Abstract
Purpose
The objective of this study is to evaluate the effects of blood flow restriction (BFR) on muscle oxygenation during low-intensity resistance exercise as well as postexercise expression of molecules related to physiological angiogenesis.
Methods
Using a randomized cross-over design, six apparently healthy young adults (22 ± 1 yr) performed 120 unilateral knee extensions at 40% of 1 repetition maximum with and without BFR (CNTRL). Near-infrared spectroscopy was used to measure oxygenation of the vastus lateralis during exercise. Serum and muscle expression of Post–Resistance vascular endothelial growth factor (VEGF) were determined preexercise, 4 h postexercise, and 24 h postexercise. Transcript (mRNA) expression of VEGF and other angiogenic genes was also determined.
Results
BFR increased muscle hemoglobin (Hb) concentrations during exercise (14.4 ± 1.6 vs. 0.9 ± 1.6, P = 0.002), driven largely by an increase in deoxygenated Hb (11.0 ± 2.5 vs. 0.5 ± 1.1, P = 0.030). BFR also increased (P < 0.05) transcript expression of VEGF, VEGF-R2, hypoxia-inducible factor 1 alpha, inducible nitric oxide synthase (NOS), and neuronal NOS. The most dramatic change in response to BFR was an increase in VEGF mRNA at 4 h postexercise (4.1 ± 0.6 vs. 0.6 ± 0.2-fold change, P = 0.028). Compared with control, transcript expression of endothelial NOS, serum VEGF, or muscle protein expression of VEGF was not altered in response to BFR (P > 0.05).
Conclusion
Acute BFR increases postexercise expression of mRNA related to skeletal muscle angiogenesis, plausibly in response to changes in muscle Hb concentrations.
Keywords: BFR, KAATSU, SKELETAL MUSCLE, RESISTANCE EXERCISE, NIRS, VEGF, NOS
Skeletal muscle function, that is, strength and endurance, is an essential component of overall health and independent living. To date, performance of high-intensity resistance exercise (RE) is the most efficacious method of maintaining skeletal muscle function. As such, the American College of Sports Medicine recommends that individuals train at or above 65% of his/her 1 repetition maximum (RM) to achieve muscle hypertrophy and strength gains (2). However, high-intensity RE may be difficult for some populations, including persons with osteoarthritis or osteoporosis or those recovering from surgery or stroke. Furthermore, limited self-efficacy often prevents these persons from engaging in high-intensity RE (25). Therefore, alternative and/or adjuvant interventions are needed that are capable of maintaining skeletal muscle function while using low-intensity loads.
As reviewed recently (29), the use of blood flow restriction (BFR) holds potential as such a therapy. RE with BFR, also known as KAATSU, involves performing low-intensity RE while externally applied compression mildly restricts blood flow to the active skeletal muscle. Because BFR eases joint stress by avoiding high-intensity loads, it may be a plausible alternative to high-intensity training. However, additional information is needed regarding the mechanistic adaptations that underlie BFR training. Prior studies have demonstrated that BFR training increases skeletal muscle mass and strength (24,32,39). Although the mechanisms that induce these changes in muscle mass and strength are yet to be fully elucidated, these changes are likely mediated at least in part by repeated increases in muscle protein synthesis that are observed after acute BFR (14,15). In addition to changes in muscle mass and strength, chronic BFR is also known to improve skeletal muscle endurance (23,38). Several mechanisms have been proposed as causal factors in this increased muscle endurance, including increased concentrations of lactate, growth hormone, and inorganic phosphate. More recently, increases in the delivery of blood and oxygen to the activated muscle tissue were demonstrated and proposed to contribute to increases in muscular endurance after BFR training (12,23,32). Interestingly, BFR also enhances muscle microvascular filtration capacity, a surrogate index of muscle capillarity (12).
The main stimuli for inducing skeletal muscle capillarization are hypoxia, sheer stress, and increases in the concentrations of growth factors such as vascular endothelial growth factor (VEGF) (21). Although high-intensity RE is known to increase transcript expression of VEGF and other growth/transcription factors associated with capillary formation, that is, angiogenesis (16), the relative impact of BFR on postresistance expression of these factors remains unclear. Therefore, the overarching purpose of the present study was to determine whether BFR potentiates the effects of low-intensity exercise on skeletal muscle expression of molecules involved in physiological angiogenesis. We hypothesized that the addition of BFR to low-intensity exercise would enhance postexercise protein expression of VEGF as well as mRNA expression of factors involved in regulating new capillary formation, for example, VEGF and its primary receptor (VEGF-R2), hypoxia-inducible factor 1 alpha (HIF-1α), and isoforms of nitric oxide synthase (NOS)—including neuronal (nNOS), inducible (iNOS), and endothelial (eNOS).
