Abstract
Background
Although the DASH (Dietary Approaches to Stop Hypertension) diet has been shown to lower blood pressure (BP) in short-term feeding studies, it has not been shown to lower BP among free-living individuals, nor has it been shown to alter cardiovascular biomarkers of risk.
Objective
To compare the DASH diet alone or combined with a weight management program with usual diet controls among participants with prehypertension or stage 1 hypertension (systolic BP, 130–159 mm Hg; or diastolic BP, 85–99 mm Hg).
Design and Setting
Randomized, controlled trial in a tertiary care medical center with assessments at baseline and 4 months. Enrollment began October 29, 2003, and ended July 28, 2008.
Participants
Overweight or obese, unmedicated outpatients with high BP (N = 144).
Interventions
Usual diet controls, DASH diet alone, and DASH diet plus weight management.
Outcome Measures
The main outcome measure is BP measured in the clinic and by ambulatory BP monitoring. Secondary outcomes included pulse wave velocity, flow-mediated dilation of the brachial artery, baroreflex sensitivity, and left ventricular mass.
Results
Clinic-measured BP was reduced by 16.1/9.9 mm Hg (DASH plus weight management); 11.2/7.5 mm (DASH alone); and 3.4/3.8 mm (usual diet controls) (P < .001). A similar pattern was observed for ambulatory BP (P < .05). Greater improvement was noted for DASH plus weight management compared with DASH alone for pulse wave velocity, baroreflex sensitivity, and left ventricular mass (all P < .05).
Conclusion
For overweight or obese persons with above-normal BP, the addition of exercise and weight loss to the DASH diet resulted in even larger BP reductions, greater improvements in vascular and autonomic function, and reduced left ventricular mass. Key words: Hypertension, Exercise, DASH diet, Blood pressure, Left ventricular mass
INTRODUCTION
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7)1 recommends that lifestyle modifications should be the initial treatment strategy for lowering blood pressure (BP). In addition to advocating weight reduction, physical activity, dietary sodium reduction, and moderation of alcohol consumption, as recommended by earlier guidelines,2 JNC-7 endorses the DASH (Dietary Approaches to Stop Hypertension) diet for patients with elevated BP. Evidence supporting the efficacy of this diet comes primarily from the DASH feeding trials, in which a diet high in low-fat dairy products, fruits, and vegetables; lower in fats; and rich in fiber significantly lowered clinic-measured BP with or without sodium reduction.3–5 The PREMIER study6 subsequently demonstrated that the “established”(JNC-6) lifestyle modifications and lifestyle modifications plus the DASH diet (JNC-7) were associated with larger BP reductions compared with “advice only” controls; however, the BP differences between the JNC-7 recommendations and the previously established (JNC-6) treatment recommendations were small (<1 mm Hg) and not statistically significant. Therefore, although the DASH diet has been shown to lower BP in short-term feeding studies, it has not been shown to lower BP independent of other lifestyle changes among free-living individuals. Because the clinical significance of high BP is derived from morbid events that are not directly caused by elevated BP, but rather the associated structural and functional changes in the heart and blood vessels, we also examined changes in cardiovascular biomarkers of risk.
METHODS
Participants
The sample consisted of healthy, but overweight, men and women with above-normal BP. Persons were eligible for study inclusion if they were not taking antihypertensive medication and had a mean systolic BP (SBP) of 130 to 159 or diastolic BP (DBP) of 85 to 99 mm Hg based on 4 screening visits. Other inclusion criteria included age 35 years or older, body mass index of 25 to 40 (calculated as weight in kilograms divided by height in meters squared), being sedentary, having no other medical comorbidities that would preclude safe participation in the trial, and use of any medications known to affect the cardiovascular system.
Trial Overview
The ENCORE (Exercise and Nutrition interventions for CardiOvasculaR hEalth) study was approved by the Institutional Review Board at Duke University Medical Center. Written informed consent was obtained from all participants.
Enrollment began October 29, 2003, and ended July 28, 2008. Participants were recruited from physician referrals, community-based screenings, and mass media advertisements. Eligibility was established during a series of screening visits that included a medical history and physical examination, measurement of height and weight, and determination of baseline clinic-measured BP. Following completion of baseline assessments, participants were randomized to the DASH diet alone (DASH-A), the DASH diet combined with a behavioral weight management program (DASH-WM), or to the usual diet control (UC) group. At the end of the 4-month treatment period, assessments were repeated.
Assessments
Clinic-Measured BP
Clinic-measured BP was determined according to JNC-7 guidelines. Potential participants were asked to refrain from smoking or ingesting caffeine for at least 30 minutes before their appointment time. Measurements were standardized for cuff size, position, environment, and time of day. After 5 minutes of quiet rest, 4 seated BP readings, each 2 minutes apart, were obtained using a standard mercury sphygmomanometer and stethoscope. We also obtained simultaneous automated BP recordings using an Accutorr Plus BP monitor (Datascope, Mahwah, New Jersey)7 to provide an objective secondary approach to clinic BP measurement. This clinic BP measurement protocol was repeated on 4 screening sessions over a 3 to 4 week period.
Ambulatory BP Monitoring
To assess BP during a typical day, participants wore an Accutracker II (Suntech Medical Inc, Raleigh, North Carolina) ambulatory BP monitor.8 The Accutracker was programmed to record BP measurements 4 times per hour throughout the waking hours and 2 times per hour during sleep. The mean BP during the entire 24-hour monitoring period, adjusted for posture, was used for the primary analysis.
Pulse Wave Velocity
Pulse wave velocity (PWV), measured using the Complior device (Artech Medical, Pantin, France), was used as an index of central artery stiffness.9 Pulse pressure waveforms were recorded from the right carotid and right femoral arteries, and PWV (meters per second) was calculated from measurements of pulse transit time (in seconds) and the distance (in meters) traveled by the pulse between the 2 recording sites.
