Abstract
Background
TH17 responses have recently been implicated to play a role in allergic airway diseases, but their local expression in the setting of allergic rhinitis (AR) and their regulation in allergic airway diseases remain unclear.
Objective
We sought to investigate the regulatory role of Clara cell 10-kDa protein (CC10), an endogenous regulator of airway inflammation, on TH17 responses in the setting of AR.
Methods
Wild-type and homozygous CC10-null mice were used to establish an ovalbumin (OVA)–induced AR model. Human recombinant CC10 was given during sensitization or challenge. TH17 responses in human subjects and mice were examined by using flow cytometry, quantitative RT-PCR assay, immunohistochemistry, and ELISA. The direct effect of CC10 on TH17 cells and CD11c+ dendritic cells (DCs) was studied by means of cell culture. Adoptive transfer was used to examine the influence of CC10-conditioned DCs on airway inflammation. The regulatory effect of CC10 on the expression of the CCL20 gene was tested by using the BEAS-2B cell line.
Results
Compared with those of control subjects, TH17 responses were enhanced in the nasal mucosa of patients with AR. CC10-null mice with AR showed enhanced TH17 responses, and CC10 treatment significantly decreased TH17 responses. CC10 had no direct effect on in vitro TH17 cell differentiation. CC10 could significantly decrease the expression of OX40 ligand, IL-23, and IL-6 but enhance CD86 and TGF-β expression in DCs. Importantly, CC10 was able to inhibit TH17 cell polarization in the presence of OVA-pulsed DCs. CC10 pretreatment inhibited TH17 responses elicited by adoptive transfer of OVA-pulsed DCs. Furthermore, CC10 decreased the expression of CCL20 in BEAS-2B cells induced by inflammatory cytokines.
Conclusion
TH17 responses are enhanced in patients with AR, and CC10 inhibits TH17 responses through modulation of the function of DCs.
Keywords: Allergic rhinitis, Clara cell 10-kDa protein, dendritic cell, inhibition, TH17 response
Allergic rhinitis (AR) is a chronic inflammatory upper airway disease that is characterized by intense eosinophil infiltration, mucus hypersecretion, and airway remodeling, which are orchestrated mainly by antigen-specific TH2 cells and their cytokines, such as IL-4, IL-5, and IL-13.1,2 Recent evidence suggests that IL-17A and TH17 cells might be also involved in AR and other TH2-mediated allergic airway diseases.3-5 Compared with wild-type mice, IL-17 receptor–deficient mice exhibit a reduced level of neutrophil and eosinophil recruitment into the airways, and IL-17A–null mice demonstrate reduced TH2 responses to antigen sensitization.3,4 TH17 cells cooperate to enhance TH2 cell–mediated eosinophil recruitment into the airways.5 In human subjects increased IL-17 levels, TH17 responses, or both have been found to be associated with chronic rhinosinusitis, allergic asthma, and AR.6-8
In different organs and under different pathophysiologic conditions, TH17 responses can be regulated by distinct mechanisms. Several molecules, such as platelet-activating factor, retinoic acid, osteopontin, thymic stromal lymphopoietin, and prostaglandin E2, have been indicated to modulate the differentiation, function, or chemotaxis of TH17 cells in patients with autoimmune diseases, dermatitis, cancers, or asthma.8-10 Despite this progress, the regulation of TH17 responses in patients with airway diseases, including AR, remains poorly understood. An improved understanding of the cellular and molecular mechanisms regulating TH2 and TH17 responses might lead to novel therapeutic strategies for the prevention, control, or both of allergic airway diseases. Clara cell 10-kDa protein (CC10) is an anti-inflammatory and immunomodulatory protein secreted by the epithelial lining of the lung and nose.11-15 Our previous studies have shown that CC10 expression is downregulated in patients with allergic airway diseases, including AR and asthma, and that CC10 suppresses TH2 responses in these patients.11,13,15-18 However, the regulatory mechanisms underlying the inhibitory effect of CC10 on allergic responses remain ill defined, and the effect of CC10 on TH17 responses is not known.
In this study we examined the involvement of TH17 responses in the setting of AR and explored whether CC10 could regulate the TH17 responses in the context of AR. We found that TH17 responses were exaggerated in patients with AR and that CC10 inhibited TH17 responses through modulating the function of dendritic cells (DCs).
METHODS
Animals
Wild-type C57BL/6 mice and homozygous CC10-deficient mice on a C57BL/6 background were obtained, as previously described.13 DO11.10 mice on a BALB/c background and naive BALB/c mice were purchased from the Jackson Laboratory (Hancock, Me). All mice were used according to protocols approved by the Animal Care and Use Committee of Tongji Medical College of Huazhong University of Science and Technology.
Patients
Twelve patients with AR and 12 control subjects, who participated in a previous study,17 were included. Further information is provided in the Methods section in this article’s Online Repository at www.jacionline.org. Inferior turbinate mucosal samples were taken during septoplasty. This study was approved by the Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology. Informed consent was obtained from every subject.
AR mouse models, tissue preparation, and RNA extraction
AR mouse models induced by ovalbumin (OVA) and house dust mite (HDM) were established, and human recombinant CC10 (R&D Systems, Minneapolis, Minn) treatment during the sensitization and challenge phases was conducted as described elsewhere.17,19 OVA-specific TH17 cells were generated from DO11.10 mice and adoptively transferred to OVA-sensitized mice, as previously described.5 Twenty-four hours after the last challenge, nasal lavage fluid (NLF) was collected and subjected to cell counting and flow cytometric analysis, as described previously.17,18,20 The sinonasal cavity structure was processed for histologic examination, as described elsewhere.20,21 In some experiments respiratory sinonasal mucosal tissue and splenocytes were harvested and subjected to real-time PCR assay, flow cytometry, or both.17,18 The kinetic expression of CC10 and IL-17A in mice after OVA challenge was analyzed. More information is provided in the Methods section in this article’s Online Repository.
