The latency to start smoking (i.e., ability to resist) is the primary outcome measure, following which ad-libitum smoking is evaluated. Using this unique paradigm, our laboratory has reliably demonstrated that negative mood reduces the ability to resist smoking and leads to more intense smoking behaviors (e.g., increased puffs, shorter inter-puff interval, and greater peak puff velocity), compared to positive mood, in daily smokers (McKee, et al., 2011). Adapting this model to examine eating behavior will provide a framework to evaluate the effects of a mood manipulation on the ability to resist eating high-calorie, palatable food. Similar to the smoking-lapse model, money was used as an alternative reinforcer to provide a sensitive test of the relative reinforcing value of high-calorie foods.
The goal of this pilot study was to examine the effects of both negative and positive mood induction on two important aspects of overeating behavior in obese and non-obese individuals: 1) the failure of the ability to resist eating high-calorie food and 2) subsequent ad-lib eating of high-calorie food. It is unknown whether mood manipulations will affect the ability to resist eating and subsequent food consumption differently in obese and non-obese individuals. We also examined whether the relationship between mood-induced eating behaviors, self-reported positive and negative emotions, and food craving differed in obese and non-obese individuals.
2. Methods
2.1. Participants
A total of 30 participants (mean age = 36.8 ± 12.6 years old; 43.3% women) completed the study. Eligible participants had to be between 18 to 65 years of age and have a Body Mass Index (BMI) between 30 and 45 (obese group) or below 30 (non-obese group) (CDC, 2011). Exclusion criteria included: current diagnosis of Axis I psychiatric disorders (except nicotine dependence), current diagnosis of anorexia nervosa and/or bulimia nervosa, significant medical conditions, including metabolic disorders (e.g., diabetes, thyroid problems, and abnormal fasting glucose value), and current use of psychotropic or illicit drugs. The ethnic composition of the sample was non-Hispanic White (56.7%) and non-Hispanic African-American (43.3%). The majority of participants had completed high school (80.0%) and reported an income of less than $60,000 (65.5%).
2.2. Procedures
The experimental protocol was approved by the Yale Human Investigation Committee, and the procedures were in compliance with the Declaration of Helsinki for human subjects. Written informed consent was obtained from all the participants.
2.2.1. Intake assessment
The Structured Clinical Interview for DSM-IV Axis I Psychiatric Disorders (SCID; First, Spitzer, Gibbon, & Williams, 1995) was used to exclude individuals who met diagnostic criteria for current psychiatric disorders (except nicotine dependence), including eating disorders. Participants were screened for metabolic disorders assessed with basic blood chemistry tests. Eligible participants were then scheduled for the laboratory sessions.
2.2.2. Script development session
A personalized guided imagery procedure was used for negative and positive mood induction (Sinha, 2009). The imagery script for negative mood induction was developed by having participants provide a detailed description about a recent negative mood-inducing experience occurring in the past 6 months that they perceived as “most stressful.” Perceived stress was rated on a 10-point Likert scale where 1 = not at all stressful and 10 = the most stress they recently felt in their life. Ratings were made relative to other experiences in the past 6 months. Only situations with perceived stress rating of ≥ 8 were accepted as appropriate for script development. Examples of stressful experiences were marital conflict or losing employment. The imagery script for positive mood induction was developed by having participants describe a personal positive mood-inducing situation that only involved themselves, such as sitting at the beach or reading in the park. Scripts were developed by a PhD-level clinician, audio-taped for presentation, and were approximately 5 minutes in length (see also McKee et al., 2011). At the end of this session, the participants completed an assessment of their preferred high-calorie sweet and salty foods.
2.2.3. Laboratory session
Each participant individually completed two 9-hour laboratory sessions (positive imagery vs. negative imagery session). The order of the sessions was counterbalanced. Participants were compensated $390 for completing the entire study. The average time between two laboratory sessions was 7.0 ± 2.9 days.
Laboratory sessions started at 7:30 AM. Participants were instructed not to eat past 10 PM the night before the laboratory session. After providing a urine drug screen and baseline assessments of self-report emotion ratings and food craving, participants received a standardized breakfast to control caloric intake and the time since last food consumption at 8:00 AM. This was followed by 3 hours of food deprivation. During the food deprivation period, participants were allowed to watch TV and read. Emotion ratings and food cravings were assessed before the imagery task at 10:45 AM (i.e., pre-imagery assessment).