METHODS
Participants
We studied six apparently healthy subjects on two separate occasions in a randomized, cross-over design. On separate occasions, three men and three women performed unilateral knee extension exercise either with BFR or without BFR (CNTRL). Before enrollment in the study, all participants provided written informed consent on the basis of documents approved by the University of Florida Institutional Review Board. Participants recruited were 1) between the ages of 18 and 30 yr, 2) were not obese (body mass index <30 kg·m−2), 3) were nonsmokers, 4) and had normal vascular function as evidenced by an ankle–brachial index (ABI) value >0.95. Exclusionary criteria included regular participation in lower body RE (>5 h in past month), participation in more than 4 h of aerobic exercise per week, active treatment for disease, bone fracture within the last 6 months, limb amputation, use of estrogen-based contraceptives or anabolic drugs, significant cognitive impairment (i.e., Mini-Mental State Examination score ≤23), excessive alcohol use (>14 drinks per week), pregnancy, tachycardia (resting heart rate >120 bpm), uncontrolled hypertension (resting systolic blood pressure >180 mm Hg or diastolic blood pressure >100 mm Hg), and current participation in a clinical trial or consumption of an investigational product within 30 d before enrollment.
During an initial screening visit, vital measures and anthropometric characteristics were assessed before familiarizing participants to the experimental protocol and determining muscle strength by measuring unilateral 1 RM on a standard leg extension machine (Paramount Fitness, Los Angeles, CA). Participants were then provided a SenseWear physical activity monitor (Body Media, Pittsburgh, PA) to wear for 7 d to verify that they were not regular exercisers. Participants had a mean age of 22 ± 1 yr, body mass of 72 ± 7 kg, body mass index of 23.7 ± 1.4, ABI of 1.05 ± 0.03, steps per day of 7073 ± 619, and 1 RM of 44 ± 7 kg.
Research design
Upon completion of the screening visit, participants were randomly assigned to complete either RE with externally applied vascular restriction or a control condition with no vascular restriction. Participants were instructed to refrain from exercise for 48 h before exercising. The evening before the exercise bout, participants were admitted to the Shands Clinical Research Unit (SCRU) of the UF Clinical and Translational Science Institute (CTSI). Participants were fed a standard dinner consisting of 12 kcal·kg−1 and a macronutrient composition of 60% carbohydrate, 20% protein, and 20% fat, as well as a late-night snack. Assessment began the following morning with participants in the fasted state.
Before exercising, participants underwent a standard blood draw and a skeletal muscle biopsy. Participants then performed the appropriate exercise bout according to random assignment. Each exercise bout consisted of a total of 120 repetitions (10 sets of 12 repetitions) of knee extensions performed with the dominant limb at 40% of 1 RM with 1-min rest periods between sets. For the BFR condition, a lower extremity pressure cuff (Kaatsu-Master Mini, Sato Sports Plaza, Tokyo, Japan) was placed around the most proximal portion of the exercised leg and inflated to a pressure of 220 mm Hg.
Before exercise, a near-infrared spectroscopy (NIRS) probe was placed on the skin superficial to the vastus lateralis to measure tissue oxygenation during exercise. Blood and muscle samples were collected 4 h postexercise, and participants were again provided a standard meal and late-night snack. The 4-h postexercise sample collection was included because most contraction-induced increases in mRNA expression peak between 3 and 6 h postexercise (3,34,40). Blood and muscle samples were repeated the following morning (24 h post-exercise) in the fasted state before release from the SCRU. After a minimum of 3 wk after completion of the first experimental condition, participants returned to complete the other condition. During each condition, female participants were tested during the midfollicular phase of their menstrual cycle to minimize the potential effects of monthly estrogenic variation on study outcomes. Serum estradiol concentrations were 111.4 ± 12.8 pg·mL−1 in the BFR condition and 103.3 ± 7.4 pg·mL−1 in the CNTRL condition (P = 0.595).
NIRS
NIRS, a noninvasive technology that uses light attenuation to determine tissue absorption and scattering, was used to determine tissue oxygenation of the vastus lateralis during exercise. The theory and application of NIRS during exercise has been extensively described elsewhere (13). By using two different near-infrared light frequencies, the concentrations of oxygenated (O2Hb) and deoxygenated hemoglobin (HHb) can be measured in skeletal muscle tissue. In addition, the total amount of hemoglobin (Hb), calculated as the sum of O2Hb and HHb, represents an index of total blood volume in the tissue at the site of measurement (9). Tissue oxygenation profiles of the vastus lateralis muscle were made using a NIRO-200 near-infrared spectrometer (Hamamatsu Photonics, Hamamatsu City, Japan) using pulsed laser diodes at wavelengths of 775 and 850 nm. First, a large area of skin was shaved and cleaned with alcohol. Then the optode holder was attached to a double-sided adhesive pad placed on the subject’s skin. Optode positions were standardized between conditions by placing the adhesive pad midway between the lateral epicondyle and greater trochanter of the femur. The tissue concentrations of O2Hb and HHb were derived through coefficients of absorption and scattering after adjusting for amplitude and frequency using a modified Lambert law. Raw NIRS data were processed offline using custom software built with LabVIEW 2009 (National Instruments Inc., Austin, TX). Data were summarized as the average O2Hb, HHb, and total Hb (THb) from the start to the end of the exercise bout, and the values include rest periods. Because the number of contractions was standardized across conditions, adjustments for oscillations due to muscle stretching were not necessary.