DC isolation
Splenic CD11c+ DCs were obtained by using anti-CD11c magnetic beads (Miltenyi Biotec, Auburn, Calif) and cultured according to the manufacturer’s instructions. In some experiments DCs from naive wild-type C57BL/6 mice were treated with 30 ng/mL human recombinant CC10 or PBS for 2 hours with or without a 2-hour pretreatment of the cells with the formyl peptide receptor 2 (FPR2) antagonist WRW4 (50 μmol/L; Tocris Bioscience, Ellisville, Mo).11,17 The cells were then pulsed with 60 μg/mL OVA or PBS for 48 hours. The culture supernatants and cells were harvested and subjected to further assays. More information is provided in the Methods section in this article’s Online Repository.
In vitro T-cell polarization and DC–T-cell culture
CD4+ T cells were isolated as mentioned elsewhere.12 In vitro TH17 cell differentiation was conducted, as previously reported, with modifications.5 CD4+ T cells were polarized under lineage-instructing cytokines with or without CC10 to explore the direct effect of CC10 on TH17 polarization.11,17 For DC–T-cell culture, DCs from naive DO11.10 mice were pulsed with OVA peptide 323-329 (GenScript, Piscataway, NJ) or PBS in the presence or absence of CC10, and then the cells were cultured with autologous naive CD4+ T cells. In some experiments the FPR2 antagonist WRW4 (50 μmol/L) was added 2 hours before CC10 treatment. Splenocytes from OVA-sensitized mice were pretreated with or without CC10, followed by stimulation of the cells with OVA and subsequent analysis of their proliferative response by using the Cell Counting Kit-8 (Boster Bio-Technology Company, Wuhan, China).11 More information is provided in the Methods section in this article’s Online Repository.
Adoptive transfer of DCs into naive mice
OVA- or PBS-pulsed DCs with or without CC10 pretreatment were generated, as previously described, with minor modifications, and transferred to naive mice, followed by OVA challenge. For further information, see the Methods section in this article’s Online Repository.22
BEAS-2B cell culture
BEAS-2B cells (a human bronchial epithelial cell line) were cultured and stimulated, as previously described.18 Before stimulation, the cells were pretreated with or without CC10. Five hours after cytokine stimulation, cells were harvested and subjected to real-time RT-PCR. For further information, see the Methods section in this article’s Online Repository.
Flow cytometric analysis
Flow cytometric analysis was conducted with an LSRII flow cytometer (Becton Dickinson, Franklin Lakes, NJ). DCs and CD4+ T cells, splenocytes, and inflammatory cells in NLF were stained with fluorescence-conjugated antibodies (see Table E1 in this article’s Online Repository at www. jacionline.org).23,24 More information is provided in the Methods section in this article’s Online Repository.
Immunohistochemistry
Immunohistochemical staining was conducted, as previously described.17,20 Rabbit anti-human IL-17A and anti-mouse CCL20 (1:200; Beijing Biosynthesis Biotechnology, Beijing, China) and anti-mouse CC10 (1:200; Santa Cruz Biotechnology, Santa Cruz, Calif) antibodies were used as primary antibodies. Numbers of IL-17A+, CC10+, and CCL20+ cells were counted, as previously described.17,18
Quantitative real-time PCR
cDNA was reverse transcribed, and quantitative PCR was performed with specific primer pairs (see Table E2 in this article’s Online Repository at www. jacionline.org), as described elsewhere.17,21 Relative gene expression was calculated by using the comparative cycle threshold method, as described previously.25
ELISA
Murine cytokine levels in culture supernatants or NLFs were determined by using ELISA, according to the manufacturer’s instructions. For further information, see the Methods section in this article’s Online Repository.
Statistical analysis
All results were expressed as means ± SEMs. The Mann-Whitney U test was used for paired sets of data. The paired t test was used in cell-culture data analysis. The Spearman test was applied to determine correlations. The P value for significance was set to .05. Data analysis was performed through the application of SPSS software for Windows (SPSS, Chicago, Ill).
RESULTS
Enhanced TH17 responses and decreased CC10 expression in the setting of AR
Our previous study demonstrated typical TH2-skewed eosinophilic inflammation in an OVA-induced AR model.17 In this study we found the percentage of TH17 cells was increased in NLF from OVA-induced mice with AR in comparison with that seen in control mice (Fig 1, A-C). It was also noted that the IL-17A levels in NLF were increased, in a time-dependent manner, during the development of AR (Fig 1, C), which was accompanied by a time-dependent downregulation of CC10 expression in the nasal mucosa (Fig 1, A). Similarly, enhanced TH17 responses were also found in an HDM-induced AR mouse model, which simulates human AR more precisely (see Fig E1 in this article’s Online Repository at www.jacionline.org). To keep the consistency between in vivo and in vitro experiments, we used the OVA-induced AR mouse model for further studies.
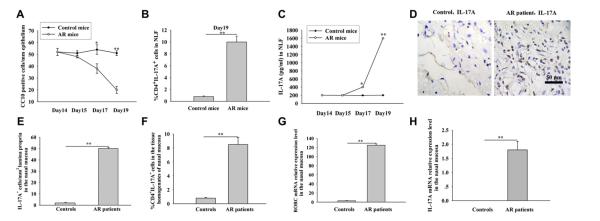
FIG 1.
Enhanced TH17 responses and decreased expression of CC10 in the setting of AR. A-C, CC10 expression in the nasal mucosa (Fig 1, A) and levels of TH17 cells (Fig 1, B) and IL-17A (Fig 1, C) in NLF. *P < .05 and **P < .01, OVA-induced mice with AR versus control animals (n = 8-10 mice per group). D, Immunohistochemical staining of IL-17A in inferior turbinate tissues. E-H, Number of IL-17A+ cells (Fig 1, E), percentage of TH17 cells (Fig 1, F), and levels of RORC (Fig 1, G) and IL-17A (Fig 1, H) mRNA expression in nasal mucosa. **P < .01, patients with AR versus control subjects (n = 12 per group).