Flow-Mediated Dilation
Brachial artery flow-mediated dilation (FMD) was assessed following overnight fasting.10 Longitudinal B-mode ultrasonographic images of the brachial artery, 4- to 6-cm proximal to the antecubital crease, were obtained using an Aspen ultrasound platform with an 11-MHz linear array transducer (Acuson, Mountain View, California). Images were obtained after 10 minutes of supine relaxation and during reactive hyperemia, induced by inflating a forearm occlusion cuff to suprasystolic pressure (approximately 200 mm Hg) for 5 minutes. End-diastolic arterial diameters were measured as the distance between the proximal and distal arterial wall intima-media interfaces using PC-based software (Brachial Analyzer, version 4.0; Medical Imaging Applications LLC, Iowa City, Iowa). Flow-mediated dilation was defined as the maximum percentage change in arterial diameter relative to resting baseline from 10 to 120 seconds after deflation of the occlusion cuff.
Baroreflex Sensitivity
Beat-by-beat SBP and heart rate (HR) were collected using the Finapres noninvasive BP monitor (model 2300; Ohmeda, Madison, Wisconsin). Recordings of beat-by-beat SBP and R-R interval (derived as 60 000/HR) were edited for artifacts, linearly interpolated, and resampled at a frequency of 4 Hz to generate an equally spaced time series. A fast Fourier transform was then applied to the interpolated data after detrending and application of a Hanning filtering window. Baroreflex sensitivity (BRS) was estimated from the magnitude of the transfer function relating R-R interval oscillations to SBP oscillations across the 0.07 to 0.1299 Hz, or low-frequency, band. Coherence between SBP and R-R interval oscillations was required to be at least 0.5 for measurements to be accepted as estimates of BRS.
Left Ventricular Mass Index
Two-dimensional echocardiograms were acquired using an Aspen imaging system and stored in a digital format for subsequent quantification by a single observer (A.H.) who was blinded to treatment group. Left ventricular (LV) end-diastolic diameter, posterior wall thickness, and interventricular septal thickness were measured at end-diastole, using a leading edge–to–leading edge convention. Left ventricular mass was estimated using a cube function model with a correction factor.11 To adjust for variations in heart size owing to differences in body size, the LV mass index was calculated as LV mass divided by height2.7 as described by de Simone et al.12
Nutritional and Weight Assessment
An independent assessment of dietary and nutritional content was obtained by 2 separate self-report measures of diet: a retrospective food frequency questionnaire13 requiring participants to recall typical consumption during a 4-week period and a 4-day food diary. The food frequency questionnaire was analyzed by NutritionQuest (Berkeley, California), and the diary data were analyzed using Food Processor SQL Edition software, version 10.3 (ESHA Research, Salem, Oregon).14 In addition, sodium, calcium, and potassium intake were estimated from urinary excretion during a 24-hour period.15 Body weight was determined by a calibrated digital scale (Detecto; Cardinal Scale Manufacturing Co, Webb City, Missouri).
Cardiorespiratory Fitness
Participants underwent a maximal graded exercise treadmill test in which workloads were increased at a rate of 1 metabolic equivalent per minute.16 Expired air was collected by mouthpiece for quantification of minute ventilation, oxygen consumption, and carbon dioxide production with the Parvo Medics TrueOne measurement system (model 2400; Parvo Medics, Sandy, Utah).
Randomization
On completion of the baseline assessments, participants were randomized in groups of 2 to 5 participants using a computer program. The size of the group was determined by how many eligible participants were available to be randomized within 4 weeks of their baseline BP assessments. Participants were provided their treatment group assignments in sealed envelopes; staff members performing the assessments were unaware of the group assignments. Assignments were stratified by clinic-measured BP, body mass index, and age.
Interventions
Immediately following randomization, participants entered a 2-week controlled feeding period in which they ate according to the assigned dietary patterns (control diet, DASH diet, or a reduced-calorie DASH diet). For the UC and DASH-A arms, participants consumed study meals isocalorically so they would not gain or lose weight, whereas participants in the DASH-WM arm consumed meals at a 500-calories-per-day deficit to allow weight loss of about 1 pound a week. During the controlled feeding period, body weight was measured every other day to monitor participants’ weight stability or loss, allowing for adjustments in caloric intake. In addition, participants who were assigned to DASH-A or DASH-WM met with the nutritionist twice weekly for instruction about the DASH pattern. A 7-day menu cycle from the DASH-Sodium study for each dietary pattern at each of the 1600, 2100, 2600, 3100, and 3600 kcal energy levels was used as the basis for the recommended diets.17 We based caloric intake on the Harris Benedict formula, using screening weights and estimated physical activity levels derived from the Seven-Day Physical Activity Recall survey.18
The control diet contained 34% of calories from fat (the average level for Americans based on the National Health and Nutrition Examination Survey Phase III19 data), whereas potassium, magnesium, calcium, and fiber levels were set at approximately the 25th percentile for average American intakes. Protein accounted for 15% of calories in the control diet and 18% in the DASH diet. The DASH diet is reduced in total fat (27%), saturated fat (6%), and cholesterol and contains about 3 times as much dietary fiber, potassium, magnesium, and calcium as the control diet. Because severe sodium restriction made a relatively small difference for those on the DASH diet,5 we used the current national recommended level (2400 mg/d per 2000 kcal). Following the initial 2 weeks of controlled feeding, participants were instructed to maintain the DASH diet on their own with (DASH-WM) or without (DASH-A) weight loss.
DASH Diet Alone
Participants in the DASH-A condition only received instruction in modifying the content of their diet to meet DASH guidelines. Participants in this group were explicitly asked not to exercise or to attempt to lose weight and to focus their attention only on what they ate. Participants received counseling on the DASH diet and were provided feedback on their adherence to the diet in weekly 30- to 45-minute small group sessions led by the study nutritionist. The goal of the weekly sessions was to assist participants in learning how to buy and prepare the appropriate foods, to enhance their motivation to choose to eat those foods, and to overcome obstacles to following the diet. Participants also were weighed each week to monitor their weight and to make adjustments in the recommended servings so that their weight would remain stable during the intervention period.
DASH Diet Plus Weight Management
Participants in the DASH-WM condition received the same instruction in the DASH diet as the DASH-A group, but their weekly 30- to 45-minute small group sessions also included a weekly cognitive-behavioral weight loss intervention and they attended supervised exercise sessions 3 times per week.
Cognitive-Behavioral Weight Loss
This intervention was based on cognitive-behavioral strategies20 and included Appetite Awareness Training,21 a self-monitoring strategy in which individuals learn to identify internal cues of moderate hunger and fullness and to use these cues to guide their eating behavior. The DASH recommendations were used to provide guidance regarding what to eat, whereas cognitive-behavioral strategies were designed to help individuals learn when/how to eat.
