Abstract
Decision-making is a complex cognitive process that is impaired in a number of psychiatric disorders. In the laboratory, decision-making is frequently assessed using “gambling” tasks that are designed to simulate real-life decisions in terms of uncertainty, reward and punishment. Here, we investigate whether lesions of the medial prefrontal cortex (PFC) cause impairments in decision-making using a rodent gambling task (rGT). In this task, rats have to decide between 1 of 4 possible options: 2 options are considered “advantageous” and lead to greater net rewards (food pellets) than the other 2 “disadvantageous” options. Once rats attained stable levels of performance on the rGT they underwent sham or excitoxic lesions and were allowed to recover for 1 week. Following recovery, rats were retrained for 5 days and then the effects of a dopamine D1-like receptor antagonist (SCH23390) or a D2-like receptor antagonist (haloperidol) on performance were assessed. Lesioned rats exhibited impaired decision-making: they made fewer advantageous choices and chose the most optimal choice less frequently than did sham-operated rats. Administration of SCH23390 (0.03 mg/kg), but not haloperidol (0.015–0.03 mg/kg) attenuated the lesion-induced decision-making deficit. These results indicate that the medial PFC is important for decision-making and that excessive signaling at D1 receptors may contribute to decision-making impairments.
Keywords: rodent gambling task, medial prefrontal cortex, dopamine, lesion, SCH23390, haloperidol
1.0 Introduction
Decision-making is a complex cognitive process that involves assessing the risk, reward, and costs associated with different alternatives and then selecting that which will likely lead to the maximal benefit for the individual [1]. Decision-making deficits are commonly observed in a number of psychiatric conditions including attention-deficit hyperactivity disorder (ADHD; [2]), substance abuse [3], Parkinson’s Disease [4,5,6], problem gambling [7,8] and schizophrenia [9, 10, 11,12]. These decision-making deficits can have devastating consequences and may contribute to the poor outcomes for afflicted individuals. Understanding the neural basis of optimal decision-making may allow us to develop strategies to treat decision-making impairments.
In the laboratory, decision-making is frequently tested using “gambling” tasks that are designed to simulate real-life decisions in terms of uncertainty, reward and punishment [1]. The Iowa Gambling Task (IGT; [13]), a task commonly used to assess decision-making in the laboratory, requires human subjects to choose between 4 different decks of cards. Unbeknownst to the subject, two of the decks of cards are associated with large financial gains, and unpredictable even larger losses such that continued choice of these decks leads to a net financial loss. The other two decks are associated with small financial gains, and unpredictable smaller losses such that continued choice of these decks leads to a net financial gain. Healthy control subjects develop a preference for the advantageous small win/small loss decks, while individuals with ventromedial PFC [13] or dorsolateral PFC [14] lesions fail to develop these preferences; instead they prefer the disadvantageous large reward/large loss decks.
Recently, rodent “gambling” tasks have been developed that share many of the features of the human gambling tasks including uncertainty, reward and punishment [15, 16]. The rodent gambling task (rGT) developed by Winstanley and colleagues [15,17] is notably similar to the IGT— there are 4 response options each of which is associated with a different reward/punishment contingency. Two of the response options are associated with small rewards (food pellets) and small, infrequent punishments (omission of reward and delay until the next trial). The other two options are associated with large rewards and large, frequent punishments. These response contingencies result in two options being relatively more “advantageous” than the other two “disadvantageous” options. Similar to healthy humans subjects, control rats develop a preference for the advantageous small reward/small punishment options over the disadvantageous large reward/large punishment options [15].
Using the rGT developed by Winstanley and colleagues [15, 17], we aimed to determine whether lesions of the medial PFC negatively affected stable choice behavior. It has previously been observed that medial PFC and orbitofrontal cortex (OFC) lesions impair the development of an advantageous decision-making strategy in rats [17,18]. Once a stable strategy had been established however, OFC lesions did not affect choice behavior [17]. Given the role of the medial PFC in human decision-making [14] and because a number of psychiatric conditions display pathology in this brain area (e.g., [2–12] we aimed to determine whether medial PFC lesions would cause a change in rats’ decision-making. Because “risk” based decision-making is modulated by dopamine [1, 15, 19] and because medial PFC lesions can cause changes in striatal dopamine transmission [20, 21, 22], we also investigated whether D1-like or D2-like receptor antagonist administration affected choice behavior in rats with sham or medial PFC lesions.