In human subjects we previously reported the diminished expression of CC10 in nasal mucosa from patients with AR.17 In the present study the same group of subjects was studied, and immunohistochemical staining revealed increased infiltration of IL-17A+ cells in nasal biopsy specimens from patients with AR compared with control subjects (Fig 1, D and E). Flow cytometric analysis demonstrated that the percentage of TH17 cells was significantly higher in tissue homogenates from patients with AR than in control subjects (Fig 1, F). Moreover, the levels of mRNA expression for both RORC and IL-17A were increased in patients with AR compared with those seen in control subjects (Fig 1, G and H). However, we did not find a correlation between IL-17A local expression levels and the symptom severity (see Fig E2 in this article’s Online Repository at www.jacionline.org).
TH17 cells exaggerate eosinophilic and neutrophilic inflammation in the setting of AR
The transfer of OVA-specific TH17 cells to sensitized mice significantly enhanced not only neutrophil but also eosinophil recruitment into the nose after OVA challenge compared with that seen in mice with AR without TH17 cell transfer (see Fig E3 in this article’s Online Repository at www.jacionline.org). Moreover, although the mRNA expression of IL-4 was not enhanced, the mRNA expression of eotaxin-1 and eotaxin-2 was upregulated in the noses of TH17 cell–transferred mice (see Fig E3).
CC10 inhibits TH17 responses in the setting of AR
Our previous report has described an exaggerated TH2-skewed eosinophilic inflammation with no significant change in the TH1 response in the nasal mucosa of CC10-deficient mice with AR compared with that seen in wild-type mice with AR.17 In the present study we concentrated on the potential regulatory effect of CC10 on TH17 responses in the setting of AR. First, we found that the percentage of TH17 cells was significantly increased in NLF from CC10-deficient mice with AR compared with that seen in wild-type mice with AR (Fig 2, A, and see Fig E4, A, in this article’s Online Repository at www.jacionline.org). We next examined the TH1, TH2, and TH17 responses in the spleen after allergen sensitization. It was found that in the splenocyte population there was no significant difference in the percentage of TH1 cells between OVA- and PBS-sensitized mice or between wild-type and CC10-deficient mice (Fig 2, B, and see Fig E4, B). On the contrary, we found that the proportion of TH17 and TH2 cells in splenocytes was markedly increased in OVA-sensitized wild-type and CC10-deficient mice compared with that seen in PBS-sensitized control mice and that, significantly, such an increase was more prominent in CC10-deficient mice (Fig 2, C and D, and see Fig E4, C and D).
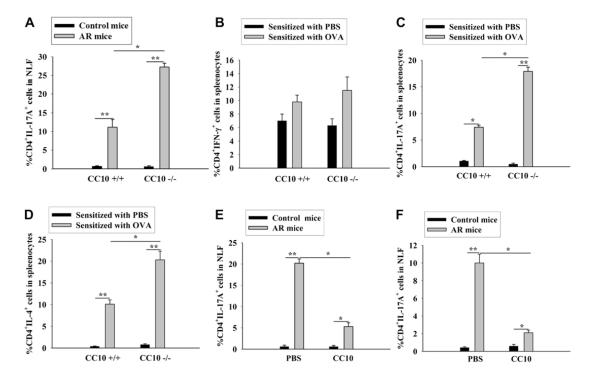
FIG 2.
CC10 inhibits TH17 responses in the setting of AR. A, TH17 cell levels in NLF from mice. B-D, Splenic TH1 (Fig 2, B), TH17 (Fig 2, C), and TH2 (Fig 2, D) cell levels in PBS- or OVA-sensitized mice. E and F, TH17 cell levels in NLF from CC10-deficient mice treated with CC10 or PBS before sensitization (Fig 2, E) and wild-type mice treated with CC10 or PBS before challenge (Fig 2, F). *P < .05 and **P < .01 (n = 8-15 mice per group).
Human recombinant CC10 was administered to CC10-deficient mice during sensitization to further define the role of CC10 in the sensitization phase. We previously reported that the TH2 responses and infiltration of eosinophils, lymphocytes, and total inflammatory cells in the nasal mucosa in OVA-challenged mice could be dramatically ameliorated by the administration of CC10 in the sensitization phase.17 Here we observed a decreased percentage of TH17 cells in NLF from CC10-treated mice with AR compared with that of PBS-treated mice with AR (Fig 2, E, and see Fig E4, E).
Finally, we investigated whether CC10 has a therapeutic role in the challenge phase. For this purpose, wild-type mice were used. Our previous study has already demonstrated that intranasal CC10 administration before OVA challenge could significantly inhibit TH2-skewed eosinophilic inflammation in the nasal mucosa.17 In this study we further revealed that CC10 administration before challenge could dramatically diminish TH17 cell numbers in NLF from mice with AR compared with numbers in those treated with PBS control (Fig 2, F, and see Fig E4, F).
CC10 suppresses TH17 responses through affecting DCs
To explore the mechanisms underlying the physiologic function of CC10, we first tested whether CC10 has a direct inhibitory effect on the differentiation of TH17 cells in vitro. We found no inhibitory effect of CC10 on the differentiation of TH17 cells (Fig 3, A, and see Fig E5 in this article’s Online Repository at www.jacionline.org). In addition, we found no significant effect of CC10 on the antigen-induced T-cell proliferative response (Fig 3, B).
FIG 3.