Biochemical analyses
Venous blood samples were collected at baseline, 4 h postexercise, and 24 h postexercise during each condition and centrifuged to obtain serum according to standard laboratory practices. Serum VEGF was determined by enzyme-linked immunosorbent assay from R&D Systems (Minneapolis, MN). A percutaneous muscle biopsy was then collected as previously described (5). Briefly, muscle samples were collected under 2% lidocaine local anesthetic using a six-gauge needle with suction applied. Samples collected at 4 h were collected from the initial incision site with an altered needle angle, whereas samples were collected at 24 h postexercise from a new incision site approximately 5 cm from the initial incision. Muscle samples were gently blotted on saline-moistened gauze to remove excess blood, weighed, and separated from adipose and/or connective tissue under microscope. Muscle was aliquoted and placed into cryovials either with or without RNAlater (Ambion, Austin, TX). Cryovials were snap frozen in liquid nitrogen and stored at −80°C until analysis.
Muscle mRNA expression was determined using quantitative real-time polymerase chain reaction (Q-PCR) with minor modification to previously published procedures (6,7). Briefly, the muscle aliquot saved in RNAlater was homogenized, and RNA was isolated using the RNeasy Fibrous Tissue Mini kit (Qiagen, Valencia, CA). Contaminating DNA was removed by DNase digestion using the RNase-Free DNase set from Qiagen. RNA integrity was assessed by RIA integrity number determined by the Agilent 2100 Bio-analyzer (Agilent Technologies, Santa Clara, CA). The mean ± SE RIA integrity number of the RNA samples was 7.9 ± 0.2, indicating good RNA integrity. Two microgram of total RNA was then reverse transcribed to synthesize cDNA using the High Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems, Carlsbad, CA). Primer sequences (Table 1) were designed using the Applied Biosystems (ABI) Primer Express 3.0 software and used to construct commercially synthesized oligonucleotide sense and antisense primers (Integrated DNA Technologies, Coralville, IA). Q-PCR analysis was performed using the Power SYBR® Green PCR Master Mix (ABI, Warrington, UK), 0.2-nm primers and nuclease-free water in a 25-L reaction. Relative expression was determined using the ABI 7300 real-time PCR system with universal cycling conditions (enzyme activation at 95°C for 10 min, 40 cycles of denaturation at 95°C for 15 s, and annealing/extension at 60°C for 1 min). All samples were examined in triplicate and melt curves performed to verify primer specificity. Sample mRNA expression of target genes was determined relative to the expression of glyceraldehyde-3-phosphate dehydrogenase (GADPH) as it has previously been shown to be a stable control gene for exercise studies (22). Validation of GADPH was performed to ensure that its expression was unaffected by the present exercise regimen. The relative amount of GADPH was calculated using the 2−ΔCT equation in which the change in threshold cycle (ΔCT) = CT time × −CT time 0 (26). This calculation should yield a value close to 1 (e.g., 20 = 1) if the exercise did not affect the control gene. The mean (95% confidence interval) across time points was 1.02 (0.88–1.16) for exercise with vascular restriction and 1.07 (0.98–1.16) for the control exercise. Postexercise changes in target mRNA expression were determined using the 2−ΔΔCT equation where ΔΔCT = (CT target − CT reference) time × −(CT target − CT reference) time 0 (26). Using this analysis, the mean fold change at time zero should also be very close to 1. This is a convenient method of checking for experimental variation (8,10). In this study, the fold change at time zero for all target genes across both conditions was 1.05 (0.95–1.14).
TABLE 1.
Primer sequences used for Q-PCR.
| Target mRNA | NCBI Accession Number | Sense Sequence (5′ → 3′) | Antisense Sequence (5′ → 3′) |
|---|---|---|---|
| GADPH | J02642 | ATG GAA ATC CCA TCA CCA TCT T | CGC CCC ACT TGA TTT TGG |
| VEGF | AF022375 | CTT GCT GCT CTA CCT CCA CCA T | ATG ATT CTG CCC TCC TCC TTC T |
| VEGF-R2 | NM_002253 | CAC CAC TCA AAC GCT GAC ATG TA | CCA ACT GCC AAT ACC AGT GGA T |
| HIF-1α | AF208487 | GCC CCA GAT TCA GGA TCA GA | TGG GAC TAT TAG GCT CAG GTG AAC |
| nNOS | NM_000620 | GAA ATC ATC TTG GCC TGA TAG CA | GAA GAG ACG AAC AGA AAT GAC ATT G |
| iNOS | NM_000625 | CCC CTT CAA TGG CTG GTA CA | GCG CTG GAC GTC ACA GAA |
For quantification of muscle VEGF protein expression, 50 μg of whole muscle protein extract was separated by gel electrophoresis on 15% polyacrylamide gels and transferred to nitrocellulose membranes using a semidry blotter (Bio-Rad, Hercules, CA). Transfer efficiency was verified by staining the gels with GelCode Blue Stain Reagent (Pierce Biotechnology, Rockford, IL) and the membranes with Ponceau S (Sigma-Aldrich, St. Louis, MO). Ponceau S staining was also used as a loading control (31). Blots were blocked for 1 h in StartingBlock T20 Blocking Buffer (Thermo Scientific, Waltham, MA) and probed with the primary antibody for VEGF at a dilution of 1:200. The VEGF antibody (catalog #SC-152) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). After primary antibody incubation, blots were incubated with antirabbit secondary antibody (Cell Signaling, Danvers, MA) conjugated with horseradish peroxidase at a dilution of 1:5000. Blots were washed in Tris-Buffered Saline-Tween 20 mixture (3 × 5 min), and protein was subsequently visualized with a DuoLux enhanced chemiluminescence kit (Vectro Laboratories, Burlingame, CA) and detected using a ChemiDoc XRS imager (BioRad, Hercules, CA). Spot density of target bands was normalized to the amount of protein loaded in each lane, as determined by densitometric analysis of the corresponding Ponceau S–stained membranes. Bands were quantified using Image Lab software from BioRad.