Supervised Exercise
Participants had supervised exercise sessions 3 times per week at a level of 70% to 85% of their initial heart rate reserve determined at the time of the baseline treadmill test. The supervised exercise routine consisted of 10 minutes of warm-up exercises, 30 minutes of biking and/or walking or jogging, and 5 minutes of cool-down exercises.
Usual Diet Controls
Participants in the UC condition were asked to maintain their usual dietary and exercise habits for 4 months until they were reevaluated. On a biweekly basis, their weight and BP were monitored and their health habits were assessed to ensure that they had not joined any exercise or weight-loss program and had maintained their body weight.
Statistical Analysis
The effect of treatment on the primary and secondary outcomes was evaluated using the general linear model function in SAS statistical software, version 9.1 (SAS Institute, Cary, North Carolina). Separate models were estimated for each outcome. The predictors in each model were 2 indicator variables carrying the orthogonal contrasts (DASH-WM and DASH-A vs UC and DASH-WM vs DASH-A) and the corresponding pretreatment value of the outcome, age, sex, and ethnicity (white vs nonwhite). We also adjusted for posture in the analysis of ambulatory BP and for arterial diameter at rest in the FMD analysis. With respect to changes in aerobic capacity, exercise endurance, and weight-related variables, where DASH-WM was expected a priori to differ from the other 2 groups, we used 2 planned contrasts: DASH-WM vs DASH-A and DASH-WM vs UC. Data for all outcomes were analyzed following the intent-to-treat principle, with missing data managed using the multiple imputation method available in SAS PROC MI. We estimated that we would have about 80% power to detect a 0.5-SD difference between the active treatments and UC and a 0.6-SD difference between the 2 active treatments.
RESULTS
Participant Flow
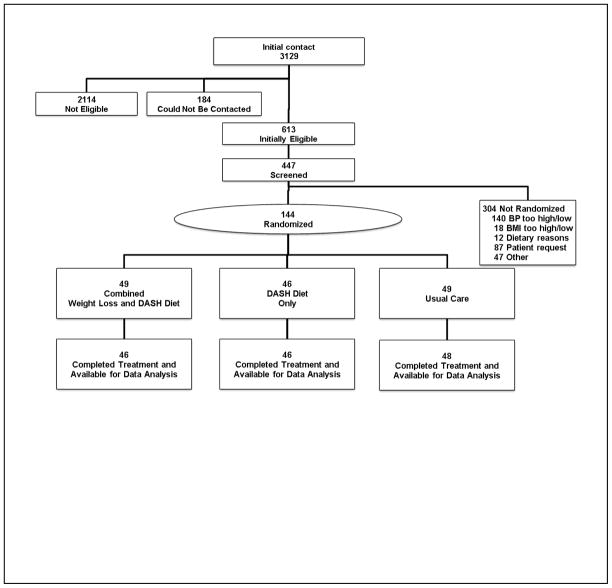
Of the 3129 participants who initially inquired about the study, 449 (32.1) met our initial inclusion criteria. After screening, 144 participants (32.2%) were randomized: 49 to the DASH-WM condition (34.0%); 46 to DASH-A (31.9%); and 49 to UC (34.0%) (Figure 1).
Figure 1.
Participant flow in the ENCORE (Exercise and Nutrition interventions for CardiOvasculaR hEalth) clinical trial. BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; DASH, Dietary Approaches to Stop Hypertension; and ITT, intent-to-treat.
Participant Characteristics
The mean age of the sample was 52 years; 38.9% of participants were African American, and 67.4% were women; the mean clinic-measured BP was 138/86 mm Hg. Most participants were college-educated and relatively affluent. The groups were generally comparable across the background variables (Table 1).
Table 1.
Background Characteristics of Sample
| N | Weight Loss + DASH N=49 | DASH Alone N = 46 | Control N = 49 | All N = 144 | |
|---|---|---|---|---|---|
| Age (years), m (SD) | 144 | 52.3 (10) | 51.8 (10) | 51.8 (9) | 52.0 (10) |
| Gender: Female | 144 | 69% (34) | 63% (29) | 69% (34) | 67% (97) |
| Ethnicity | 144 | ||||
| Caucasian | 69% (34) | 50% (23) | 59% (29) | 60% (56) | |
| African American | 31% (15) | 48% (22) | 39% (19) | 39% (86) | |
| Asian | 0% (0) | 2% (1) | 2% (1) | 1% (2) | |
| Hispanic | 144 | 4% (2) | 6% (3) | 3% (5) | |
| Level of Education | 143 | ||||
| High School | 31% (15) | 30% (14) | 42% (20) | 34% (49) | |
| Some College | 8% (4) | 9% (4) | 14% (7) | 11% (15) | |
| Completed College | 29% (14) | 30% (14) | 18% (9) | 22% (32) | |
| Post-Graduate School | 20% (10) | 28% (13) | 20% (10) | 24% (34) | |
| Other | 12% (6) | 13% (6) | 2% (1) | 9% (13) | |
| Annual Household Income | 123 | ||||
| < $20K | 10% (5) | 13% (6) | 20% (10) | 15% (21) | |
| $20–50K | 14% (7) | 4% (2) | 8% (4) | 11% (13) | |
| $50–99K | 6% (3) | 13% (6) | 18% (9) | 15% (18) | |
| > $100K | 55% (27) | 50% (23) | 43% (21) | 49% (71) | |
| Weight (kg), m (SD) | 144 | 93.9 (14) | 93.0 (14) | 92.6 (15) | 93.1 (14.1) |
| BMI (kg/m2), m (SD) | 144 | 33.5 (4.4) | 32.8 (3.4) | 33.0 (3.9) | 33.1 (3.9) |
| Clinic SBP (mmHg), m (SD) | 144 | 138.7 (8.2) | 137.6 (9.0) | 138.0 (9.5) | 138.1 (8.8) |
| Clinic DBP (mmHg), m (SD) | 144 | 85.5 (6.8) | 86.1 (6.1) | 85.6 (5.8) | 85.8 (6.2) |
| Ambulatory SBP (mmHg), m (SD) | 138 | 134.3 (12.0) | 136.7 (12.5) | 139.6 (11.6) | 136.9 (12.1) |
| Ambulatory DBP (mmHg), m (SD) | 138 | 79.8 (8.2) | 81.6 (8.8) | 82.7 (7.9) | 81.3 (8.3) |
| Diabetes | 144 | 2% (1) | 0% (0) | 0% (0) | < 1% (1) |
| Hyperlipidemia | 144 | 33% (16) | 22% (10) | 33% (16) | 29% (42) |
| Arthritis | 144 | 29% (14) | 13% (6) | 22% (11) | 22% (31) |
Attendance at Diet and Exercise Sessions
Attendance at the exercise and diet classes was excellent. Of the 42 expected exercise sessions, the median number attended was 38 sessions (90%). The median percentage time spent in the target HR range during exercise was 94%. The DASH class attendance also was excellent; for both intervention groups, the median number of sessions attended was 12 (92%).