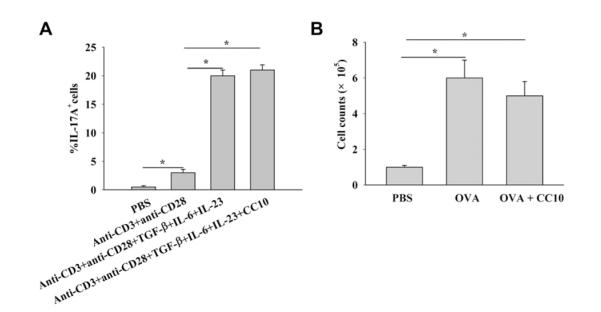
CC10 does not affect cytokine-induced TH17 cell differentiation. A, Generation of TH17 cells from cytokine-treated naive CD4+ T cells with or without CC10 treatment (n = 3-4). B, Proliferation assay of OVA-stimulated splenocytes with or without CC10 pretreatment. Pooled spleen cells from 3 OVA-sensitized mice were used, and 3 independent experiments were repeated. *P < .05.
Next we examined whether CC10 could modulate TH17 cell differentiation through DCs. We found that the mRNA expression of IL-23 and IL-6 was significantly upregulated whereas TGF-β mRNA expression was markedly downregulated in spleen CD11c+ DCs from CC10-null mice with AR compared with those from wild-type mice with AR (Fig 4, A). No difference in IL-12 expression was found (Fig 4, A). When OVA-pulsed CD11c+ DCs generated from naive mice were pretreated with CC10, we found that CC10 enhanced CD86 expression, whereas it decreased OX40 ligand (OX40L) expression and had no significant effect on CD80 expression (Fig 4, B, and see Fig E6, A, in this article’s Online Repository at www.jacionline.org). Moreover, we found that TGF-β production was significantly upregulated whereas IL-6 and IL-23p19 production was markedly downregulated when antigen-pulsed DCs were pretreated with CC10 (Fig 4, C). However, the production of IL-12 was not affected by CC10 pretreatment (Fig 4, C).
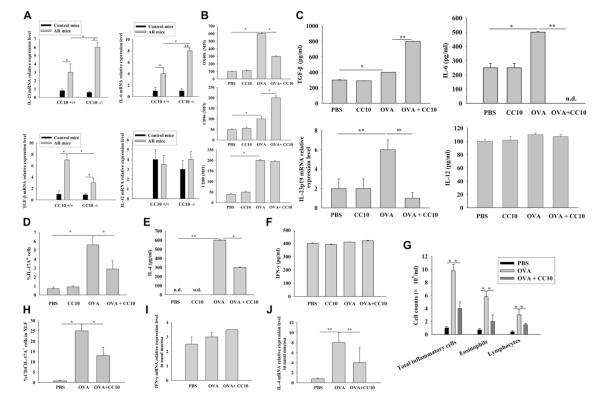
FIG 4.
CC10 affects TH17 responses through modulating DC function. A, Relative mRNA expression levels of cytokines in murine splenic CD11c+ DCs (n = 8-15 mice per group). B and C, Expression of surface markers on DCs (Fig 4, B) and protein levels of cytokines in culture supernatants and IL-23p19 mRNA expression in DCs (Fig 4, C). D-F, Generation of TH17 cells (Fig 4, D) and IL-4 (Fig 4, E) and IFN-γ (Fig 4, F) levels in DC–T-cell cocultures (n = 3-5). G-J, Analysis of infiltrating cells (Fig 4, G) and TH17 cells (Fig 4, H) in NLF and IFN-γ (Fig 4, I) and IL-4 (Fig 4, J) mRNA expression in the nasal mucosa of DC-transferred mice (n = 8-12 mice per group). *P < .05 and **P < .01.
DCs from naive DO11.10 mice were stimulated with OVA peptide 323-329 in the presence or absence of CC10 and then cocultured with autologous naive CD4+ T cells to examine whether CC10 would influence antigen-pulsed DCs and the subsequent generation of TH17 cells in vitro. As expected, we found that the percentage of TH17 cells was increased in culture with antigen-pulsed DCs when compared with that seen in DCs without antigen stimulation, and a significant reduction in the level of TH17 cell population was noted when CC10 was added in culture during antigen stimulation of DCs (Fig 4, D, and see Fig E6, B). Similarly, we found that IL-4 levels were increased in the supernatants of antigen-pulsed DC–T-cell cocultures, and such upregulation was also significantly ameliorated when DCs were treated with CC10 (Fig 4, E). However, IFN-γ levels were not affected by antigen stimulation or CC10 treatment (Fig 4, F).
OVA-pulsed DCs with or without CC10 pretreatment were adoptively transferred into naive mice followed by nasal OVA challenge to further investigate whether CC10-modulated DCs can influence TH17 responses in the context of allergic reactions in vivo. OVA-pulsed DC transfer could elicit marked TH2 and TH17 responses compared with PBS-pulsed DC transfer. Compared with the mice transferred with DCs without CC10 pretreatment, the mice transferred with CC10-pretreated DCs showed a decreased number of total inflammatory cells, eosinophils, and lymphocytes in NLF (Fig 4, G); a lower percentage of TH17 cells in NLF (Fig 4, H, and see Fig E6, C); and reduced levels of IL-4 and comparable levels of IFN-γ in nasal mucosa (Fig 4, I and J).
Regulatory effect of CC10 on DC function is not mediated through FPR2
The putative receptors for CC10 remain elusive. Because CC10 exhibits its biological effect through interacting with FPR-like 1 in some human cell types,26 we investigated whether CC10 influenced TH17 responses through FPR2 (the counterpart of human FPR-like 1 in the mouse) on DCs. We found that FPR2 was expressed on CD11c+ DCs (see Fig E7, A, in this article’s Online Repository at www.jacionline.org). Furthermore, we found that pretreatment with the FPR2 antagonist WRW4 did not influence the mRNA expression of TGF-β, IL-6, and IL-23p19 in DCs (see Fig E7, B) and TH17 cell differentiation induced by antigen-pulsed DCs (see Fig E7, C and D). CC10 could dramatically inhibit TH17-polarizing cytokine expression in DCs and DC-instructed TH17 cell differentiation, and this effect was not altered by WRW4 pretreatment (see Fig E7, B-D). The efficiency of the FPR2 antagonist WRW4 was confirmed by a control experiment, and the results are shown in Fig E8 in this article’s Online Repository at www.jacionline.org.