Statistical analysis
NIRS data were analyzed using paired Student’s t-tests as were fold changes in muscle protein content at each time point. Serum VEGF data were log transformed and then analyzed by ANCOVA with repeated measures, adjusted for baseline level of the outcome. Muscle VEGF data were also analyzed by repeated-measures ANCOVA. Because mRNA data were nonnormally distributed, fold changes in gene expression at each time point were compared using the nonparametric Wilcoxon test. Differences between conditions were considered significant at P < 0.05 (two tailed). Data were analyzed using SPSS 19 for Windows (IBM, Chicago, IL) and are presented as means ± SE.
RESULTS
Tissue oxygenation and serum VEGF
Across the entire bout, mean O2Hb was 0.3 ± 0.8 μmol·cm during CNTRL and 3.4 ± 2.0 μmol·cm during BFR (P > 0.05, Fig. 1). Meanwhile, HHb was significantly increased by BFR (11.0 ± 2.5 μmol·cm) compared with CNTRL (0.5 ± 1.1 μmol·cm, P = 0.030). Accordingly, THb was significantly (P = 0.002) higher during exercise with BFR (14.4 ± 1.6 μmol·cm) than with CNTRL (0.9 ± 1.6 μmol·cm). For serum VEGF, baseline concentrations of VEGF were similar between CNTRL and BFR conditions (absolute concentrations displayed in Table 2). Although absolute serum VEGF values increased to a greater extent in the BFR group, the time × condition interaction was significant, indicating no difference from CNTRL (P > 0.05, Fig. 3A).
FIGURE 1.
Mean concentrations of Hb in the vastus lateralis muscle during the exercise bouts as measured by NIRS. Black columns indicate the effects of low-intensity exercise (CNTRL), whereas white columns indicate the effects of adding BFR. Columns indicate means and bars indicate SE. *Significantly different from CNTRL (P < 0.05).
TABLE 2.
Absolute concentrations of serum VEGF.
| CNTRL | BFR | |
|---|---|---|
| Baseline | 181.8 ± 47.1 | 184.6 ± 46.8 |
| 4 h postexercise | 187.2 ± 43.8 | 208.6 ± 51.4 |
| 24 h postexercise | 180.5 ± 47.8 | 191.4 ± 48.1 |
Concentrations are in picograms per milliliter and expressed as mean ± SE.
FIGURE 3.
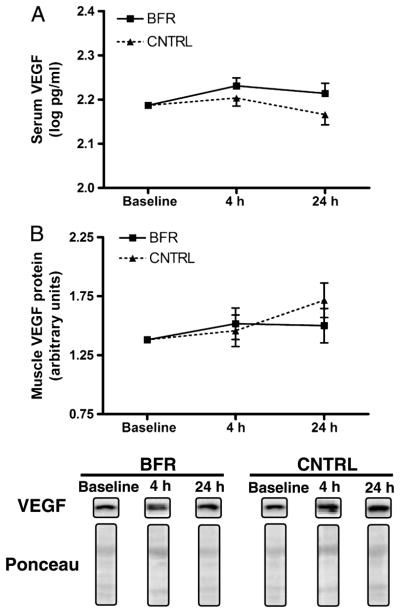
Skeletal muscle expression of VEGF in serum (A) and muscle (B) at baseline, 4 h, and 24 h after low-intensity exercise either with BFR or without BFR (CNTRL). Serum VEGF data are represented as the log of the absolute concentrations. Means are adjusted for baseline level of expression. Boxes indicate means and bars indicate SE. Representative blots for VEGF and Ponceau staining are provided below panel B.