At 10:55 AM, participants were instructed to clear their mind of any worrying thoughts and to focus on deep breathing. At 11:00 AM, they were instructed as follows for the guided imagery: “You will soon hear a situation being described to you. Your task is to close your eyes and imagine yourself in the situation being described, ‘as if’ it were happening right now. Allow yourself to become completely involved in the situation, by involving your mind and body in actually doing what is being described. Continue imagining until you are asked to stop.” Then, the participant listened to the script (negative or positive) over headphones. Following the script, participants rated how clearly they were able to imagine the scene on a 140-mm visual analog scale (VAS). Mean vividness ratings were 113.77 ± 19.71 for the negative mood imagery and 119.00 ± 15.63 for the positive mood imagery (p > .05). At 11:05 AM, post-imagery assessments of mood and food craving were completed.
At 11:30 PM, ad-lib food consumption began by presenting the individual participant’s preferred three choices of high-calorie sweet foods (e.g., cookies, snack cakes, chocolate pudding) and three choices of high-calorie salty foods (e.g., potato chips, popcorn with butter, peanuts). Snacks were portioned to five servings of each item, with possible caloric intake ranging from 3950 kcal to 5750 kcal, depending on the combination of food choices. Participants were told that they could start eating at any time they wish over the next three hours. However, for each minute they delayed or “resisted” eating, they would receive monetary rewards. Monetary reinforcement was scheduled as $0.20/minute for the first hour, $0.10/minute for the second hour, and $0.05/min for the third hour. The participants could thus earn up to $21 over the 3-hour period. We determined the value of monetary reinforcement based on our previous smoking-lapse models, and chose this de-escalating schedule of reinforcement to model how the ability to resist eating high-calorie food decreases over time. The end of delay period was marked when the participants could no longer resist and decided to start eating, and their emotion ratings and food craving were assessed at this time (immediately prior to food consumption). Participants were then allowed to eat as much as they wished until the end of the 3-hour ad-lib eating period. After the 3-hour ad-lib eating period, the food was removed, and thus participants no longer had access to the food. Participants were required to remain in the lab for an additional 2 hours to add a response cost if they chose not to consume any food during this period.
2.3. Measures
2.3.1. Eating behaviors
Ability to resist eating was calculated as the latency between the presentation of the preferred foods and the decision to start eating. When subjects decided to start eating, time was recorded in minutes and seconds (range = 0 – 180 min). In addition, total calories consumed (sweet and salty foods combined) over the 3-hour ad-lib eating period were recorded.
2.3.2. Self-report measures
The Revised Differential Emotion Scale (RDES; Izard, 1972) was used to measure positive and negative emotional states throughout the session. The RDES consists of 30 negative (e.g., irritated, distressed, upset, sad or depressed, angry) and positive (e.g., pleasant, happy, joyful, relaxed, comfortable) emotion words, and participants were asked to rate them on a 100-mm VAS. To measure food craving, we developed a food craving scale based on a 10-item Tiffany Questionnaire of Smoking Urges (QSU-Brief; Cox, Tiffany, & Christen, 2001) by replacing the word “a cigarette” with “food,” and the word “smoke” with “eat.” Although the factor structure of this food craving scale has not been tested, corresponding with the original QSU-Brief, we calculated a global food craving score (Chronbach’s alpha = 0.97). In addition, the Eating Disorder Examination Questionnaire with Instructions (EDE-Q-I; Goldfein, Devlin, & Kamenetz, 2005), a psychometrically sound measure with good test-retest reliability (Reas, Grilo, & Masheb, 2006), was used to measure if participants had recently engaged in dieting (Item 1: “how many days out of the past 28 days have you been deliberately trying to limit the amount of food you eat to influence your shape or weight?”) or experienced binge eating episodes (Item 15: “over the past 28 days, how many days have such episodes of overeating occurred [i.e., you have eaten an usually large amount of food and have had a sense of loss of control at the time]?). The Dutch Eating Behavior Questionnaire (DEBQ; Van Strien, Frijters, Bergers, & Defares, 1986), a scale of eating habits with demonstrated validity (Van Strien et al., 1986; Wardle, 1987), was used to characterize restraint and emotional eating in our sample.
2.4. Statistical Analysis
Repeated measures analysis of covariance (ANCOVA) was conducted to compare the latency to start eating between positive and negative mood imagery conditions (within-subject effect) by obesity status (between-subject effect). Repeated measures ANCOVA was also used to examine the within-subject effect of the imagery condition and time (pre-mood induction, post-mood induction, and end of delay [i.e., made the decision to eat, but had not yet started to eat]) on positive and negative emotion ratings, as well as global food craving. When participants successfully resisted eating for the entire 3 hours, values for emotion ratings and food craving scores were censored at the end of the 3-hour eating period. In addition, Pearson correlation analyses were conducted, separately for obese and non-obese groups, to examine the relationship of eating behaviors with emotion ratings and food craving. In all analyses, gender, session order, age, eating behaviors in the past 28 days (i.e., dieting and binge eating), and eating habits (i.e., restraint and emotional eating) were evaluated as covariates and were retained only if they reduced residual variance.