CC10 curbs the expansion of TH17 responses at local sites
The expression of a TH17 cell chemokine (CCL20) and an expansion factor, IL-23, in the nasal mucosa was explored. The CCL20 protein expression and IL-23 mRNA expression levels were increased in the nasal mucosa of mice with AR compared with those of control mice, and this increase was more prominent in CC10-deficient mice than in wild-type mice (Fig 5, A-D). Immunohistochemical staining showed that CCL20 was mainly produced by epithelial cells in murine nasal mucosa (Fig 5, A). Similarly, we found the CCL20 and IL-23 mRNA expression was upregulated in nasal mucosa from patients with AR compared with that seen in control subjects (Fig 5, E and F). Furthermore, we found that CC10 could significantly inhibit the upregulation of CCL20 expression in the BEAS-2B cell line induced by IFN-γ, TNF-α, IL-4, IL-1β, and IL-13 (Fig 5, G).
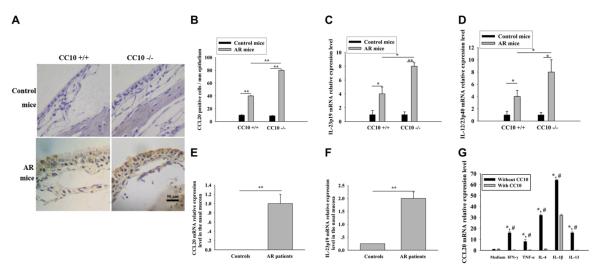
FIG 5.
Increased local expression of IL-23 and CCL20 in the setting of AR. A, Immunohistochemical staining of CCL20 in murine nasal tissues. B-D, Number of CCL20+ cells (Fig 5, B) and relative mRNA expression of IL-23p19 (Fig 5, C) and IL-23p40 (Fig 5, D) in murine nasal mucosa. *P < .05 and **P < .01 (n = 8-10 mice per group). E and F, Relative mRNA expression of CCL20 (Fig 5, E) and IL-23p19 (Fig 5, F) in the nasal mucosa of control subjects (n = 12) and patients with AR (n = 12). **P < .01. G, CC10 inhibits CCL20 mRNA expression in BEAS-2B cells. *P < .05 compared with medium controls, #P < .05 compared with the corresponding condition with CC10 treatment (n = 3-5).
DISCUSSION
A few previous studies have demonstrated increased IL-17 levels in peripheral blood in patients with AR and in NLF in a murine AR model.6,27 In the present study we have demonstrated enhanced TH17 responses in nasal mucosa in the setting of AR not only in human subjects but also in animal models. Furthermore, an adoptive transfer experiment using TH17 cells demonstrated that TH17 responses could enhance not only neutrophilic but also eosinophilic inflammation in mice with AR, which might result from the local upregulation of eotaxin-1 and eotaxin-2 expression. These findings are consistent with those found in a mouse model with allergic lower airway inflammation and confirm an effector role of TH17 responses in the setting of AR.5 In this study no correlation between local IL-17A expression and disease severity was found. It should be noted that the sample size was small in our study, and the concomitant nasal septal deviation in our patients with AR could be an additional factor affecting the severity of symptoms. Therefore a larger-scale study of a better defined patient population could more properly address the relationship between IL-17A expression and disease severity.
Despite the progress in our understanding of the pathophysiologic functions of TH17 cells, the regulation of TH17 responses, particularly in the context of specific diseases, has been relatively poorly understood. CC10 has been previously demonstrated to inhibit allergic inflammation through dampening TH2 responses in the setting of AR.17 In the present study we examined the regulatory function of CC10 on TH17 responses. We found that local IL-17A levels were increased whereas local CC10 expression was decreased in a time-dependent manner during the development of AR, suggesting an inverse correlation between the expression of CC10 and TH17 responses. Further studies with CC10-deficient mice and the use of recombinant CC10 treatment confirmed that in addition to its well-defined effect on TH2 responses, CC10 could also suppress TH17 responses at both the sensitization and challenge phases in the context of AR. Interestingly, the regulatory activity of CC10 on TH17 responses was mediated through its ability to modulate DC function.
DCs are known to play a primary role in determining the nature of T-lymphocyte differentiation in the face of allergen exposure.28 CD11c+ DCs, but not plasmacytoid DCs, have been indicated to perform an essential role in the development of AR.29,30 Also, we found that adoptive transfer of OVA-pulsed CD11c+ DCs strongly induced nasal eosinophilia and TH2 and TH17 responses, supporting the role of CD11c+ DCs in the setting of AR. We found that CC10 could significantly inhibit the expression of IL-6 and IL-23, the crucial TH17 lineage–instructing cytokines,31 in antigen-pulsed CD11c+ DCs, which might contribute to the inhibitory effect of CC10 on TH17 differentiation.
IL-23 is a heterodimeric protein consisting of p19 and p40 subunits that promotes survival and proliferation of TH17 cells.31 CCR6 has been shown to be mainly expressed on TH17 cells, and CCR6-CCL20 interaction has been shown to be critical in mediating TH17 cell migration into inflamed tissues.31 In the present study we found that IL-23 and CCL20 expression levels were increased locally in both human subjects and mice with AR and that CC10 could inhibit IL-23 and CCL20 expression in mice with AR and inflammatory cytokine-induced CCL20 expression in airway epithelial cells. These results suggest that CC10 can also affect TH17 cell chemotaxis and local expansion.