2.0 Materials and Methods
2.1 Rats
Twenty-seven male Sprague-Dawley rats born at Oberlin College were used. Rats were maintained on a 14-h/10-h light-dark cycle (lights on at 0700h) and were group housed until post-natal day (PND) 55; during this time they had unlimited access to food (Purina Rat Chow) and water. On PND 55 rats were pair housed until the time of surgery at which time they were housed singly. Upon pair housing and throughout the experiment rats were food restricted to ~85% of their free feeding weight; rats were fed after daily training sessions. Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals [23] and Oberlin College policies.
2.2 Drugs
Ibotenic acid and haloperidol were purchased from Sigma-Aldrich (St. Louis, MO). Ibotenic acid was dissolved in physiological saline (0.9%) to a final concentration of 10 μg/μl. Haloperidol was dissolved in 75% dimethyl sulfoxide to concentrations of 0.015 mg/ml and 0.03 mg/ml. SCH23390 was purchased from Tocris (Ellisville, MO) and was dissolved in saline (pH 6.5) to final concentrations of 0.03 mg/ml and 0.06 mg/ml.
2.3 Surgery
Rats were anesthetized with sodium pentobarbital (65 mg/kg, IP). The skull was exposed and burr holes drilled above the medial PFC. Rats received bilateral injections of either saline (n=9) or ibotenic acid (n=12) at the following coordinates (mm relative to bregma): AP + 2.8, ML =±0.75, DV −3.3 and DV −2.3 [24]. Rats received two bilateral infusions, one at each DV coordinate. Each infusion delivered 0.4 μl/side of 10 μg/μl ibotenic acid at a rate of 0.2 μl/min. The infuser remained in place for an additional 2 min following each infusion and then was raised for the second infusion or removed. Once the infuser was removed, the scalp was sutured shut.
2.4 Rodent Gambling Task (rGT)
The rGT was based upon that developed by Winstanely and colleagues [15, 17]. Rats were trained and tested in 5-hole operant chambers enclosed in sound attenuating chambers (Med Associates, Vermont); the center hole was occluded throughout all experiments.
Rats were first trained to retrieve food pellets (45-mg, Bio-Serv, Frenchtown NJ) from the food magazine over two consecutive days; during these sessions all holes were occluded. Next, rats were trained to nose poke in the holes under a continuous reinforcement schedule. In order to encourage equal nose poking in all 4 holes the two outer holes were occluded on the first session and the two inner holes occluded on the second session. On the third session and all subsequent sessions all 4 holes were open. The continuous reinforcement task began with the delivery of 1 food pellet and the illumination of the houselight and magazine light. Retrieval of the food pellet extinguished the magazine light and initiated a 5-sec inter-trial interval (ITI). At the end of the ITI, lights at the rear of the holes (aperture lights) were illuminated and rats had 10 sec to make a response in any hole. Responses resulted in the delivery of a food pellet reward (Bio-Serv). Rats were trained on the continuous reinforcement task until they received 100 rewards with less than 20 omissions in ~30 min.
Once rats reached criterion performance on the continuous reinforcement task they underwent 4 days of forced choice training on the full rGT. In each forced choice session only a single hole was available; this measure ensured that rats had experience with the contingencies associated with each hole prior to allowing rats to choose freely (see below). For the full rGT, sessions began with the delivery of a single food pellet and illumination of the houselight and the magazine light. Pellet retrieval extinguished the magazine light and initiated a 5-sec ITI. At the end of the ITI the 4 aperture lights were illuminated and rats had 10 sec (limited hold) to make a response in any hole. A response resulted in the assigned outcome (reward/punishment) for that hole (see Table 1 for example). On rewarded trials the aperture lights were all extinguished, the magazine light was illuminated and the appropriate number of pellets was delivered to the magazine. On punished trials no pellets were delivered and the houselight and all aperture lights, except the one from the chosen hole, were extinguished for the duration of the punishment period (i.e., the chosen hole remained illuminated throughout the punishment period). The next trial commenced at the end of the punishment period or once the rat retrieved the food pellet reward. Failing to respond within the limited hold was scored as an omission and responses occurring during the ITI were scored as premature responses. Omissions and premature responses were punished with a 5-sec time out during which the houselight, magazine light and all aperture lights were extinguished. Each session lasted for a total of 60 min.
Table 1.
Example of response contingencies across nose poke holes
| Hole (H) 1 | H2 | H3 | H4 | |
|---|---|---|---|---|
| # of Rewards | 4 | 1 | 3 | 2 |
| Punishment Duration (sec) | 40 | 5 | 30 | 10 |
| Probability of Punishment | 0.6 | 0.1 | 0.5 | 0.2 |
Note: There were 4 different spatial arrangements of outcomes; one such arrangement is depicted here. An individual rat was assigned a single arrangement for the duration of the experiment. On rewarded trials the number of pellets indicated were delivered for that choice; on punished trials the no pellets were delivered.