3. Results
3.1. Sample characteristics
Table 1 summarizes the sample characteristics by obesity status. There were no significant differences in demographic characteristics between obese and non-obese individuals, except that the obese group was significantly older and, as designed, had greater BMI scores.
Table 1.
Sample characteristics for obese and non-obese participants.
| Obese (n = 15) | Non-Obese (n = 15) | |
|---|---|---|
| Age | 41.9 (11.6) † | 31.7 (11.9) |
| % female | 40.0 | 46.7 |
| % White | 53.3 | 46.7 |
| % African-American | 60.0 | 40.0 |
| BMI Ranges |
35.1 (3.70) † 30-44 |
23.0 (1.96) 20-26 |
| Number of days engaging the following behaviors in the past 28 days at intake (from EDE-Q-I) | ||
| Recent dieting (item 1) | 6.45 (9.16) | 7.00 (10.81) |
| Binge eating (item 15) | 2.71 (6.19) | 0.87 (1.25) |
| Eating habits (from DEBQ) | ||
| Restraint eating | 24.67 (9.90) | 23.60 (10.38) |
| Emotional eating | 31.33 (17.77) | 31.93 (13.83) |
| Negative and positive emotion at the time of arrival to the laboratory session | ||
| Negative emotion (negative) | 11.20 (8.79) | 8.73 (3.67) |
| Positive emotion (negative) | 68.26 (22.17) | 59.79 (21.03) |
| Negative emotion (positive) | 7.48 (3.78) | 8.90 (3.44) |
| Positive emotion (positive) | 63.63 (19.65) | 65.51 (24.21) |
Notes. Numbers in parentheses indicate standard deviation. BMI = body-mass-index. EDE-Q-I = the Eating Disorder Examination Questionnaire with Instructions (Goldfein et al., 2005). DEBQ = Dutch Eating Behavior Questionnaire (Van Strien et al., 1986), with score ranges from 10-50 (restraint eating) and 13-65 (emotional eating), respectively.
= significantly different from non-obese group at p < .05.
3.2. Manipulation check
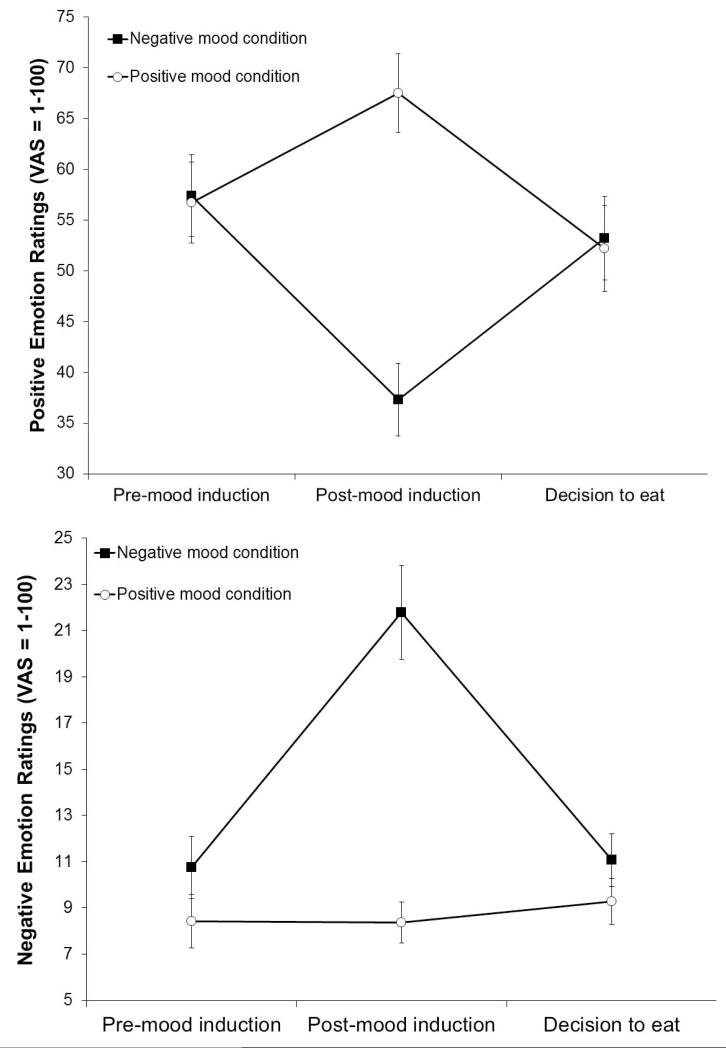
Positive emotion ratings (imagery condition-by-time; F[2, 52] = 33.75, p < .01, partial η2 = 0.57 [large], with a significant quadratic trend) and negative emotion ratings (imagery condition-by-time; F[2, 52] = 13.94, p < .01, partial η2 = 0.35 [large], with a significant quadratic trend) differed by imagery condition, regardless of obesity status. Confirming the imagery manipulation, positive emotion ratings increased after positive mood induction and decreased after negative mood induction (Figure 1, upper); negative emotion ratings increased after negative mood induction, but showed no changes after positive mood induction (Figure 1, lower). Both positive and negative emotion ratings returned to the pre-imagery level at the end of delay when participants made the decision to eat, but had not yet started to eat.