Changes in Body Weight
Adjusting for initial weight, age, sex, and ethnicity, the mean posttreatment weight for the DASH-WM group was significantly lower (84.5 kg) compared with the DASH-A (92.9 kg; P <.001) and UC (94.1 kg; P <.001) groups. The weight change was −8.7 kg in the DASH-WM group, −0.3 kg in the DASH-A group, and 0.9 kg in the UC group.
Changes in Dietary Intake and Urinary Excretion
Participants in the 2 DASH treatment conditions showed excellent adherence to the DASH guidelines compared with those randomized to UC (Table 2). Participants in the DASH-WM condition also consumed significantly fewer total calories, less total protein, and fewer carbohydrates compared with those in DASH-A.
Table 2.
Daily dietary intake after treatment, adjusted for age, gender, ethnicity, and pretreatment level of outcome
| Weight Loss + DASH | DASH Alone | Usual Care | Active Treatments vs. Usual Care | Weight Loss + DASH vs. DASH Alone | |
|---|---|---|---|---|---|
| Calories, kcal | 1648 1521–1774 |
1962 1833–2090 |
2095 1961–2228 |
< .001 | < .001 |
| Protein, g | 80 74–86 |
95 89–100 |
87 81–93 |
.776 | < .001 |
| % Protein Calories | 19.5 18.7–20.4 |
19.4 18.6–20.3 |
16.7 15.9–17.6 |
< .001 | .797 |
| Carbohydrates, g | 232 214–250 |
266 247–284 |
241 222–259 |
.475 | .011 |
| % Carb. Calories | 56.0 54.4–58.2 |
53.8 51.9–55.7 |
46.4 44.5–48.4 |
< .001 | .071 |
| Fat, g | 49 42–56 |
60 53–67 |
87 79–94 |
< .001 | .028 |
| % Fat Calories | 26.3 24.7–27.9 |
27.8 26.1–29.4 |
36.8 35.1–38.6 |
< .001 | .215 |
| Saturated Fat, g | 14 12–17 |
18 15–20 |
29 26–31 |
< .001 | .058 |
| Dietary Cholesterol, mg | 198 162–234 |
256 220–293 |
346 309–384 |
< .001 | .023 |
| Fiber, g | 25 22–27 |
26 24–29 |
16 14–19 |
< .001 | .349 |
| Sodium, mg | 1990 1772–2208 |
2044 1825–2262 |
2922 2698–3146 |
< .001 | .734 |
| Potassium, mg | 3476 3217–3735 |
4047 3785–4309 |
2637 2370–2903 |
< .001 | .003 |
| Magnesium, mg | 363 333–394 |
432 393–454 |
282 251–313 |
< .001 | .008 |
| Calcium, Mg | 1023 914–1132 |
1175 1065–1284 |
861 750–972 |
< .001 | .058 |
| Phosphorous, mg | 1390 1284–1491 |
1632 1529–1735 |
1361 1254–1467 |
.023 | < .001 |
Compared with UC, DASH-WM resulted in significantly lower urinary sodium levels and higher urinary potassium excretion (P < .01) (Table 3). The DASH-WM and DASH-A participants did not differ on any of the urinary excretion measures.
Table 3.
Urinary excretion after treatment, adjusted for age, gender, ethnicity, and pretreatment level of outcome
| Weight Loss + DASH | DASH | Usual Care | Active Treatments vs. Usual Care | Weight Loss + DASH vs. DASH Alone | |
|---|---|---|---|---|---|
| Sodium (mmol/day) | 113.7 97.1–130.4 |
125 108.4–141.5 |
145 129.3–161.3 |
.010 | .355 |
| Potassium (mmol/day) | 71.1 63.5–78.6 |
74.9 67.3–82.5 |
52.9 45.6–60.3 |
.001 | .488 |
| Phosphorous (mg/day) | 783 698–868 |
860 775–945 |
813 731–895 |
.866 | .087 |
| Calcium (mg/day) | 138 116–161 |
167 144–189 |
155 133–177 |
.868 | .216 |
| Creatinine (g/day) | 1.3 1.2–1.4 |
1.3 1.2–1.4 |
1.3 1.2–1.5 |
.599 | .987 |
| Urea nitrogen (g/day) | 9.2 8.3–10.0 |
10.2 9.3–10.1 |
10.1 8.3–9.9 |
.270 | .095 |
Changes in Aerobic Fitness
The DASH-WM group exhibited greater improvements in peak V O2 (oxygen consumption) and exercise endurance compared with the DASH-A and UC groups. Adjusting for pretreatment levels, age, sex, and ethnicity, the mean posttreatment peak V O2 was higher among the DASH-WM participants (29 mL/kg/min) compared with those randomized to the DASH-A (23 mL/kg/min; P <.001) or UC (22 mL/kg/min; P <.001) condition. Participants in the DASH-WM group showed a 19% increase in peak V O2 compared with negligible changes for participants in the DASH-A (−1.2%) and UC (−3.2%) conditions.