Four different spatial arrangements of the outcomes across holes were used. An individual rat however, was only trained and tested on a single spatial arrangement of outcomes. Two of the holes had relatively “advantageous” outcomes (increased total number of pellets over the entire 1 hour session) due to the low probability (0.1–0.2) and short duration (5–10 sec) of punishment periods (see Table 1). The advantageous choices were associated with smaller rewards (1–2 pellets). Two holes were associated with relatively “disadvantageous” outcomes due to the high probability (0.5–0.6) and long duration (30–40 sec) of punishment. The disadvantageous choices were associated with larger rewards (3–4 pellets). The % responses at each hole and % advantageous responses ((# advantageous responses/total responses) * 100) were used as indicators of decision-making. Once % advantageous responding stabilized (< 5% variability across days), rats underwent surgery and were allowed to recover for 5–7 days prior to beginning testing.
2.5 Testing
2.5.1 Effects of medial PFC Lesions on choice behavior
After a 5–7 day recovery period rats were retested on the rGT for 5 consecutive days in order to assess the effects of medial PFC lesions on decision-making.
2.5.2 Effects of SCH23390 on choice behavior
Next, the ability of the D1-like receptor antagonist SCH23390 to affect decision-making behavior in sham-operated and lesioned rats was assessed. SCH23390 (0.0, 0.03 and 0.06 mg/kg, IP) was administered 30 min prior to testing; doses were administered in a counterbalanced fashion. Rats were given at least 2 drug-free days between consecutive drug doses.
2.5.3 Effects of haloperidol on choice behavior
Finally, the ability of the D2-like receptor antagonist haloperidol to affect decision-making behavior in sham-operated and lesion rats was assessed. Haloperidol (0.0, 0.015 and 0.03 mg/kg, SC) was administered 30 min prior to testing; doses were administered in a counterbalanced fashion. Rats were given at least 2 drug-free days between consecutive drug doses.
2.6 Histology
Following the last test session rats were anesthetized with sodium pentobarbital (100 mg/kg, IP) and transcardially perfused with 0.9% NaCl followed by 4% paraformaldehyde. Following perfusions, brains were removed, post-fixed for 24 h and then cryoprotected in 30% sucrose prior to slicing on a microtome. Sections (40 μm) were mounted on slides, stained with cresyl violet and the extent of the lesion assessed.
2.7 Statistical Analyses
rGT data were analyzed using repeated measures ANOVAs with Day, Dose and/or % Choice per hole as the within-subjects factor and Condition (sham-operated, lesioned) as the between-subjects factor. Significant effects were further analyzed using an estimated marginal means procedure with a Bonferroni correction.
Measures of interest included: % advantageous responses, % choice per hole ((# responses per hole/total responses)*100), % omissions ((# omissions/total responses)*100) and % premature responses ((# premature responses/total responses)*100).
3.0 Results
3.1 Histology
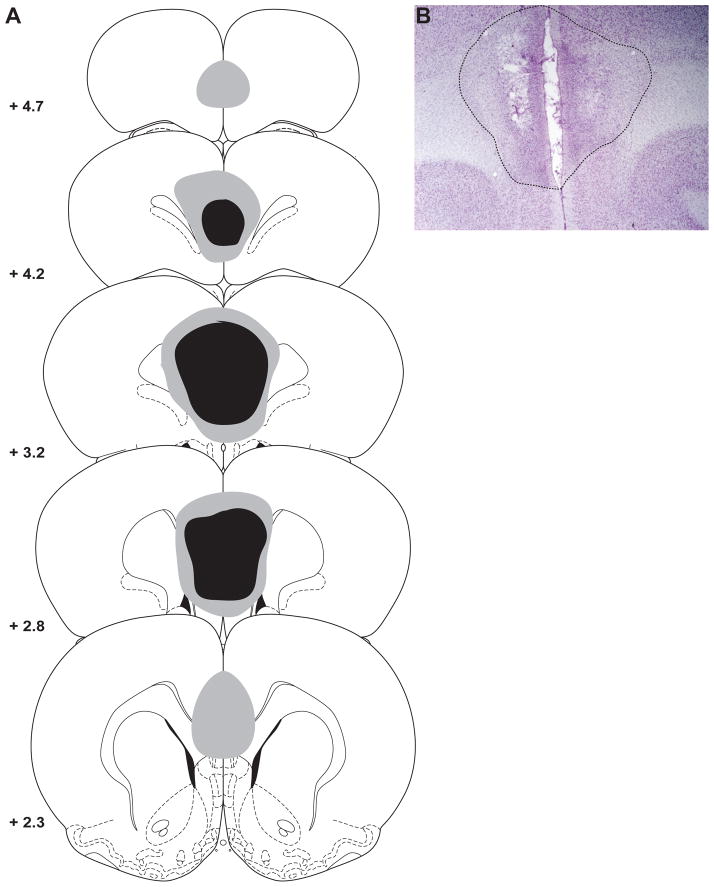
Figure 1 depicts the extent of the medial PFC lesions. Six rats were excluded from the experiment because their choice behavior never reached the pre-surgery stability criterion. One lesioned rat was excluded because of a unilateral lesion and two lesioned rats were excluded because of excessive damage that included an enlargement of the lateral ventricles. Thus, 9 rats remained in each group for the statistical analyses. One additional sham-operated rat was excluded from the statistical analysis on the haloperidol data because his behavior became unstable following SCH23390 administration.