Figure 1.
Means and standard errors of positive emotion ratings (upper) and negative emotion ratings (lower) by imagery conditions by time (pre-mood induction, post-mood induction, decision to eat).
3.3. Eating behaviors
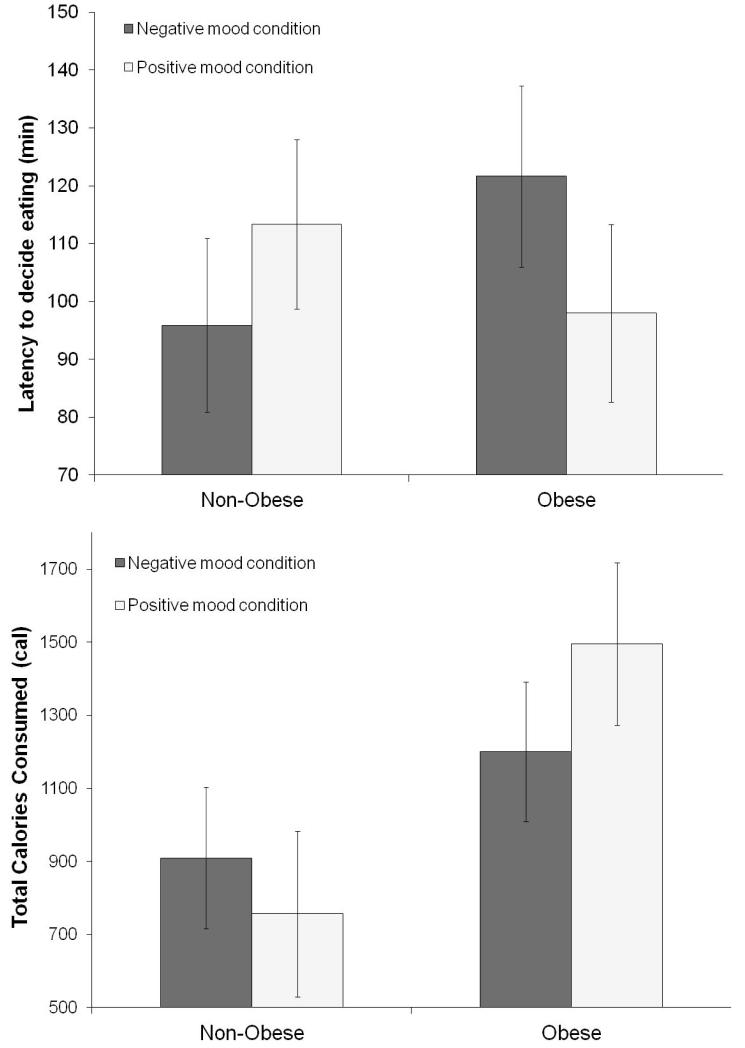
Obesity status interacted with imagery condition on latency to eat, F(1, 24) = 7.39, p < .05, partial η2 = 0.24 (large).1 Obese individuals were less able to resist eating in the positive mood condition compared to the negative mood condition (Figure 2, upper). Conversely, non-obese individuals were less able to resist eating in the negative mood condition compared to the positive mood condition (Figure 2, upper). Obesity status also interacted with imagery condition on total calorie consumption, F(1, 24) = 4.36, p < .05, partial η2 = 0.15 (large).2 Obese individuals consumed significantly more calories compared to non-obese individuals, particularly after the positive mood induction (Figure 2, lower). Obese individuals also consumed more calories in the positive mood condition than in the negative mood condition. No other significant between- and within-subject effects were found for “resistance to eat” or total calories consumed (all p > .05).
Figure 2.