Primary End Points
Blood Pressure
Compared with UC, the active treatments significantly lowered SBP (P < .001) and DBP (P < .001) (Figure 2). In addition, compared with DASH-A, the DASH-WM intervention resulted in significantly lower SBP (P = .02) and DBP (P < .048). Expressed as adjusted change from pretreatment to posttreatment, the reduction in SBP was 16.1 (95% confidence interval, 13.0–19.2) mm Hg in the DASH-WM group, 11.2 (8.1–14.3) mm Hg in the DASH-A group, and 3.4 (0.4–6.4) mm Hg in the UC group; the reduction in DBP was 9.9 (8.1–11.6) mm Hg in the DASH-WM group, 7.5 (5.8–9.3) mm Hg in the DASH-A group, and 3.8 (2.2–5.5) mm Hg in the UC group. The contrast between active treatments (DASH-A and DASH-WM) and UC also was significant for machine-read SBP (P < .001) and DBP (P < .001); in addition, the machine-read BP values were lower for the DASH-WM group compared with the DASH-A group for SBP (P = .01) and DBP (P = .06). Moreover, at the end of treatment, 19 (38.8%) UC group participants were classified as hypertensive (clinic BP >140/90 mm Hg) compared with only 6 (12.2%) in the DASH-WM and 7 (15.2%) in the DASH-A groups.
Figure 2.
Comparison of posttreatment means and 95% confidence intervals for clinic-measured blood pressure (BP) using an intent-to-treat model, adjusted for age, sex, ethnicity, and pretreatment BP. The contrasts between all active treatment groups and the usual diet control (UC) group were significant for both systolic (A) and diastolic (B) BP (P < .001), as were the contrasts between DASH-WM (Dietary Approaches to Stop Hypertension plus weight management) vs DASH-A (DASH alone) for systolic BP (P = .02) and diastolic BP (P = .048). The right panels display the pairwise differences (mean difference and 95% confidence interval) between the treatment groups calculated from the adjusted posttreatment means.
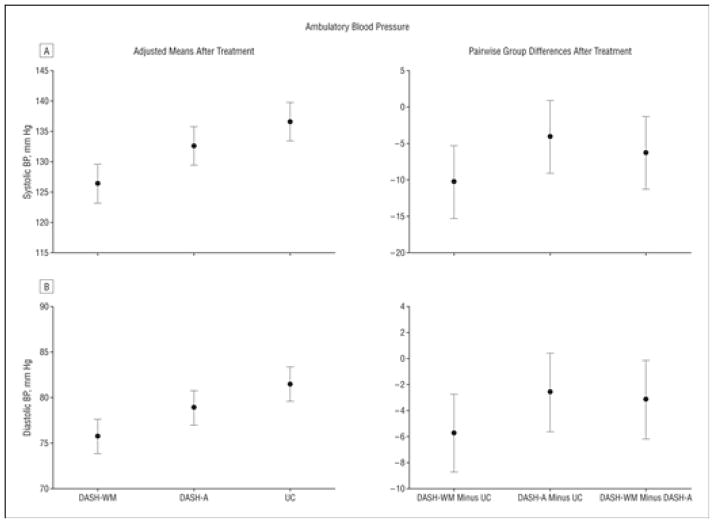
Eighteen participants had missing or inadequate ambulatory BP readings either at baseline or posttreatment. These missing assessments were imputed using a multiple imputation model. Compared with the UC group, participants in the active treatment groups had significantly lower ambulatory SBP and DBP (P < .001) (Figure 3). Ambulatory BPs were lower in the DASH-WM group compared with the DASH-A group for SBP (P = .01) and DBP (P = .03). Expressed as adjusted change from pretreatment to posttreatment, the reductions in ambulatory BP were: DASH-WM group, 10.2 (95% confidence interval, 6.8 to 13.6)/5.4 (3.4 to 7.4) mm Hg; DASH-A group, 5.3 (2.0 to 8.6)/2.9 (1.0 to 4.9) mm Hg; and UC group, 0.2 (3.4 to 7.4)/0.003 (−1.8 to 1.9) mm Hg.
Figure 3.
Comparison of posttreatment means and 95% confidence intervals for 24-hour ambulatory blood pressure (BP) using an intent-to-treat model, adjusted for age, sex, ethnicity, percentage of time in sitting or standing position, and pretreatment BP. The contrast between all active treatment groups and the usual diet control (UC) group was significant for systolic (A) and diastolic (B) BP (P < .001), as were the contrasts between DASH-WM (Dietary Approaches to Stop Hypertension plus weight management) vs DASH-A (DASH alone) for systolic BP (P = .01) and diastolic BP (P = .03). The right panels display the pairwise differences (mean difference and 95% confidence intervals) between the treatment groups calculated from the adjusted posttreatment means.
Cardiovascular Biomarkers
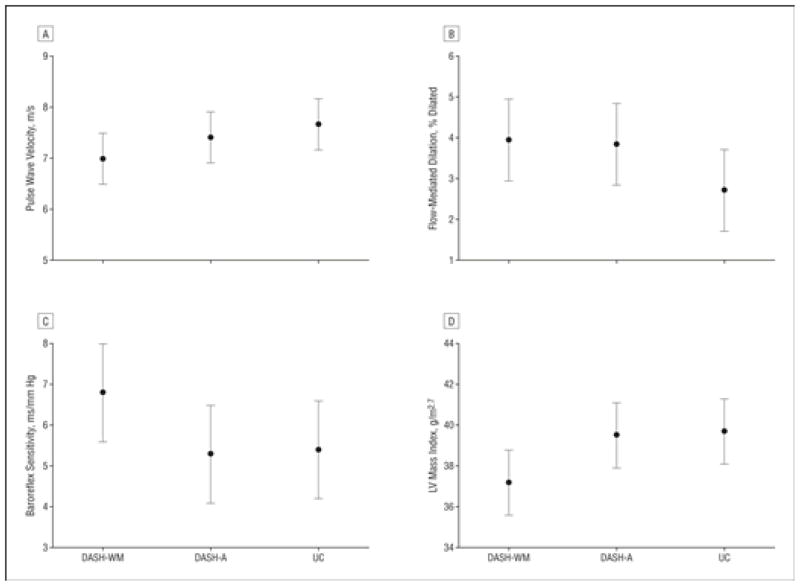
The 2 DASH diet interventions resulted in lower PWV compared with the UC group (P = .001), and PWV was lower in the DASH-WM group compared with the DASH-A group (P = .045) (Figure 4). The 2 DASH treatment groups also tended to exhibit larger improvements in FMD than the UC group (P = .06), but they did not differ from one another (P = .99). For BRS, posttreatment results in the active treatment groups were not different than those in the UC group (P = .38); however, greater improvements were seen in DASH-WM compared with DASH-A (P = .01). The active treatment groups had lower posttreatment values for LV mass index compared with the UC group, but this difference was not significant (P = .26); however, the DASH-WM intervention resulted in lower LV mass than did DASH-A (P = .02).