Figure 1.
The location and extent of ibotenic acid lesions of the medial prefrontal cortex (PFC). A) Schematic showing the largest (grey) and smallest (black) extent of the lesion on any particular section through the medial PFC. B) Photomicrograph depicting the average extent of damage caused by ibotenic acid infusions. Adapted from [24].
3.2 Post-Lesion Tests
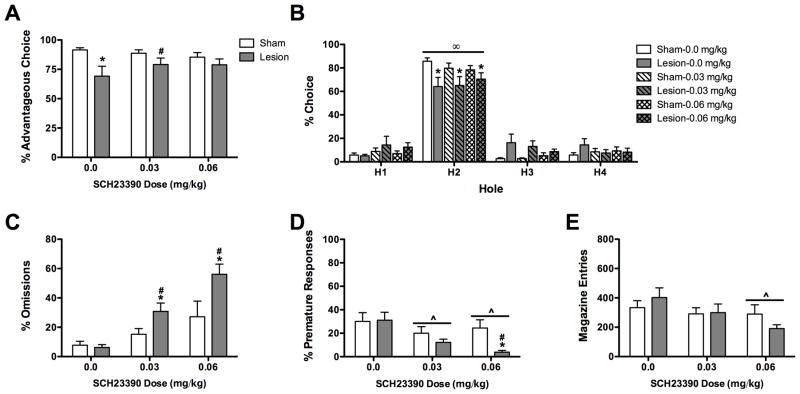
The % advantageous responses differed across day of test (F(5, 80) = 2.36, P < 0.05; see Figure 2A) and there was a trend towards a difference between sham-operated and lesioned rats (F(1, 16) = 4.00, P = 0.06). Importantly there was a significant day X condition interaction (F(5, 80) = 3.15, P < 0.05). Post-hoc analysis on the interaction revealed that lesioned rats had lower % advantageous responses on day 4 following surgery (P < 0.05) and tended to have lower % advantageous responses on day 5 following surgery (P = 0.07) relative to their pre-surgery baseline performance. In addition, lesioned rats had lower % advantageous responses on post-lesion day 1 (P = 0.07), post-lesion day 4 (P < 0.05) and post-lesion day 5 (P = 0.05) compared to sham-operated rats.
Figure 2.
Effects of medial PFC lesions on performance in the rodent gambling task. A) % Advantageous responses. Lesioned rats exhibit a decrease in their choice of advantageous options that progressively worsened across testing. B) % Choice of each hole (H). All rats chose hole 2 (most optimal) more than they chose the other 3 options. Lesioned rats chose hole 3 more frequently than sham-operated rats on post-lesion days 1, 4 and 5 and chose hole 1 less frequently than sham-operated rats on post-lesion day 3. There was no effects of either condition or day of testing on the % Omissions (C), the % Premature responses (D), or the number of magazine entries made (E). *P < 0.05 sham-operated vs lesion; #P < 0.05 lesion compared to baseline; ^P < 0.05 both groups compared to baseline; ‡P < 0.5, sham-operated vs lesion hole 1; †P < 0.05 sham-operated vs lesion hole 3; ∞P < 0.05 hole 2 vs all other holes.
When the % choice of each hole were analyzed there was a significant effect of hole (F(3, 48)= 181.25, P < 0.01; see Figure 2B) and a significant hole X day interaction (F(15, 240) = 1.89, P < 0.05). On all days, rats chose hole 2 more frequently than they chose all other holes (all P < 0.05); an effect that did not differ across days (all P > 0.05). Furthermore, there was a trend for a three-way condition X hole X day interaction (F(15,240) = 1.67, P = 0.06). Post-hoc analyses revealed that lesioned rats chose hole 3 more frequently than sham-operated rats on post-lesion days 1, 4 and 5 (all P ≤ 0.05) and lesioned rats chose hole 1 less frequently than sham-operated rats on post-lesion day 3 (P = 0.05). All other main effects and interactions were not significant (all F < 1.56, all P > 0.05).