Means and standard errors of latency to decide eating (upper) and total calorie consumption (lower) by obesity status (obese, non-obese) by imagery conditions (positive, negative).
3.4. Food craving
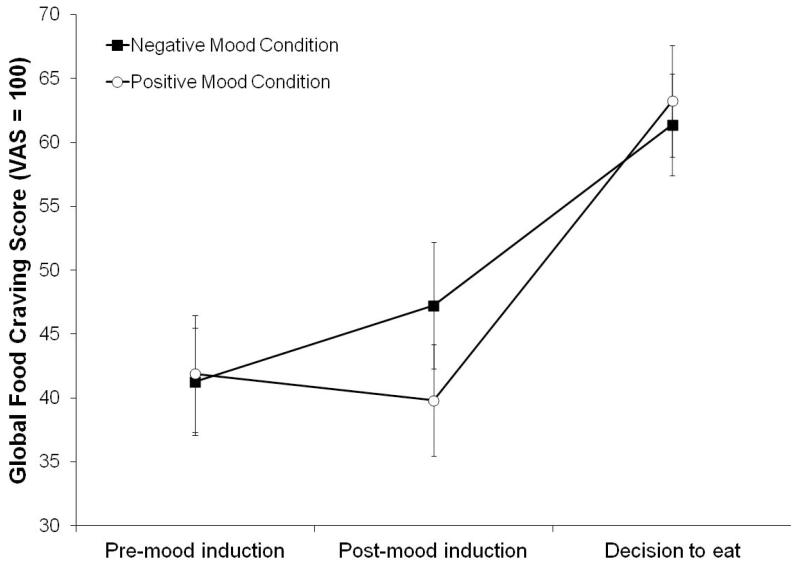
For global food craving scores, there was a significant imagery condition-by-time interaction (Figure 3), F(2, 25) = 3.66 (multivariate test), p < .05, partial η2 = 0.23 (large). Regardless of obesity status, food craving ratings increased after negative mood induction, and showed little changes after positive mood induction. Following the decision to eat, food craving increased in both imagery conditions.
Figure 3.
Means and standard errors of global food craving score by imagery conditions (positive, negative) by time (pre-mood induction, post-mood induction, decision to eat).
3.5. Correlation analysis
Table 2 summarizes the results of correlation analysis.3 In the negative mood condition, less ability to resist eating was significantly correlated with higher negative emotion ratings before mood induction and greater food craving at the end of delay in obese individuals. Greater total calorie consumption was significantly correlated with greater food craving after negative mood induction in obese individuals. In the positive mood condition, greater total calorie consumption was significantly correlated with greater food craving at all three assessment time points. No significant correlations were found among non-obese individuals in the negative or positive mood condition.
Table 2.
Correlation between eating behaviors, mood, and food craving.
| Negative Mood Induction |
Positive Mood Induction |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative emotion ratings | Food craving | Positive emotion ratings | Food craving | |||||||||
|
|
|
|||||||||||
| Pre | Post | End | Pre | Post | End | Pre | Post | End | Pre | Post | End | |
| Obese | ||||||||||||
| Latency | −.60† | −.31 | −.53 | −.19 | −.32 | −.67† | −.10 | −.12 | .02 | −.40 | −.53 | −.35 |
| Calories | .44 | .20 | .24 | .42 | .63† | .45 | −.27 | −.05 | −.35 | .66† | .77† | .64† |
| Non-obese | ||||||||||||
| Latency | .03 | .06 | −.05 | −.20 | −.34 | −.49 | −.15 | .18 | .01 | .17 | .14 | .06 |
| Calories | .03 | .36 | −.15 | −.17 | .00 | .20 | .07 | −.04 | .17 | −.20 | −.37 | −.36 |
Notes. Pre = pre-mood induction; Post = post-mood induction; End = end of delay (i.e., decision to eat).
p < .05.
4. Discussion
This pilot laboratory study was the first to examine the effects of positive and negative mood induction on the ability to resist high-calorie foods, in addition to ad-libitum consumption of preferred high-calorie food. Interestingly, obese individuals showed less ability to resist eating high-calorie foods and subsequently consumed more calories in the positive mood condition than in the negative mood condition. There are only two studies that examined the effects of positive mood induction on eating behaviors in laboratory settings, with both examining calorie consumption in normal weight individuals (Cools, et al., 1992; Yeomans & Coughlan, 2009). Our findings suggest that positive mood may contribute to increased consumption of high-calorie food in obese individuals by reducing their ability to resist eating. Negative mood did not reduce the ability to resist eating in our obese sample. Vicennati et al. (2009) has suggested that not all obese individuals are susceptible to negative-mood related eating behaviors. However, in our non-obese sample, the negative mood induction reduced the ability to resist eating when compared with the positive mood induction, but this did not translate to increased calorie consumption. Findings suggest that negative mood may be a more salient cue to consume high-caloric snacks in this sample. Together, the present findings support that mood, regardless of valence, can negatively affect the ability to resist eating high-calorie food, and there are great individual differences in whether positive or negative mood contributes to increased susceptibility to high-calorie food intake.