Figure 4.
Comparison of posttreatment mean (95% confidence interval) values for pulse wave velocity (A), flow-mediated dilation (B), baroreflex sensitivity (C), and left ventricular (LV) mass index (D) by treatment group, adjusted for age, sex, ethnicity, and pretreatment level of response variable. Flow-mediated dilation of the brachial artery also was adjusted for pretreatment arterial diameter at rest. Results of contrasts were as follows: for pulse wave velocity (A), all treatments vs usual diet controls (UC), P = .002, and DASH-WM (Dietary Approaches to Stop Hypertension plus weight management) vs DASH-A (DASH alone), P = .045; for flow-mediated dilation (B), all treatments vs UC, P = .06, and DASH-WM vs DASH-A, P = .99; for baroreflex sensitivity (C), all treatments vs UC, P = .38, and DASH-WM vs DASH-A, P = .01; and for left ventricular mass index (D), all treatments vs UC, P = .26, and DASH-WM vs DASH-A, P = .02.
DISCUSSION
Results of this randomized controlled trial demonstrate that the DASH diet produces significant reductions in BP compared with a typical American diet among unmedicated, overweight or obese men and women with high BP and that weight loss and exercise combined with the DASH diet produce additional BP lowering. Compared with UC, we observed a 12.5/5.9 mm Hg net benefit in clinic-measured BP with the DASH-WM program consisting of aerobic exercise, caloric restriction, and cognitive-behavioral intervention and a 7.7/3.6 mm Hg net benefit with DASH-A. These findings confirm the value of the DASH diet in reducing BP and provide evidence for the significant “added value” associated with exercise and weight loss in the context of the DASH diet.
The efficacy of the DASH diet initially was established on the basis of several controlled feeding trials designed to examine the effects of dietary patterns on BP among unmedicated persons with higher-than-optimal DBP or with stage 1 hypertension; as a result of these studies, the DASH diet was adopted as part of current national recommendations for the prevention and treatment of high BP.1 The subsequent PREMIER study6 demonstrated the feasibility of implementing the DASH diet in daily life, but the small and nonsignificant BP differences between the DASH diet and the “established” intervention (which also involved some dietary changes) raised doubts about the added value of the DASH diet in optimizing BP. Because participants in the DASH plus “established” intervention lost more weight than the “established” intervention alone, the effects of the DASH diet could not be determined. The ENCORE trial has now extended the PREMIER study by not only examining the extent to which lifestyle modifications can be adopted in the home environment but also by manipulating the DASH diet intervention and weight loss independently. Our results confirm the findings of the earlier DASH feeding studies: participants who ate the DASH diet achieved significant BP reductions.3–5 However, adding exercise and weight loss led to an even greater decrease in BP.
The BP reductions achieved in our DASH-A and DASH-WM interventions were greater than those described in the PREMIER study and in other trials of lifestyle modification.22–24 The reasons for the greater benefit from the current ENCORE intervention could be attributed to the greater weight loss and excellent adherence to the DASH diet and exercise sessions. The 12/6 mm Hg relative reduction in BP that we observed among participants randomized to DASH-WM is equivalent to the BP lowering that physicians could expect from a high dose of an antihypertensive drug.25 Similar BP reductions have been achieved in placebo-controlled treatment trials and have resulted in a lowering of stroke risk by approximately 40% and a reduction in ischemic heart disease events by about 25%.26
In addition to BP lowering, we demonstrated improvements in important cardiovascular biomarkers. One of the structural consequences of high BP, left ventricular hypertrophy (LVH), is the strongest known predictor, other than advancing age, of cardiovascular morbidity and mortality. Increased LV mass predicts these clinical outcomes in hypertensive27 and healthy individuals,28 independent of other conventional risk factors. Drug therapy that results in LVH regression29 is associated with improved cardiovascular outcomes. For example, Verdecchia et al30 found a lower risk of cardiovascular events in hypertensive participants who had a decrease in LV mass during treatment, independent of baseline BP or the degree of BP reduction. Similarly, in a substudy of the Losartan Intervention of Endpoint Reduction in Hypertension (LIFE) trial, lowered LV mass was associated with decreased rates of cardiovascular events.31
Arterial stiffness also has been shown to be a strong independent predictor of cardiovascular morbidity and mortality.32–35 The DASH-A and the DASH-WM interventions resulted in greater reductions in PWV than did UC, with more pronounced reductions among the DASH-WM participants. Dietary sodium intake was reduced by approximately 30% compared with UC, and participants in the DASH-WM group achieved a 19% improvement in aerobic capacity, which may have augmented the benefits of the DASH diet and weight loss on arterial stiffness. The observed reductions in PWV may be a result of the direct impact of diet and exercise as well as the lower BP resulting from these lifestyle changes. A reduction in arterial stiffness may also contribute to regression of LVH. The Ohasama study showed that arterial stiffness measured by PWV was related to LVH, independent of age and BP, in a population of 798 older adults.36 Ongoing trials should help clarify whether reducing arterial stiffness contributes to a lowered risk for cardiovascular events.37
Impairment of the sensitivity of the baroreflex system is an early consequence of hypertension38– 41 and likely reflects reduced viscoelastic properties of the vascular wall housing the baroafferent stretch receptors owing to arterial stiffness and atherosclerosis.42– 44 The DASH-A intervention did not alter BRS, but DASH-WM improved BRS by 33%. The improvements in BRS may result from reduced vascular stiffness45– 47 or improved parasympathetic cardiac control through improved insulin sensitivity and glucose metabolism secondary to exercise and weight loss.48
The present study is limited by its relatively small sample of highly motivated participants along with a labor-intensive treatment program that may be difficult to fully implement in clinical practice. The ENCORE study was not powered to detect differences in “hard” clinical end points, such as stroke, myocardial infarction, and death. Trials of pharmacologic therapy, however, demonstrate that BP lowering reduces the risk of cardiovascular events and that the magnitude of BP reduction and reversal of cardiovascular structural changes associated with hypertension are key determinants of the effectiveness of therapy.49 Ultimately, the effects of the DASH diet and weight management will need to be evaluated prospectively in a larger sample of participants; longer-term follow-up of ENCORE study participants is currently ongoing. The present findings suggest that the DASH diet, particularly when augmented by exercise and weight loss, can offer considerable benefit to patients with high BP, not only through reductions in BP but through favorable modification of biomarkers of disease risk.