Moreover, we found that CC10 was able to enhance the expression of CD86 on CD11c+ DCs, suggesting that CC10 can promote the maturation and antigen-presenting function of DCs. Despite exhibiting similar binding affinity to the CD28 ligand, CD80 and CD86 can exhibit different biochemical characteristics that can result in different T-cell functional outcomes.32 Therefore the influence of distinct effects of CC10 on CD86 and CD80 expression awaits further study. Furthermore, DCs are known to express OX40L, which interacts with OX40 on naive T cells and influences TH2 lineage commitment.33 Our results indicate that CC10 might suppress TH2 response through downregulation of OX40L expression on CD11c+ DCs. It is also known that IL-12–producing DCs are capable of inducing TH1 responses. In the present study we found that CC10 had no obvious effect on IL-12 expression in antigen-pulsed CD11c+ DCs, which is in line with the observation that CC10 had no significant influence on TH1 polarization in vitro and in vivo. It is possible that CC10 might also affect the engraftment rate and trafficking pattern of DCs to the draining lymph nodes, where DCs present antigens to T lymphocytes and initiate cognate T-cell responses. However, this possibility awaits further investigation.
The putative receptors for CC10 remain elusive. A study indicated that in human subjects CC10 interacts with a low-affinity receptor for formyl peptide.26 Therefore we investigated whether CC10 could influence DC function through FPR2, the mouse counterpart of the human receptor. We found that FPR2 was expressed on CD11c+ DCs, but FPR2 antagonist treatment had no influence on CC10’s effect on TH17-polarizing cytokine production in antigen-pulsed DCs and TH17 cell differentiation instructed by antigen-pulsed DCs, indicating that CC10 does not affect DC function through FPR2. Therefore the intracellular mechanisms underlying the regulatory effect of CC10 on DC function need further investigation.
In conclusion, the evidence from both in vivo and in vitro studies suggests an inhibitory role of CC10 in TH17 responses in the setting of AR. CC10 prevents TH17 polarization through modulation of DC function in terms of costimulatory molecule and cytokine expression. CC10 also affects TH17 cell chemotaxis and local expansion in the setting of AR. However, the biological signaling pathway, which underlies the function of CC10, needs further investigation. CC10 is relatively small, resistant to proteases, and stable to extremes of heat and pH and can be produced by recombinant methods. These characteristics make CC10 an excellent candidate for clinical development to treat allergic airway diseases.
Supplementary Material
FIG E1. Enhanced TH17 and TH2 responses in the nose in mice with dust mite–induced AR. The relative mRNA expression of retinoic acid–related orphan receptor γt (RORγt), IL-17A, IL-4, and IFN-γ in the nasal mucosa and the percentage of TH17 cells and numbers of inflammatory cells in NLF from mice are shown. *P < .05 (n = 8-10 mice per group).
FIG E2. No significant correlation between IL-17A expression in the nasal mucosa and VAS symptom scores in 12 patients with AR.
FIG E3. Antigen-specific TH17 cells enhance antigen-induced eosinophil and neutrophil recruitment into the upper airways of sensitized mice. OVA- or PBS-sensitized mice were transferred with OVA-specific TH17 cells or PBS controls. The number of inflammatory cells in the NLF and the goblet cell hyperplasia and mRNA expression of cytokines in the nasal mucosa were evaluated. *P < .05 (n = 4 mice per group).
FIG E4. CC10 inhibits TH17 responses in the setting of AR. A, A representative result of flow cytometric experiments of TH17 cells in NLF from mice. B-D, Representative results of flow cytometric experiments of splenic TH1 (Fig E4, B), TH17 (Fig E4, C), and TH2 (Fig E4, D) cells in PBS- or OVA-sensitized mice. E, A representative result of flow cytometric experiments of TH17 cells in NLF from CC10-deficient mice with CC10 or PBS treatment 2 hours before sensitization. F, A representative result of flow cytometric experiments of TH17 cells in NLF from wild-type mice with CC10 or PBS treatment 2 hours before challenge.
FIG E5. CC10 does not affect cytokine-induced in vitro TH17 cell differentiation. Representative results of flow cytometric experiments of TH17 cells differentiated from naive CD4+ T cells in the presence of polarizing cytokines with or without CC10 treatment.
FIG E6. CC10 affects TH17 responses through modulation of the function of CD11c+ DCs. A, Representative results of flow cytometric experiments of surface marker expression on CD11c+ DCs. B, Representative results of flow cytometric experiments of TH17 cells differentiated from naive CD4+ T cells cocultured with CD11c+ DCs. C, A representative result of flow cytometric experiments of TH17 cells in NLF from DC-transferred mice.
FIG E7. FPR2 is not involved in the regulatory effect of CC10. A, A representative result of flow cytometric experiments of the expression of FPR2 on CD11c+ DCs (n = 4-5 mice). B, mRNA levels of cytokines in CD11c+ DCs. *P < .05 and **P < .01 (n = 4-5). C and D, Representative results of flow cytometric experiments (Fig E7, C) and quantification of the percentage of TH17 cells differentiated from naive CD4+ T cells cocultured with CD11c+ DCs (Fig E7, D). *P < .05 (n = 4-5).
FIG E8. WRW4 inhibits WKYMVM-induced cytosolic calcium increase in murine CD11c+ DCs. Murine CD11c+ DCs were stimulated with WKYMVM in the presence or absence of WRW4. The [Ca2+]i value was determined fluorometrically by using fura-2/AM. The peak [Ca2+]i value was monitored. *P < .05 (n = 3).
TABLE E1. Anti-mouse antibodies used in flow cytometry
TABLE E2. Primer sequences for real-time PCR
Clinical implications: CC10 is able to ameliorate allergic airway inflammation in a mouse model of AR through modulating DC functions, resulting in the inhibition of TH17 and TH2 responses. CC10 might be an excellent candidate for drug development.
Acknowledgments
Supported by National Natural Science Foundation of China (NSFC) grants 81020108018 and 30872847 and the program for New Century Excellent Talents in University from the State Education Ministry grant NCET-07-0326 (to Z.L.), a grant from Ministry of Health of China (201202005), and, in part, National Institutes of Health grants AI052468 and AI073610 (to S.-K.H).