Day of testing significantly affected % omissions (F(5, 80) = 3.86, P < 0.01; see Figure 2C). Post-hoc analyses did not reveal that the % omissions were different between any two days (all P > 0.05). There was no effect of condition or a condition X day interaction (both F < 1.17, both P > 0.05).
There were no significant effects or interactions for % premature responses (all F < 1.06, P > 0.05; see Figure 2D).
There was a significant effect of day for the number of magazine entries (F(5, 80) = 3.06, P < 0.05; Figure 2E). However, the number of magazine entries made on any given day did not differ from those made on any other day (all P > 0.05). There was no effect of condition nor a condition X day interaction (both F < 0.05, both P > 0.05).
3.3 Effects of SCH23390 administration on decision-making
Following SCH23390 administration there was a main effect of condition (F(1,16) = 4.32, P=0.05; see Figure 3A) whereby sham-operated rats had higher % advantageous responses than lesion rats. In addition, there was a significant SCH23390 X condition interaction (F(2,32) = 3.57, P < 0.05). Post-hoc analysis on the interaction revealed that lesioned-operated rats made fewer advantageous responses than did sham-operated rats after 0.0 mg/kg SCH23390 (P < 0.05), but not after either 0.03 or 0.06 mg/kg SCH23390 (both P > 0.05). In addition, lesioned rats made more advantageous responses following 0.03 mg/kg than they did following 0.0 mg/kg SCH23390 (P < 0.05). There was no effect of SCH23390 on sham-operated rats (F(2,32) = 0.63, P > 0.05).
Figure 3.
Effects of SCH23390 on choice behavior in the rodent gambling task. A) Lesioned rats chose the advantageous holes less frequently than sham-operated rats; this effect was attenuated by SCH23390 administration. B) All rats chose hole 2 more frequently than all other holes. In addition sham-operated rats chose hole 2 more frequently than lesioned rats. SCH23390 did not affect hole choice. C) SCH23390 increased the % omissions in lesioned, but not sham-operated, rats. D) SCH23390 administration significantly decreased the % premature responding; this effect was potentiated in lesioned rats. E) SCH23390 administration significantly reduced the number of magazine entries. *P < 0.05 sham-operated vs lesion; #P < 0.05 lesion compared to baseline; ^P < 0.05 both groups compared to baseline; ∞P < 0.05 hole 2 vs all other holes.
When the % choice of each hole were analyzed there was a significant main effect of hole (F(3,48) = 131.48, P < 0.01; see Figure 3B) and a significant condition X hole interaction (F(3,48) = 3.28, P < 0.05). Sham-operated rats chose hole 2 more frequently and tended to choose hole 3 less frequently than lesioned rats (P < 0.05 and P = 0.07, respectively). Both groups responded more in hole 2 than they did in any other hole (both P < 0.05). SCH23390 did not affect % choice of each hole nor were there any drug by condition interactions (all F < 1.76, all P > 0.05).
SCH23390 administration dose-dependently affected % omissions (F(2, 32) = 17.34, P < 0.01; see Figure 3C). In addition there was a main effect of condition (F(1, 16) = 7.69, P < 0.05) and a condition X dose interaction (F(2, 32) = 3.37, P < 0.05). Regardless of dose, lesioned rats omitted more trials after SCH23390 administration than after vehicle administration (both P < 0.05). In addition, lesioned rats omitted more trials than sham-operated rats after both 0.03 and 0.06 mg/kg SCH23390 administration (both P < 0.05). Importantly, SCH23390 did not affect the % omissions in sham-operated rats (both P > 0.05).
SCH23390 administration significantly affected % premature responses (F(2,32) = 8.13, P < 0.01; see Figure 3D). In addition, there was a trend for SCH23390 administration to affect premature responding differently in sham-operated and lesioned rats (F(2,32) = 3.02, P = 0.06). Regardless of condition, rats made fewer premature responses after either 0.03 mg/kg or 0.06 mg/kg SCH23390 than they did after 0.0 mg/kg SCH23390 (both P < 0.05). Analysis of the dose X condition interaction revealed that the effect of dose was primarily due to the effect of SCH23390 administration in lesioned rats. Lesioned rats made fewer premature responses following 0.06 mg/kg than they did following 0.0 mg/kg SCH23390. Moreover, following administration of 0.06 mg/kg SCH23390 lesion rats made fewer premature responses than did sham-operated rats. There was no effect of condition on the % premature responses made (F(1,16) = 2.23, P > 0.05).