Regardless of obesity status, food craving increased after the negative mood induction, while it showed little changes after the positive mood induction. When an individual made a decision to eat, food craving significantly increased in both positive and negative mood conditions, suggesting the strong role of food craving in one’s decision to eat. Further, correlation analyses revealed that reduced ability to resist eating high-calorie food was significantly associated with food craving in obese individuals at the point when they decided to eat following negative mood manipulation. Thus, negative mood may still have an adverse impact on the ability to resist eating high-calorie food in obese individuals with heightened food craving reactivity in response to negative mood. As intuitive as the finding may be, this is the first laboratory study to demonstrate the relationship between food craving and the ability to resist eating in obese individuals. The finding also adds experimental evidence to a retrospective self-report study with overweight dieters which demonstrated that situations which caused emotional ‘upset’ were antecedents of overeating (Grilo, et al., 1989). While there were no baseline differences in negative emotion ratings between our obese and non-obese samples, there was a negative association with pre-induction levels of negative emotion and the ability to resist eating in the negative mood condition among obese individuals. This is somewhat parallel to Jansen et al. (2008), who found increased calorie consumption after exposure to palatable food cues in non-disordered overweight/obese women with high levels of negative affect as compared to overweight/obese women with low levels of negative affect. Thus, for obese individuals, pre-existing levels of negative emotion may are important to consider when evaluating individual differences in the ability to resist eating after experiencing negative mood inducing situations.
The study further demonstrated significant correlations between total calorie consumption and food craving in obese individuals. In the negative mood condition, higher level of food craving following mood induction was associated with greater total calorie consumption. Thus, increases in food craving may play a key role in negative mood-related eating in obese individuals. In contrast, higher levels of food craving at all three assessment points were associated with greater total calorie intake during the positive mood condition. Thus, greater total calorie consumption in the positive mood condition may be attributable to generally elevated food craving due to the three hours of food deprivation. Surprisingly, the relationship between mood-induced food craving and eating behaviors has not been well-explored in human laboratory studies on obesity. Given absence of significant correlations in non-obese individuals, the findings from correlation analyses suggest that, similar to addictive behaviors, food craving may have important mechanistic implications in mood-induced high-calorie food consumption, especially in obese individuals.
In both obese and non-obese individuals, the negative mood induction increased negative emotion ratings and decreased positive emotion ratings. Once participants decided to eat, but had not yet consumed food, both positive and negative emotion ratings reverted back to pre-induction levels. A similar pattern of findings was demonstrated when examining the effects of mood induction on smoking behavior (McKee et al., 2011). Reward anticipation has been shown to activate the ventral striatum (O’Doherty, 2004), which has also been linked to anticipatory increase in positive affect (Burgdorf & Panksepp, 2006). We can speculate that improvement in emotional states once participants made the decision to eat may reflect reward anticipation. Alternatively, it may be related to alleviation of stress/negative emotion that had increased while resisting temptation to eat. While further research on underlying mechanisms is needed, consistent findings between two distinct appetitive behaviors suggest that changes in emotion in this self-administration paradigm may be useful in characterizing the common aspect of reward anticipation in addictive behaviors.
Our study has several strengths and limitations that serve as context for our findings. We adapted a validated smoking paradigm (McKee et al., 2011), which utilizes a well-studied method to induce negative mood, to evaluate the ability to resist eating and ad-lib consumption of preferred high-caloric snacks. In contrast to previous research focusing those with binge eating disorders or with specific characteristics (e.g., high restraint eaters, emotional eaters), this study examined mood-eating relationship in obese and non-obese individuals without eating disorders. In addition, a substantial proportion of our sample was ethnic minorities. Together, while the study results cannot be generalized to clinical population, they provide insight into mood-induced overeating in the general population, which has important implications for current obesity epidemic. As this was a pilot study, the study sample was relatively small but comparable to similar investigations (Appelhans, Pagoto, Peters, & Spring, 2010; Wallis & Hetherington, 2004, 2009). The advantage of well-controlled within-subject laboratory investigations is that robust effects can be demonstrated with relatively modest sample sizes. In our investigation, robust effect sizes of our primary outcomes were demonstrated.