Acknowledgments
This study was supported by grant HL074103 from the National Heart, Lung, and Blood Institute; grant M01-RR-30 from the General Clinical Research Center; grant 5UL1RR024128-03 from the National Center for Research Resources; and the National Institutes of Health Roadmap for Medical Research.
The contents of this study are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or the National Institutes of Health.
The following ENCORE research staff members assisted with this trial: Lara LaCaille, PhD; Krista Barbour, PhD; Teresa Edenfield, PhD; Emily York-Crowe, PhD; Deborah Sebring, PhD; Marquisha Green, MA; Jennifer Jawanda, MA; Simon Bacon, PhD; Elizabeth Gullette, PhD; Steve Taxman, PA-C; Mark Feinglos, MD; Eugene Oddone, MD; Emily Ballard, BS; Beth Polisson, BA; Brian Beckman, BA; Katie Earnhardt, BA; Catherine Wu, BA; Kylie Stott, MA; Emily Hill, MA; Laura Jost, BA; Elizabeth Brenner, BS; Mark Taylor, MA; Jessica Tucker, MA; Sandra Kennedy, MA; Brad Gregory, MA; Jessica Hawkins, MA; Michael Ellis, BA; Susan Rohn, MA; and Elise Pangborn, MA. The data and safety monitoring board members are Mark Appelbaum, PhD; David Sheps, MD; and David Krantz, PhD. We thank the participants in this trial for their commitment to the trial and for their contribution to this research.
References
- 1.Joint National Committee on Prevention, Evaluation, and Treatment of High Blood Pressure. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: US Dept of Health and Human Services; 2004. NIH publication 04-5230. [Google Scholar]
- 2.The Sixth Report of the Joint National Committee on Prevention. Detection, Evaluation, and Treatment of High Blood Pressure [published correction appears in Arch Intern Med. 1998;158(6):573] Arch Intern Med. 1997;157(21):2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 3.Appel LJ, Moore TJ, Obarzanek E, et al. DASH Collaborative Research Group, A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 4.Harsha DW, Lin PH, Obarzanek E, Karanja NM, Moore TJ, Caballero B. DASH Collaborative Research Group, Dietary Approaches to Stop Hypertension: a summary of study results. J Am Diet Assoc. 1999;99(8 suppl):S35–S39. doi: 10.1016/s0002-8223(99)00414-9. [DOI] [PubMed] [Google Scholar]
- 5.Sacks FM, Svetkey LP, Vollmer WM, et al. DASH-Sodium Collaborative Research Group, Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 6.Appel LJ, Champagne CM, Harsha DW, et al. Writing Group of the PREMIER Collaborative Research Group, Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289(16):2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 7.Anwar YA, Tendler BE, McCabe EJ, Mansoor GA, White WB. Evaluation of the Datascope Accutorr Plus according to the Association for the Advancement of Medical Instrumentation. Blood Press Monit. 1997;2(2):105–110. [PubMed] [Google Scholar]
- 8.White WB, Lund-Johansen P, McCabe EJ, Omvik P. Clinical evaluation of the Accutracker II ambulatory blood pressure monitor: assessment of performance in two countries and comparison with sphygmomanometry and intra-arterial blood pressure at rest and during exercise. J Hypertens. 1989;7(12):967–975. doi: 10.1097/00004872-198912000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Asmar R, Topouchian J, Pannier B, Benetos A, Safar M. Scientific, Quality Control, Coordination and Investigation Committees of the Complior Study, Pulse wave velocity as endpoint in large-scale intervention trial. J Hypertens. 2001;19(4):813–818. doi: 10.1097/00004872-200104000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 11.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57(6):450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 12.de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20(5):1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 13.Eck LH, Klesges RC, Hanson CL, Slawson D, Portis L, Lavasque ME. Measuring short-term dietary intake: development and testing of a 1-week food frequency questionnaire. J Am Diet Assoc. 1991;91(8):940–945. [PubMed] [Google Scholar]
- 14.Bazzano LA, He J, Ogden LG, et al. Agreement on nutrient intake between the databases of the First National Health and Nutrition Examination Survey and the ESHA Food Processor. Am J Epidemiol. 2002;156(1):78–85. doi: 10.1093/aje/kwf003. [DOI] [PubMed] [Google Scholar]
- 15.Luft FC, Sloan RS, Lang CL, et al. Influence of home monitoring on compliance with a reduced sodium intake diet. Arch Intern Med. 1984;144(10):1963–1965. [PubMed] [Google Scholar]
- 16.Blumenthal JA, Rejeski WJ, Walsh-Riddle M, et al. Comparison of high- and low-intensity exercise training early after acute myocardial infarction. Am J Cardiol. 1988;61(1):26–30. doi: 10.1016/0002-9149(88)91298-2. [DOI] [PubMed] [Google Scholar]
- 17.Karanja NM, Obarzanek E, Lin PH, et al. DASH Collaborative Research Group, Descriptive characteristics of the dietary patterns used in the Dietary Approaches to Stop Hypertension trial. J Am Diet Assoc. 1999;99(8 suppl):S19–S27. doi: 10.1016/s0002-8223(99)00412-5. [DOI] [PubMed] [Google Scholar]
- 18.Blair SN, Haskell WL, Ho P, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122(5):794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 19.McDowell MA, Briefel RR, Alaimo K, et al. Energy and macronutrient intakes of persons ages 2 months and over in the United States: Third National Health and Nutrition Examination Survey, Phase 1, 1988–91. Adv Data. 1994;(255):1–24. [PubMed] [Google Scholar]
- 20.Fairburn CG. Cognitive-Behavioral Treatment of Obesity: a Clinician’s Guide. New York, NY: Guilford Press; 2003. [Google Scholar]
- 21.Allen HN, Craighead LW. Appetite monitoring in the treatment of binge eating disorder. Behav Ther. 1999;30:253–272. [Google Scholar]