S.-K. Huang has received grants AI052468 and AI073610 from the National Institutes of Health. Z. Liu has received National Natural Science Foundation of China (NSFC) grants 81020108018 and 30872847 and a Program for New Century Excellent Talents in University grant (NCET-07-0326) from the State Education Ministry.
Abbreviations used
- AR
Allergic rhinitis
- CC10
Clara cell 10-kDa protein
- DC
Dendritic cell
- HDM
House dust mite
- FITC
Fluorescein isothiocyanate
- FPR2
Formyl peptide receptor 2
- NLF
Nasal lavage fluid
- OVA
Ovalbumin
- OX40L
OX40 ligand
- PE
Phycoerythrin
Footnotes
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Durham SR, Ying S, Varney VA, Jacobson MR, Sudderick RM, Mackay IS. Cytokine messenger RNA expression for IL-3, IL-4, IL-5, and granulocyte-macrophage-colony-stimulating factor in the nasal mucosa after local allergen provocation: relationship to tissue eosinophilia. J Immunol. 1992;148:2390–4. [PubMed] [Google Scholar]
- 2.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008. Allergy. 2008;63(suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 3.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–87. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 4.Schnyder-Candrian SD, Togbe I, Couillin I, Mercier F, Brombacher V, Quesniaux F, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–25. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008;178:1023–32. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- 6.Ciprandi G, De Amici M, Murdaca G, Fenoglio D, Ricciardolo F, Marseglia G, et al. Serum interleukin-17 levels are related to clinical severity in allergic rhinitis. Allergy. 2009;64:1375–8. doi: 10.1111/j.1398-9995.2009.02010.x. [DOI] [PubMed] [Google Scholar]
- 7.Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009;124:478–84. doi: 10.1016/j.jaci.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 8.John FA, Christopher RC, Jay KK. TH17 cells in asthma and COPD. Ann Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 9.Singh TP, Huettner B, Koefeler H, Mayer G, Bambach I, Wallbrecht K, et al. Platelet-activating factor blockade inhibits the T-helper type 17 cell pathway and suppresses psoriasis-like skin disease in K5.hTGF-β1 transgenic mice. Am J Pathol. 2011;178:699–708. doi: 10.1016/j.ajpath.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206:2067–77. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung CH, Chen LC, Zhang Z, Chowdhury B, Lee WL, Plunkett B, et al. Regulation of TH2 responses by the pulmonary Clara cell secretory 10-kd protein. J Allergy Clin Immunol. 2004;114:664–70. doi: 10.1016/j.jaci.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 12.Johansson S, Wennergren G, Aberg N, Rudin A. Clara cell 16-kd protein downregulates T(H)2 differentiation of human naive neonatal T cells. J Allergy Clin Immunol. 2007;120:308–14. doi: 10.1016/j.jaci.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Chen LC, Zhang Z, Myers AC, Huang SK. Cutting edge: altered pulmonary eosinophilic inflammation in mice deficient for Clara cell secretory 10-kDa protein. J Immunol. 2001;167:3025–8. doi: 10.4049/jimmunol.167.6.3025. [DOI] [PubMed] [Google Scholar]
- 14.Mandal AK, Zhang Z, Ray R, Choi MS, Chowdhury B, Pattabiraman N, et al. Uteroglobin represses allergen-induced inflammatory response by blocking PGD2 receptor-mediated functions. J Exp Med. 2004;199:1317–30. doi: 10.1084/jem.20031666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Lu X, Zhang XH, Bochner BS, Long XB, Zhang F, et al. Clara cell 10-kDa protein expression in chronic rhinosinusitis and its cytokine-driven regulation in sinonasal mucosa. Allergy. 2009;64:149–57. doi: 10.1111/j.1398-9995.2008.01847.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Kim J, Sypek JP, Wang IM, Horton H, Oppenheim FG, et al. Gene expression profiles in human nasal polyp tissues studied by means of DNA microarray. J Allergy Clin Immunol. 2004;114:783–90. doi: 10.1016/j.jaci.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Lu X, Yu HJ, Hua XY, Cui YH, Huang SK, et al. The expression of osteopontin and its association with Clara cell 10 kDa protein in allergic rhinitis. Clin Exp Allergy. 2010;40:1632–41. doi: 10.1111/j.1365-2222.2010.03549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Long XB, Cao PP, Wang N, Liu Y, Cui YH, et al. Clara cell 10-kD protein suppresses chitinase 3-like 1 expression associated with eosinophilic chronic rhinosinusitis. Am J Respir Crit Care Med. 2010;181:908–16. doi: 10.1164/rccm.200904-0597OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Lu X, Cao PP, Chu Y, Long XB, Zhang XH, et al. Histological and immunological observations of bacterial and allergic chronic rhinosinusitis in the mouse. Am J Rhinol. 2008;22:343–8. doi: 10.2500/ajr.2008.22.3184. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Lu X, Wang H, You XJ, Gao QX, Cui YH. Group II subfamily secretory phospholipase A2 enzymes: expression in chronic rhinosinusitis with and without nasal polyps. Allergy. 2007;62:999–1006. doi: 10.1111/j.1398-9995.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- 22.Koya T, Matsuda H, Takeda K, Matsubara S, Miyahara N, Balhorn A, et al. IL-10-treated dendritic cells decrease airway hyperresponsiveness and airway inflammation in mice. J Allergy Clin Immunol. 2007;119:1241–50. doi: 10.1016/j.jaci.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 23.Shalev I, Liu H, Koscik C, Bartczak A, Javadi M, Wong KM, et al. Targeted deletion of fgl2 leads to impaired regulatory T cell activity and development of autoimmune glomerulonephritis. J Immunol. 2008;180:249–60. doi: 10.4049/jimmunol.180.1.249. [DOI] [PubMed] [Google Scholar]