One important limitation is that the present study did not include measures of the hypothalamic-pituitary-adrenal axis (HPA) reactivity. Epel et al. (2001) and Newman et al. (2007) found that only women who showed high cortisol response consumed more calories after psychological stress, suggesting a critical role of individual differences in glucocorticoids response in the effects of mood induction on overeating and obesity. Inclusion of a neutral mood condition might also have provided a reference point to clarify whether positive or negative mood induction led to overconsumption of highly-palatable foods. However, we have evaluated the use of neutral conditions (both neutral imagery and no-imagery) in our smoking lapse paradigm, and find little difference between positive and neutral conditions. Finally, our food craving scale was developed through modifying a well-validated smoking craving questionnaire (Cox et al., 2001). Although the internal consistency of the scale was high, further evaluation of its psychometric properties is necessary. Future studies should also investigate whether factors suggested as risk for loss of control over eating and overconsumption of palatable food, such as impulsivity (Guerrieri, Nederkoorn, Schrooten, Martijn, & Jansen, 2009; Guerrieri, et al., 2007), reward sensitivity (Davis, et al., 2007; Davis, Strachan, & Berkson, 2004), and dietary restraint (Hill, 2004), may influence eating behaviors in our model.
4.1. Conclusion
This pilot study demonstrated that the influence of positive and negative mood induction on the ability to resist eating and ad-lib consumption of high-caloric foods varied by obesity status. We also demonstrated strong associations between food craving and these eating behaviors, particularly after following a negative mood induction in obese individuals. This is the first study to utilize a human laboratory model of eating behavior that incorporates a behavioral measure of the ability to resist eating. Frequent snacking of high-caloric foods has been associated with obesity (Berteus Forslund, Torgerson, Sjostrom, & Lindroos, 2005; de Graaf, 2006). In addition to increased caloric intake per eating episode, reduced ability to resist eating may lead to increases in frequency of high-caloric food intake, which can in turn contribute to excessive calorie intake in the long-term. Collectively, our experimental findings support clinical observations for overweight dieters (Grilo et al., 1989) that different mood states can elicit urges to overeat. Our study also highlights that the ability to resist eating in response to mood cues represents a critical factor for understanding the mechanisms underlying cue-induced eating behaviors. This model requires further replication and future investigations should also examine relationships between individual differences in eating habits and physiological measures of stress response. The self-administration paradigm adapted in this study has been previously used to evaluate the effects of medication on smoking behavior (McKee, 2009) and this unique approach to study eating behaviors in laboratory settings may ultimately have important clinical applicability in developing behavioral and pharmacologic methods to reduce craving and enhance one’s ability to resist high-calorie foods.
Footnotes
The model included session order, age, and binge eating symptoms as they contributed to reducing residuals, and they were all statistically significant (p < .05).
The model included session order, age, gender, and emotional eating as they contributed to reducing residuals, but no covariates were statistically significant (p > .05).
All correlation analyses included gender and session order as covariates.
References
- Appelhans BM, Pagoto SL, Peters EN, Spring BJ. HPA axis response to stress predicts short-term snack intake in obese women. Appetite. 2010;54:217–220. doi: 10.1016/j.appet.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berteus Forslund H, Torgerson JS, Sjostrom L, Lindroos AK. Snacking frequency in relation to energy intake and food choices in obese men and women compared to a reference population. International Journal of Obesity. 2005;29:711–719. doi: 10.1038/sj.ijo.0802950. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neuroscience and Biobehavioral Reviews. 2006;30:173–187. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention . Healthy Weight - it’s not a diet, it’s a lifestyle! 2011. Retrieved from http://www.cdc.gov/healthyweight/assessing/index.html. [Google Scholar]