Skeletal muscle mRNA and protein expression
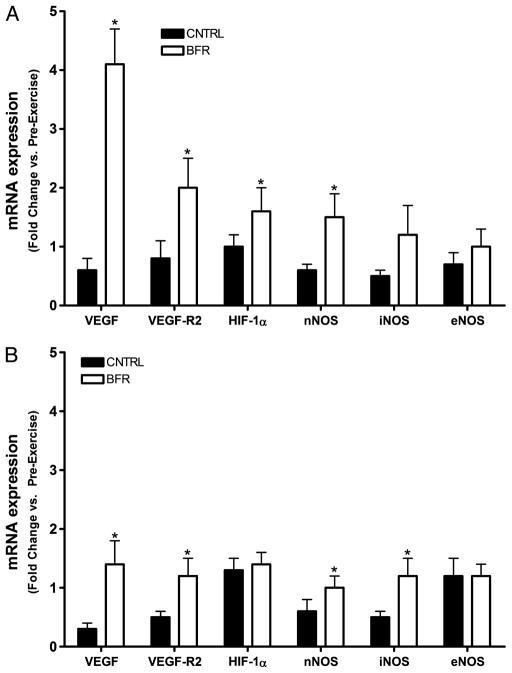
The addition of BFR to low-intensity RE increased postexercise skeletal muscle expression of several mRNA related to vascular adaptations including VEGF, VEGF-R2, HIF-1α, nNOS, and iNOS. The most dramatic increase in expression was observed for VEGF at 4 h postexercise, which demonstrated a more than fourfold up-regulation in response to BFR (Fig. 2). BFR also potentiated the expression of VEGF-R2, HIF-1α, and nNOS at 4 h postexercise. These changes persisted 24 h postexercise for VEGF, VEGF-R2, and nNOS (Fig. 3). In addition, expression of iNOS was significantly increased by BFR 24 h after exercise. Expression of eNOS did not differ between BFR and CNTRL at either 4 or 24 h postexercise (P > 0.05). In addition, BFR did not alter muscle VEGF levels after exercise relative to CNTRL because the time × condition interaction was not significant (P > 0.05, Fig. 3B).
FIGURE 2.
Skeletal muscle mRNA expression of angiogenesis-related genes after acute low-intensity exercise. Changes in mRNA content are expressed as a fold change from baseline relative to GADPH at 4 h (A) and 24 h (B) postexercise. Black columns indicate the effects of low-intensity exercise (CNTRL), whereas white columns indicate the effects of adding BFR. Columns indicate means and bars indicate SE. *Significantly different from CNTRL (P < 0.05).
DISCUSSION
Physical exercise, particularly aerobic in nature, is a well-described method of enhancing the capillary network within skeletal muscle (36). Additional evidence indicates that high-intensity RE also induces angiogenic signaling within the activated muscle (16). Because high-intensity training is not a viable option for some individuals, we aimed to determine whether BFR—a proposed alternative to high-intensity training—enhances angiogenic signaling after low-intensity exercise. Consequently, the present study tested the hypothesis that BFR used during acute low-intensity RE potentiates postexercise skeletal muscle expression of molecules involved in angiogenic cell signaling. The primary findings of this study were that compared with CNTRL, unilateral BFR (a) increased muscle Hb concentrations, (b) increased postexercise muscle expression of angiogenic transcript expression, and (c) did not alter postexercise serum or muscle VEGF levels. This study is the first to report that, compared with CNTRL, acute BFR enhances transcription of genes involved in signaling pathways that contribute to exercise-induced angiogenesis.
BFR has been proposed as a potentially viable strategy for maintaining skeletal muscle function among various groups of individuals, including astronauts (27), persons with neuromuscular disease or injury (29), and older adults for the treating sarcopenia (35). Numerous studies have reported BFR as an efficacious method of improving skeletal muscle strength. To this end, several recent studies have investigated the effects of acute and chronic BFR on skeletal muscle mechanisms involved in regulating myofibrillar protein balance (14,15,30). Recently, investigators have also become interested in the effects of BFR on skeletal muscle vascular function because of its relevance to muscle endurance (12,23,32). These studies have reported the BFR training improves muscle oxygen delivery to and microvascular filtration capacity of skeletal muscle (12,23). However, these studies have not provided data regarding the acute effects of BFR on expression of molecules involved in stimulating physiological angiogenesis, a central contributor to improved muscle microvascular function.
Three primary stimuli are known to stimulate the cellular and molecular events that stimulate increases in muscle capillarity: 1) changes in vascular wall tension and/or shear stress, 2) metabolic stimuli such as low PO2, and 3) tension developed during muscle contraction (21,28). Compared with high-intensity contractions, low-intensity RE does not produce significant muscular tension. Similarly, we demonstrate here using the NIRS technology that low-intensity RE does not alter muscle oxygenation. As a result, angiogenic gene expression did not increase (and actually slightly decreased) after standard low-intensity exercise. However, the addition of BFR to low-intensity exercise increased expression of several targeted transcripts. It is possible, if not likely, that the changes in muscle Hb concentrations created by BFR induced these changes in transcript expression.