SCH23390 administration significantly affected the number of magazine entries made (F(2,32) = 5.73, P < 0.01; see Figure 3E). Rats made fewer magazine entries after 0.06 mg/kg SCH23390 than they did after 0.0 mg/kg SCH23390 (P < 0.05). There was no effect of condition nor was there a condition X dose interaction (both F < 2.51, both P > 0.05).
3.4 Effects of haloperidol administration on decision-making
Following haloperidol administration lesioned rats made fewer advantageous responses than did sham-operated rats (F(1,15) = 7.33, P < 0.05; see Figure 4A), but the % advantageous responses was not affected by the dose of haloperidol nor was there a condition X dose interaction (both F < 1.0, both P > 0.05).
Figure 4.
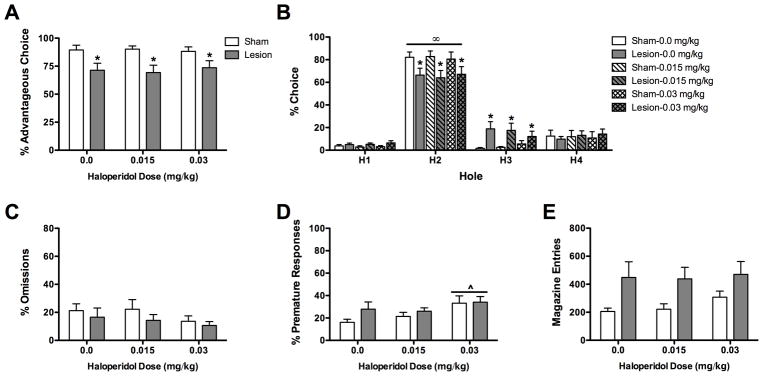
Effects of haloperidol on choice behavior in the rodent gambling task. A) Lesioned rats chose the advantageous holes less frequently than sham-operated rats; this effect was not affected by haloperidol administration. B) All rats chose hole 2 more than all other holes. In addition sham-operated rats chose hole 2 more frequently and chose hole 3 less frequently than lesioned rats. Haloperidol did not affect hole choice. C) Haloperidol did not affect % omissions. D) Haloperidol (0.03 mg/kg) increased the % premature responding. E) There was a tendency for lesioned rats to make more magazine entries than sham-operated rats; this was not affected by haloperidol administration. *P < 0.05 sham-operated vs lesion; #P < 0.05 lesion compared to baseline; ^P < 0.05 both groups compared to baseline; ∞P < 0.05 hole 2 vs all other holes.
When the % choice of each hole were analyzed there was a significant % choice X condition interaction (F(3,45) = 6.27, P < 0.01; see Figure 4B). Lesioned rats chose hole 2 less frequently and hole 3 more frequently than sham-operated rats (both P < 0.05). Both groups chose hole 2 more than all other options (all P < 0.01). Haloperidol dose and all interactions involving haloperidol were not significant (all F < 1.25, all P > 0.05).
The % omissions committed were not affected by either haloperidol dose or condition (all F < 2.48, P > 0.05; see Figure 4C).
Haloperidol significantly affected the % premature responses committed (F(2,30) = 6.21, P < 0.01; see Figure 4D); rats made more premature responses following 0.03 mg/kg than they did following 0.0 mg/kg haloperidol (P < 0.05). There was no effect of condition nor was there a condition X dose interaction (both F < 1.20, both P > 0.05).
Haloperidol significantly affected the number of magazine entries made (F(2,30) = 3.70, P < 0.05; see Figure 4E); however there were no significant differences between doses (all P > 0.05). In addition, there was a trend for lesioned animals to make more magazine entries than sham-operated rats (F(1,15) = 3.99, P = 0.06). There was however no condition X dose interaction (F(2, 30)= 1.27, P > 0.05).
4.0 Discussion
The current experiment investigated the effects of medial PFC lesions on decision-making behavior in the rGT in well-trained rats. After receiving a lesion of the medial PFC, rats gradually shifted their choices from the most advantageous options to the disadvantageous options. Moreover, optimal decision-making was restored by administration of the D1-like receptor antagonist SCH23390, but not by administration of the D2-like receptor antagonist haloperidol. Neither SCH23390 nor haloperidol, at the doses used in the current experiment, affected decision-making when administered to sham-operated control rats.