- 22.Whelton PK, Appel LJ, Espeland MA, et al. TONE Collaborative Research Group, Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly. JAMA. 1998;279(11):839–846. doi: 10.1001/jama.279.11.839. [DOI] [PubMed] [Google Scholar]
- 23.Burke V, Beilin LJ, Cutt HE, Mansour J, Wilson A, Mori TA. Effects of a lifestyle programme on ambulatory blood pressure and drug dosage in treated hypertensive patients: a randomized controlled trial. J Hypertens. 2005;23(6):1241–1249. doi: 10.1097/01.hjh.0000170388.61579.4f. [DOI] [PubMed] [Google Scholar]
- 24.Kuller LH, Kinzel LS, Pettee KK, et al. Lifestyle intervention and coronary heart disease risk factor changes over 18 months in postmenopausal women: the Women On the Move through Activity and Nutrition (WOMAN study) clinical trial. J Womens Health (Larchmt) 2006;15(8):962–974. doi: 10.1089/jwh.2006.15.962. [DOI] [PubMed] [Google Scholar]
- 25.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326(7404):1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins R, Peto R. Antihypertensive drug therapy: effects on stroke and coronary heart disease. In: Swales JD, editor. Textbook of Hypertension. Hoboken, NJ: Blackwell Scientific Publications; 1994. pp. 1156–1164. [Google Scholar]
- 27.Casale PN, Devereux RB, Milner M, et al. Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann Intern Med. 1986;105(2):173–178. doi: 10.7326/0003-4819-105-2-173. [DOI] [PubMed] [Google Scholar]
- 28.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 29.Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med. 2003;115(1):41–46. doi: 10.1016/s0002-9343(03)00158-x. [DOI] [PubMed] [Google Scholar]
- 30.Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation. 1998;97(1):48–54. doi: 10.1161/01.cir.97.1.48. [DOI] [PubMed] [Google Scholar]
- 31.Devereux RB, Wachtell K, Gerdts E, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292(19):2350–2356. doi: 10.1001/jama.292.19.2350. [DOI] [PubMed] [Google Scholar]
- 32.Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39(1):10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 33.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 34.Laurent S, Katsahian S, Fassot C, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34(5):1203–1206. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 35.Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Health ABC Study, Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111(25):3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 36.Watabe D, Hashimoto J, Hatanaka R, et al. Electrocardiographic left ventricular hypertrophy and arterial stiffness: the Ohasama study. Am J Hypertens. 2006;19(12):1199–1205. doi: 10.1016/j.amjhyper.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Topouchian J, El Feghali R, Pannier B, et al. Arterial stiffness and pharmacological interventions: the TRanscend Arterial stiffNess Substudy (TRANS study) Vasc Health Risk Manag. 2007;3(4):381–387. [PMC free article] [PubMed] [Google Scholar]
- 38.Randall OS, Esler MD, Bulloch EG, et al. Relationship of age and blood pressure to baroreflex sensitivity and arterial compliance in man. Clin Sci Mol Med Suppl. 1976:3357s–360s. doi: 10.1042/cs051357s. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein DS. Arterial baroreflex sensitivity, plasma catecholamines, and pressor responsiveness in essential hypertension. Circulation. 1983;68(2):234–240. doi: 10.1161/01.cir.68.2.234. [DOI] [PubMed] [Google Scholar]
- 40.Bristow JD, Gribbin B, Honour AJ, Pickering TG, Sleight P. Diminished baroreflex sensitivity in high blood pressure and ageing man. J Physiol. 1969;202(1):45P–46P. [PubMed] [Google Scholar]
- 41.Takeshita A, Tanaka S, Kuroiwa A, Nakamura M. Reduced baroreceptor sensitivity in borderline hypertension. Circulation. 1975;51(4):738–742. doi: 10.1161/01.cir.51.4.738. [DOI] [PubMed] [Google Scholar]
- 42.Katsube Y, Saro H, Naka M, et al. Decreased baroreflex sensitivity in patients with stable coronary artery disease is correlated with the severity of coronary narrowing. Am J Cardiol. 1996;78(9):1007–1010. doi: 10.1016/s0002-9149(96)00525-5. [DOI] [PubMed] [Google Scholar]
- 43.Monahan KD, Tanaka H, Dinenno FA, Seals DR. Central arterial compliance is associated with age- and habitual exercise–related differences in cardiovagal baroreflex sensitivity. Circulation. 2001;104(14):1627–1632. doi: 10.1161/hc3901.096670. [DOI] [PubMed] [Google Scholar]
- 44.Hunt BE, Farquhar WB, Taylor JA. Does reduced vascular stiffening fully explain preserved cardiovagal baroreflex function in older, physically active men? Circulation. 2001;103(20):2424–2427. doi: 10.1161/01.cir.103.20.2424. [DOI] [PubMed] [Google Scholar]
- 45.Grassi G, Seravalle G, Calhoun DA, Mancia G. Physical training and baroreceptor control of sympathetic nerve activity in humans. Hypertension. 1994;23(3):294–301. doi: 10.1161/01.hyp.23.3.294. [DOI] [PubMed] [Google Scholar]
- 46.Jingu S, Takeshita A, Imaizumi T, Nakamura M, Shindo M, Tanaka H. Exercise training augments cardiopulmonary baroreflex control of forearm vascular resistance in middle-aged subjects. Jpn Circ J. 1988;52(2):162–168. doi: 10.1253/jcj.52.162. [DOI] [PubMed] [Google Scholar]
- 47.Pagani M, Somers V, Furlan R, et al. Changes in autonomic regulation induced by physical training in mild hypertension. Hypertension. 1988;12(6):600–610. doi: 10.1161/01.hyp.12.6.600. [DOI] [PubMed] [Google Scholar]
- 48.Arone LJ, Mackintosh R, Rosenbaum M, Leibel RL, Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol. 1995;269(1 pt 2):R222–R225. doi: 10.1152/ajpregu.1995.269.1.R222. [DOI] [PubMed] [Google Scholar]
- 49.Turnbull F, Neal B, Algert C, et al. Blood Pressure Lowering Treatment Trialists’ Collaboration, Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med. 2005;165(12):1410–1419. doi: 10.1001/archinte.165.12.1410. [DOI] [PubMed] [Google Scholar]