The most notable transcriptional change in response to BFR was for VEGF. Importantly, VEGF is the central growth factor involved in regulating angiogenesis. This potent growth factor is a critical signal in vascular remodeling because it maintains vascular integrity and stimulates the production of the vasodilatory mediator nitric oxide (NO). In the present study, we observed a fourfold increase in VEGF mRNA expression after exercise—an elevation that remained 24 h after exercise. This finding demonstrates that BFR causes increases in VEGF transcription after low-intensity contractions. These findings regarding the effect of acute BFR on VEGF expression are similar to those observed in humans after standard high-intensity exercise (16), cycling with BFR (18), and an experimental model of RE using a pressure chamber to induce BFR (1). Likewise, others have demonstrated in a rat model that the use of intermittent pneumatic leg compressions—somewhat conceptually similar to BFR—increases skeletal muscle VEGF expression (37). It is intriguing that, despite a dramatic increase in VEGF transcription in response to BFR, VEGF protein content did not differ between conditions at the chosen time points. One potential explanation is the inherent limitation of noncontinuous sampling necessitated by the invasiveness of the muscle biopsy procedure. It is possible that more frequent or alternatively timed biopsies may reveal divergent results.
Regulation of VEGF expression in response to hypoxia is largely regulated by the transcription factor HIF-1α. The VEGF gene contains an upstream regulatory sequence that increases VEGF mRNA expression when bound by HIF-1α. During normoxic conditions, HIF-1α is ubiquitinated and subsequently degraded in less than 5 min. However, under hypoxic conditions, HIF-1α is stabilized and becomes an important stimulus for expression of VEGF and more than 100 other target genes involved in processes including but not limited to angiogenesis, erythropoiesis, and glucose metabolism (28). To our knowledge, this is the first study to demonstrate that BFR potentiates increases in expression of HIF-1α after low-intensity RE. Two prior studies reported that low-intensity RE with and without BFR increased HIF-1α; however, no significant difference was observed between conditions (1,11). Additional analysis of our data indicates that the significant result for HIF-1α was driven largely by two individuals with a robust response to the BFR exercise. Thus, the significant result herein should be considered tenuous at best, and replication is needed to confirm or refute this finding.
We also observed in this study a significant effect of BFR on the postexercise expression of VEGF-R2 (KDR). Although intramuscular VEGF can bind multiple tyrosine kinase receptors, the majority of biologic activity is signaled through binding to VEGF-R2 (KDR). Once bound by VEGF, KDR stimulates a signaling cascade that ultimately results in increased production of NO. Previously, Gavin et al. (16) reported a 1.5-fold increase in KDR expression after high-intensity RE. Our findings demonstrate that BFR induced an even greater (twofold) increase in KDR transcript expression after exercise. This response is similar to that reported by Gustafsson et al. (17) after knee extensor contractions with pressure chamber-induced BFR.
After the binding of VEGF to KDR, a signaling cascade ensues, resulting in increased NO production through calcium mobilization and phosphorylation of NOS isoforms. In addition, NO production is stimulated independent of VEGF through sheer stress—a process that reciprocally regulates VEGF expression. Three isoforms of NOS exist: nNOS, iNOS, and eNOS. In healthy persons under basal conditions, skeletal muscle contains significant nNOS protein with lesser and minimal levels of eNOS and iNOS, respectively. Although iNOS expression is up-regulated in response to acute exercise, chronic training actually reduces iNOS because excessive levels are associated with chronic inflammatory processes (19,33). Meanwhile, low skeletal muscle content of nNOS and eNOS are associated with metabolic and vascular dysfunction (4,20). This study indicates that the addition of BFR to acute low-intensity exercise increases postexercise muscle expression of nNOS and iNOS, but not eNOS. It will be interesting to determine the chronic effects of the BFR paradigm on skeletal muscle expression of the NOS isoforms.
The present study is the first to demonstrate that the addition of BFR to low-intensity RE increases skeletal muscle expression of mRNA related to angiogenesis while inducing a hypoxic intramuscular environment. The study has several strengths, including a randomized, cross-over design and the use of a clinical research center to control for potentially confounding factors such as differences in diet and physical activity. Still, despite these strengths, the study is not without limitations. First, although the relatively small sample size was adequate to detect differences in gene expression between conditions, it may have limited our ability to detect differences in serum and muscle VEGF concentrations. Second, the acute nature of the study limits our ability to evaluate the functional significance of these findings. Indeed, acute changes in transcript expression cannot be extrapolated to suggest the chronic effects on protein expression of these factors, angiogenesis, or skeletal muscle endurance. Future studies are needed to evaluate these questions.
In conclusion, this study is the first to report that BFR acutely enhances transcription of angiogenesis-related genes after a bout of low-intensity RE. BFR also increased muscle Hb concentrations during bout, possibly contributing to the increase in gene expression. Continued study is needed to determine whether these acute changes result in functional adaptations at the cellular or whole muscle levels after long-term training.
Acknowledgments
This work was funded by a grant from the American College of Sports Medicine awarded to TWB. Additional support was provided by the University of Florida Older Americans Independence Center (NIH 1P30AG028740) and CTSI (NIH 5UL1RR029890). TWB’s effort for the project was partially supported by an award from the CTSI (NIH 1KL2RR029888).