4.1 Decision-making on the rodent gambling task
Rodent gambling tasks, like their human counterparts such as the IGT, require rats develop an optimal decision-making strategy based upon the expected reward value of each choice option. In the rGT the expected value of the reward can be determined by weighing the probability of attaining reward in any particular trial against the magnitude of the reward for that option. The optimal decision-making strategy is that which results in the maximum number of rewards over the entire session, rather than the option that results in the maximum number of rewards in any particular trial. By this definition, the optimal choice in our version of the rGT is hole 2; exclusive choice of holes 1, 3 and 4 result in fewer cumulative pellets over the course of the session compared to exclusive choice of hole 2 (see [15]). “Advantageous” decision-making is defined as responding in either of the two holes that lead to the greatest cumulative rewards (i.e., holes 1 and 2) over the session. Due to the relatively short duration and relatively low probability of punishment, the choice of options that result in the smallest rewards on any given trial lead to the greatest net reward over the entire session. Importantly, rats used in the current experiment were able to develop stable optimal decision-making strategies: they chose hole 2 more than the other three holes and displayed more advantageous responses than disadvantageous responses.
4.2 Effects of medial PFC lesions on decision-making
We found that large lesions of the medial PFC, which included both the ventral (infralimbic) and dorsal (prelimbic) portions, impaired stable decision-making in the rGT. Rats with medial PFC lesions chose the advantageous options (holes 1 and 2) less frequently than they did prior to surgery and less frequently than did sham-operated control rats. Initially the decrease in advantageous responding was caused by lesioned animals increasing their choice of hole 3 (a disadvantageous response); in later testing lesioned rats also decreased their choice of hole 2 (an advantageous response). Combined, this strategy results in fewer rewards being obtained over the course of the session. These data add to a growing body of evidence finding impaired decision-making following damage to the medial PFC. Indeed, either excitotoxic lesions or temporary inactivation of the medial PFC impairs the acquisition of an optimal decision-making strategy [18, 25]. To date, it is unclear whether the impairment in decision-making resulted primarily from damage to the prelimbic or infralimbic cortex. Although the damage to the prelimbic cortex has been found to impair decision-making [18, 25], it is not clear whether damage to the ventral portions of the medial PFC (i.e., infralimbic cortex) affect decision-making [17, 18]. Nevertheless, our data are consistent from the clinical literature whereby people with damage to the dorsolateral prefrontal cortex, analogous to the rodent medial PFC, perform suboptimally on the IGT [14].
The lesion-induced change in decision-making occurred gradually over the course of several days suggesting that the change in decision-making was not simply due to a failure to remember the contingencies associated with each response hole. Prior to surgery, rats chose the advantageous options on approximately 90% of the trials. Following surgery, lesioned rats gradually sampled more from the disadvantageous options. Increased choice of the disadvantageous responses may have occurred either because lesioned rats were more sensitive to the size of the reward or because they were less sensitive to the punishment (i.e., they were more “risky”) associated with these options. To our knowledge there is no evidence that rats with medial PFC lesions exhibit an enhanced preference for large rewards over small rewards relative to sham-operated rats. Rather, in a delayed reinforcement task, rats with medial PFC lesions exhibited a reduced preference for large rewards when the delay to the large reward was 0 sec compared to sham-operated control rats [26]. Similarly, in a sustained response task, rats with medial PFC lesions exhibited a reduced preference for large rewards compared to sham-operated control rats [27]. Both of these results suggest that medial PFC lesions may actually cause a preference for small rewards over large rewards, a result opposite of that observed in the current experiment. Furthermore, reduced preference for large rewards in the lesioned rats cannot be explained by an inability to distinguish between reward magnitudes [28]. Combined, these data indicate that an enhanced sensitivity or motivation to obtain the large reward is unlikely to explain the shift in decision-making strategy observed following medial PFC lesions.
Alternatively, rats may have increased responding for the disadvantageous options (options 3 and 4) because they were less sensitive to the negative consequences associated with these options, namely the increased risk of obtaining no reward at all. There is limited evidence that rats in which the medial PFC had been temporarily inactivated or lesioned exhibit enhanced risk taking behavior [28, 29], in some, but not all tasks of “risky-decision making” [28]. These data suggest that a reduced sensitivity to the probabilistic nature of the reward may have contributed to the shift in decision-making strategy observed by rats with medial PFC lesions. That is, lesioned rats may not have been as sensitive to the duration and/or frequency of the punishment, and thus may have chosen the larger reward on more trials.
4.3 Ability of Dopamine Receptor Antagonists to Attenuate Decision-Making Deficits
Large medial PFC lesions (including both prelimbic and infralimbic cortex) can affect dopamine signaling, particularly in the striatum. Indeed there is evidence of increased DA release [20, but see 30], increased D2 receptor expression [21] and increased D1 receptor sensitivity [22] in the striatum following either ablative or excitotoxic lesions of the medial PFC. Moreover, both the prelimbic cortex and infralimbic cortex project to the ventral tegmental area [31]. Thus, we sought to determine if administration of either a D1-like receptor antagonist, SCH23390, or a D2-like receptor antagonist, haloperidol, could attenuate the decision-making deficit observed following medial PFC lesions.