- 24.Bleck B, Tse DB, Gordon T, Ahsan MR, Reibman J. Diesel exhaust particle-treated human bronchial epithelial cells upregulate Jagged-1 and OX40 ligand in myeloid dendritic cells via thymic stromal lymphopoietin. J Immunol. 2010;185:6636–45. doi: 10.4049/jimmunol.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang XH, Zhang YN, Li HB, Hu CY, Wang N, Cao PP, et al. Overexpression of miR-125b, a novel regulator of innate immunity, in eosinophilic chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2012;185:140–51. doi: 10.1164/rccm.201103-0456OC. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Huang J, Kuo M, Wang J, Huang H, Huang SK. Interaction of Clara cell 10kd protein (CC10) and formyl peptide receptor-like1 (FPRL1) suppresses lung fibroblast cytokine release and neutrophil superoxide generation. J Allergy Clin Immunol. 2009;123(suppl):S49. [Google Scholar]
- 27.Quan SH, Zhang YL, Han DH, Iwakura Y, Rhee CS. Contribution of interleukin 17A to the development and regulation of allergic inflammation in a murine allergic rhinitis model. Ann Allergy Asthma Immunol. 2012;108:342–50. doi: 10.1016/j.anai.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Gill MA. The role of dendritic cells in asthma. J Allergy Clin Immunol. 2012;129:889–901. doi: 10.1016/j.jaci.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 29.KleinJan A, Willart M, van Rijt LS, Braunstahl GJ, Leman K, Jung S, et al. An essential role for dendritic cells in human and experimental allergic rhinitis. J Allergy Clin Immunol. 2006;118:1117–25. doi: 10.1016/j.jaci.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 30.Moser M. Dendritic cells in immunity and tolerance: do they display opposite functions? Immunity. 2003;19:5–8. doi: 10.1016/s1074-7613(03)00182-1. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007;120:247–54. doi: 10.1016/j.jaci.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 32.Rate A, Upham JW, Bosco A, McKenna KL, Holt PG. Airway epithelial cells regulate the functional phenotype of locally differentiating dendritic cells: implications for the pathogenesis of infectious and allergic airway disease. J Immunol. 2009;182:72–83. doi: 10.4049/jimmunol.182.1.72. [DOI] [PubMed] [Google Scholar]
- 33.Jenkins SJ, Perona-Wright G, Worsley AGF, Ishii N, MacDonald AS. Dendritic cell expression of OX40 ligand acts as a costimulatory, not polarizing, signal for optimal Th2 priming and memory induction in vivo. J Immunol. 2007;179:3515–23. doi: 10.4049/jimmunol.179.6.3515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIG E1. Enhanced TH17 and TH2 responses in the nose in mice with dust mite–induced AR. The relative mRNA expression of retinoic acid–related orphan receptor γt (RORγt), IL-17A, IL-4, and IFN-γ in the nasal mucosa and the percentage of TH17 cells and numbers of inflammatory cells in NLF from mice are shown. *P < .05 (n = 8-10 mice per group).
FIG E2. No significant correlation between IL-17A expression in the nasal mucosa and VAS symptom scores in 12 patients with AR.
FIG E3. Antigen-specific TH17 cells enhance antigen-induced eosinophil and neutrophil recruitment into the upper airways of sensitized mice. OVA- or PBS-sensitized mice were transferred with OVA-specific TH17 cells or PBS controls. The number of inflammatory cells in the NLF and the goblet cell hyperplasia and mRNA expression of cytokines in the nasal mucosa were evaluated. *P < .05 (n = 4 mice per group).
FIG E4. CC10 inhibits TH17 responses in the setting of AR. A, A representative result of flow cytometric experiments of TH17 cells in NLF from mice. B-D, Representative results of flow cytometric experiments of splenic TH1 (Fig E4, B), TH17 (Fig E4, C), and TH2 (Fig E4, D) cells in PBS- or OVA-sensitized mice. E, A representative result of flow cytometric experiments of TH17 cells in NLF from CC10-deficient mice with CC10 or PBS treatment 2 hours before sensitization. F, A representative result of flow cytometric experiments of TH17 cells in NLF from wild-type mice with CC10 or PBS treatment 2 hours before challenge.
FIG E5. CC10 does not affect cytokine-induced in vitro TH17 cell differentiation. Representative results of flow cytometric experiments of TH17 cells differentiated from naive CD4+ T cells in the presence of polarizing cytokines with or without CC10 treatment.
FIG E6. CC10 affects TH17 responses through modulation of the function of CD11c+ DCs. A, Representative results of flow cytometric experiments of surface marker expression on CD11c+ DCs. B, Representative results of flow cytometric experiments of TH17 cells differentiated from naive CD4+ T cells cocultured with CD11c+ DCs. C, A representative result of flow cytometric experiments of TH17 cells in NLF from DC-transferred mice.
FIG E7. FPR2 is not involved in the regulatory effect of CC10. A, A representative result of flow cytometric experiments of the expression of FPR2 on CD11c+ DCs (n = 4-5 mice). B, mRNA levels of cytokines in CD11c+ DCs. *P < .05 and **P < .01 (n = 4-5). C and D, Representative results of flow cytometric experiments (Fig E7, C) and quantification of the percentage of TH17 cells differentiated from naive CD4+ T cells cocultured with CD11c+ DCs (Fig E7, D). *P < .05 (n = 4-5).
FIG E8. WRW4 inhibits WKYMVM-induced cytosolic calcium increase in murine CD11c+ DCs. Murine CD11c+ DCs were stimulated with WKYMVM in the presence or absence of WRW4. The [Ca2+]i value was determined fluorometrically by using fura-2/AM. The peak [Ca2+]i value was monitored. *P < .05 (n = 3).
TABLE E1. Anti-mouse antibodies used in flow cytometry
TABLE E2. Primer sequences for real-time PCR