The authors would like to thank all study participants for their time and effort in completing the study protocol. The authors are also very grateful to Deborah Hiatt-Jensen for assistance with muscle biopsies, Launa Clough for assistance with data analysis, as well as the nurses and dieticians of the SCRU for their attention to detail in standardizing the experimental procedures.
Footnotes
There are no conflicts of interest to disclose.
The results of the present study do not constitute endorsement by the American College of Sports Medicine.
References
- 1.Ameln H, Gustafsson T, Sundberg CJ, et al. Physiological activation of hypoxia inducible factor-1 in human skeletal muscle. FASEB J. 2005;19(8):1009–11. doi: 10.1096/fj.04-2304fje. [DOI] [PubMed] [Google Scholar]
- 2.American College of Sports Medicine. Position Stand: progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 3.Bickel CS, Slade J, Mahoney E, Haddad F, Dudley GA, Adams GR. Time course of molecular responses of human skeletal muscle to acute bouts of resistance exercise. J Appl Physiol. 2005;98(2):482–8. doi: 10.1152/japplphysiol.00895.2004. [DOI] [PubMed] [Google Scholar]
- 4.Bradley SJ, Kingwell BA, Canny BJ, McConell GK. Skeletal muscle neuronal nitric oxide synthase micro protein is reduced in people with impaired glucose homeostasis and is not normalized by exercise training. Metabolism. 2007;56(10):1405–11. doi: 10.1016/j.metabol.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Buford TW, Cooke MB, Manini TM, Leeuwenburgh C, Willoughby DS. Effects of age and sedentary lifestyle on skeletal muscle NF-{kappa}B signaling in men. J Gerontol A Biol Sci Med Sci. 2010;65(5):532–537. doi: 10.1093/gerona/glp196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buford TW, Cooke MB, Shelmadine BD, Hudson GM, Redd L, Willoughby DS. Effects of eccentric treadmill exercise on inflammatory gene expression in human skeletal muscle. Appl Physiol Nutr Metab. 2009;34(4):745–53. doi: 10.1139/H09-067. [DOI] [PubMed] [Google Scholar]
- 7.Buford TW, Cooke MB, Willoughby DS. Resistance exercise-induced changes of inflammatory gene expression within human skeletal muscle. Eur J Appl Physiol. 2009;107(4):463–71. doi: 10.1007/s00421-009-1145-z. [DOI] [PubMed] [Google Scholar]
- 8.Bustin SA, Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech. 2004;15(3):155–66. [PMC free article] [PubMed] [Google Scholar]
- 9.De Blasi RA, Cope M, Elwell C, Safoue F, Ferrari M. Noninvasive measurement of human forearm oxygen consumption by near infrared spectroscopy. Eur J Appl Physiol Occup Physiol. 1993;67(1):20–5. doi: 10.1007/BF00377698. [DOI] [PubMed] [Google Scholar]
- 10.Dheda K, Huggett JF, Bustin SA, Johnson MA, Rook G, Zumla A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques. 2004;37(1):112–4. 116, 118–9. doi: 10.2144/04371RR03. [DOI] [PubMed] [Google Scholar]
- 11.Drummond MJ, Fujita S, Abe T, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc. 2008;40(4):691–8. doi: 10.1249/MSS.0b013e318160ff84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans C, Vance S, Brown M. Short-term resistance training with blood flow restriction enhances microvascular filtration capacity of human calf muscles. J Sports Sci. 2010;28(9):999–1007. doi: 10.1080/02640414.2010.485647. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29(4):463–87. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 14.Fry CS, Glynn EL, Drummond MJ, et al. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol. 2010;108(5):1199–209. doi: 10.1152/japplphysiol.01266.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita S, Abe T, Drummond MJ, et al. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol. 2007;103(3):903–10. doi: 10.1152/japplphysiol.00195.2007. [DOI] [PubMed] [Google Scholar]
- 16.Gavin TP, Drew JL, Kubik CJ, Pofahl WE, Hickner RC. Acute resistance exercise increases skeletal muscle angiogenic growth factor expression. Acta Physiol (Oxf) 2007;191(2):139–46. doi: 10.1111/j.1748-1716.2007.01723.x. [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson T, Ameln H, Fischer H, Sundberg CJ, Timmons JA, Jansson E. VEGF-A splice variants and related receptor expression in human skeletal muscle following submaximal exercise. J Appl Physiol. 2005;98(6):2137–46. doi: 10.1152/japplphysiol.01402.2004. [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson T, Puntschart A, Kaijser L, Jansson E, Sundberg CJ. Exercise-induced expression of angiogenesis-related transcription and growth factors in human skeletal muscle. Am J Physiol. 1999;276(2 Pt 2):H679–85. doi: 10.1152/ajpheart.1999.276.2.H679. [DOI] [PubMed] [Google Scholar]