- Cools J, Schotte DE, McNally RJ. Emotional arousal and overeating in restrained eaters. Journal of Abnormal Psychology. 1992;101:348–351. doi: 10.1037//0021-843x.101.2.348. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Davis C, Patte K, Levitan R, Reid C, Tweed S, Curtis C. From motivation to behaviour: a model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite. 2007;48:12–19. doi: 10.1016/j.appet.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Davis C, Strachan S, Berkson M. Sensitivity to reward: implications for overeating and overweight. Appetite. 2004;42:131–138. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- de Graaf C. Effects of snacks on energy intake: an evolutionary perspective. Appetite. 2006;47:18–23. doi: 10.1016/j.appet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV. American Psychiatric Press; Washington, D. C.: 1995. Patient Edition. [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Goldfein JA, Devlin MJ, Kamenetz C. Eating Disorder Examination-Questionnaire with and without instruction to assess binge eating in patients with binge eating disorder. International Journal of Eating Disorders. 2005;37:107–111. doi: 10.1002/eat.20075. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Shiffman S, Wing RR. Relapse crises and coping among dieters. Journal of Consulting and Clinical Psychology. 1989;57:488–495. doi: 10.1037//0022-006x.57.4.488. [DOI] [PubMed] [Google Scholar]
- Guerrieri R, Nederkoorn C, Schrooten M, Martijn C, Jansen A. Inducing impulsivity leads high and low restrained eaters into overeating, whereas current dieters stick to their diet. Appetite. 2009;53:93–100. doi: 10.1016/j.appet.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Guerrieri R, Nederkoorn C, Stankiewicz K, Alberts H, Geschwind N, Martijn C, Jansen A. The influence of trait and induced state impulsivity on food intake in normal-weight healthy women. Appetite. 2007;49:66–73. doi: 10.1016/j.appet.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Habhab S, Sheldon JP, Loeb RC. The relationship between stress, dietary restraint, and food preferences in women. Appetite. 2009;52:437–444. doi: 10.1016/j.appet.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Hill AJ. Does dieting make you fat? British Journal of Nutrition. 2004;92:S15–18. doi: 10.1079/bjn20041135. [DOI] [PubMed] [Google Scholar]
- Izard CE. Patterns of emotions: A new analysis of anxiety and depression. Academic Press; San Diego, CA: 1972. [Google Scholar]
- Jansen A, Vanreyten A, van Balveren T, Roefs A, Nederkoorn C, Havermans R. Negative affect and cue-induced overeating in non-eating disordered obesity. Appetite. 2008;51:556–562. doi: 10.1016/j.appet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Lattimore P, Caswell N. Differential effects of active and passive stress on food intake in restrained and unrestrained eaters. Appetite. 2004;42:167–173. doi: 10.1016/j.appet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addiction Biology. 2009;14:99–107. doi: 10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, Wanzer J. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. Journal of Psychopharmacology. 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, O’Connor DB, Conner M. Daily hassles and eating behaviour: the role of cortisol reactivity status. Psychoneuroendocrinology. 2007;32:125–132. doi: 10.1016/j.psyneuen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Currrent Opinion in Neurobiology. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. Psychosomatic Medicine. 2000;62:853–865. doi: 10.1097/00006842-200011000-00016. [DOI] [PubMed] [Google Scholar]
- Pandit R, de Jong JW, Vanderschuren LJ, Adan RA. Neurobiology of overeating and obesity: the role of melanocortins and beyond. European Journal of Pharmacology. 2011;660:28–42. doi: 10.1016/j.ejphar.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Piernas C, Popkin BM. Snacking increased among U.S. adults between 1977 and 2006. Journal of Nutrition. 2010;140:325–332. doi: 10.3945/jn.109.112763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reas DL, Grilo CM, Masheb RM. Reliability of the Eating Disorder Examination-Questionnaire in patients with binge eating disorder. Behaviour Research and Therapy. 2006;44:43–51. doi: 10.1016/j.brat.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Schotte DE, Cools J, McNally RJ. Film-induced negative affect triggers overeating in restrained eaters. Journal of Abnormal Psychology. 1990;99:317–320. doi: 10.1037//0021-843x.99.3.317. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction Biology. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Strien T, Frijters JE, Bergers GP, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders. 1986;5:295–315. [Google Scholar]
- Vicennati V, Pasqui F, Cavazza C, Pagotto U, Pasquali R. Stress-related development of obesity and cortisol in women. Obesity. 2009;17:1678–1683. doi: 10.1038/oby.2009.76. [DOI] [PubMed] [Google Scholar]
- Wardle J. Eating style: a validation study of the Dutch Eating Behaviour Questionnaire in normal subjects and women with eating disorders. Journal of Psychosomatic Research. 1987;31:161–169. doi: 10.1016/0022-3999(87)90072-9. [DOI] [PubMed] [Google Scholar]
- Wallis DJ, Hetherington MM. Stress and eating: the effects of ego-threat and cognitive demand on food intake in restrained and emotional eaters. Appetite. 2004;43:39–46. doi: 10.1016/j.appet.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Wallis DJ, Hetherington MM. Emotions and eating. Self-reported and experimentally induced changes in food intake under stress. Appetite. 2009;52:355–362. doi: 10.1016/j.appet.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Coughlan E. Mood-induced eating. Interactive effects of restraint and tendency to overeat. Appetite. 2009;52:290–298. doi: 10.1016/j.appet.2008.10.006. [DOI] [PubMed] [Google Scholar]