- 19.Hesslinger C, Strub A, Boer R, Ulrich WR, Lehner MD, Braun C. Inhibition of inducible nitric oxide synthase in respiratory diseases. Biochem Soc Trans. 2009;37(Pt 4):886–91. doi: 10.1042/BST0370886. [DOI] [PubMed] [Google Scholar]
- 20.Hickner RC, Kemeny G, McIver K, Harrison K, Hostetler ME. Lower skeletal muscle nutritive blood flow in older women is related to eNOS protein content. J Gerontol A Biol Sci Med Sci. 2003;58(1):20–5. doi: 10.1093/gerona/58.1.b20. [DOI] [PubMed] [Google Scholar]
- 21.Hudlicka O, Brown MD. Adaptation of skeletal muscle microvasculature to increased or decreased blood flow: role of shear stress, nitric oxide and vascular endothelial growth factor. J Vasc Res. 2009;46(5):504–12. doi: 10.1159/000226127. [DOI] [PubMed] [Google Scholar]
- 22.Jemiolo B, Trappe S. Single muscle fiber gene expression in human skeletal muscle: validation of internal control with exercise. Biochem Biophys Res Commun. 2004;320(3):1043–50. doi: 10.1016/j.bbrc.2004.05.223. [DOI] [PubMed] [Google Scholar]
- 23.Kacin A, Strazar K. Frequent low-load ischemic resistance exercise to failure enhances muscle oxygen delivery and endurance capacity. Scand J Med Sci Sports. 2011;21(6):e231–41. doi: 10.1111/j.1600-0838.2010.01260.x. [DOI] [PubMed] [Google Scholar]
- 24.Karabulut M, Abe T, Sato Y, Bemben MG. The effects of low-intensity resistance training with vascular restriction on leg muscle strength in older men. Eur J Appl Physiol. 2010;108(1):147–55. doi: 10.1007/s00421-009-1204-5. [DOI] [PubMed] [Google Scholar]
- 25.Lees FD, Clarkr PG, Nigg CR, Newman P. Barriers to exercise behavior among older adults: a focus-group study. J Aging Phys Act. 2005;13(1):23–33. doi: 10.1123/japa.13.1.23. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Loenneke JP, Pujol TJ. KAATSU: rationale for application in Astronauts. Hippokratia. 2010;14(3):224. [PMC free article] [PubMed] [Google Scholar]
- 28.Lundby C, Calbet JA, Robach P. The response of human skeletal muscle tissue to hypoxia. Cell Mol Life Sci. 2009;66(22):3615–23. doi: 10.1007/s00018-009-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manini TM, Clark BC. Blood flow restricted exercise and skeletal muscle health. Exerc Sport Sci Rev. 2009;37(2):78–85. doi: 10.1097/JES.0b013e31819c2e5c. [DOI] [PubMed] [Google Scholar]
- 30.Manini TM, Vincent KR, Leeuwenburgh CL, et al. Myogenic and proteolytic mRNA expression following blood flow restricted exercise. Acta Physiol (Oxf) 2011;201(2):255–63. doi: 10.1111/j.1748-1716.2010.02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marzetti E, Carter CS, Wohlgemuth SE, et al. Changes in IL-15 expression and death-receptor apoptotic signaling in rat gastrocnemius muscle with aging and life-long calorie restriction. Mech Ageing Dev. 2009;130(4):272–80. doi: 10.1016/j.mad.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patterson SD, Ferguson RA. Increase in calf post-occlusive blood flow and strength following short-term resistance exercise training with blood flow restriction in young women. Eur J Appl Physiol. 2010;108(5):1025–33. doi: 10.1007/s00421-009-1309-x. [DOI] [PubMed] [Google Scholar]
- 33.Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med. 2001;7(10):1138–43. doi: 10.1038/nm1001-1138. [DOI] [PubMed] [Google Scholar]
- 34.Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279(4):E806–14. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- 35.Pillard F, Laoudj-Chenivesse D, Carnac G, et al. Physical activity and sarcopenia. Clin Geriatr Med. 2011;27(3):449–70. doi: 10.1016/j.cger.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Prior BM, Lloyd PG, Yang HT, Terjung RL. Exercise-induced vascular remodeling. Exerc Sport Sci Rev. 2003;31(1):26–33. doi: 10.1097/00003677-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Roseguini BT, Mehmet Soylu S, Whyte JJ, Yang HT, Newcomer S, Laughlin MH. Intermittent pneumatic leg compressions acutely upregulate VEGF and MCP-1 expression in skeletal muscle. Am J Physiol Heart Circ Physiol. 2010;298(6):H1991–2000. doi: 10.1152/ajpheart.00006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sumide T, Sakuraba K, Sawaki K, Ohmura H, Tamura Y. Effect of resistance exercise training combined with relatively low vascular occlusion. J Sci Med Sport. 2009;12(1):107–12. doi: 10.1016/j.jsams.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol. 2000;88(6):2097–106. doi: 10.1152/jappl.2000.88.6.2097. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol. 2005;98(5):1745–52. doi: 10.1152/japplphysiol.01185.2004. [DOI] [PubMed] [Google Scholar]