4.3.1 Effects of SCH23390 administration on decision-making
Enhanced D1 receptor sensitivity likely contributed to the decision-making deficit observed following medial PFC lesions. Consistent with a previous report [15], D1 receptor antagonism did not affect decision-making in sham-operated control rats. In contrast, D1 receptor antagonism significantly improved decision-making in lesioned rats. Following administration of SCH23390 the % advantageous choices made by lesioned rats was not significantly different from sham-operated controls rats. In addition, the low dose of SCH23390 significantly increased the % advantageous responses lesioned rats made relative to administration of vehicle; SCH23390 did not affect % advantageous responses in sham-operated rats. Surprisingly however, SCH23390 administration did not significantly increase lesioned rats’ choice of the most optimal hole (hole 2). Instead the increase in % advantageous responses appears to result from small, but not significant, decreases in the choice of the least optimal holes (holes 3 and 4) and a small, but not significant, increase in the least ‘risky’ hole (hole 1). This may suggest that administration of SCH23390 increased advantageous decision-making by causing lesioned rats to be more risk averse. Consistent with this hypothesis SCH23390 administration caused rats to be risk averse in a probabilistic reinforcement task [32].
Other rGT performance measures also suggest that lesioned rats exhibited enhanced D1 receptor number or sensitivity relative to sham-operated rats. While sham-operated rats treated with SCH23390 exhibited slightly increased omissions and decreased premature responses, these effects were potentiated in the lesioned rats. Combined this pattern of performance deficits suggests that lesioned rats were also more sensitive to the locomotor impairing effects of D1 receptor antagonism [33] thereby providing additional evidence that D1 receptors signaling was enhanced following the lesion [22]. Because responding in each of the apertures requires the same motor response, it is unlikely that the SCH23390-induced motor deficit contributed to the improvement in decision-making. That is, lesioned rats likely exhibited improved decision-making following SCH23390 administration despite displaying impaired locomotion.
4.3.2 Effects of haloperidol administration on decision-making
The results from the current experiment suggest that upregulation of D2-like receptor signaling does not contribute to the decision-making impairment observed following medial PFC lesions. Not only did the D2-like receptor antagonist haloperidol fail to affect decision-making in both sham-operated and lesioned rats, there was no evidence that it differentially affected any performance measure in the two groups. Indeed, the only measure affected by haloperidol administration was premature responses; this measure was affected equally in both groups. It is possible that we did not use a high enough dose of haloperidol to observe a change in decision-making; problematically, higher doses of haloperidol are known to cause performance deficits in an attention task [34] and induced catalepsy [35]. Moreover, if lesioned rats exhibited an upregulation of D2 receptors [21] they would be expected to display cognitive enhancement and/or motor impairments at lower doses of haloperidol than would sham-operated rats (as was observed with SCH23390). Because we did not observe enhanced sensitivity haloperidol following medial PFC lesions, we speculate that D2 receptors were unaltered by the lesion and thereby did not contribute to the decision-making deficit observed in the current experiment.
We were surprised by the lack of effect of haloperidol on decision-making in the current experiment because the D2 receptor antagonist eticlopride has been found to improve decision-making in both the rodent gambling task [15] and a probabilistic reinforcement task [32]. The discrepancy in the effects of eticlopride and haloperidol may lie in their affinities for different D2-like receptor subtypes; eticlopride is much more selective for the D2 receptor than is haloperidol [36]. Indeed, haloperidol has affinity for D3 and D4 receptors in addition to D2 receptors [37], while eticlopride has marginal affinity for these receptors [36]. In another decision-making task the effect of D3 receptor stimulation was opposite that of D2 receptor stimulation [32]. Thus, haloperidol may not affect decision-making in this task because it binds to both D2 and D3 receptors; these receptors appear to have opposite effects on decision-making.
4.4 Summary and Conclusions
In summary, we observed that excitotoxic lesions of the medial PFC caused impairments in decision-making in rats that displayed a stable pattern of optimal decision-making prior to undergoing surgery. Moreover, it is likely that the impairment in decision-making was mediated, at least in part, by increased sensitivity or upregulation of dopamine D1 receptors; the D1-like receptor antagonist SCH23390 restored optimal decision-making in lesioned rats. Consistent with previous findings SCH23390 did not affect decision-making in sham-operated control rats. The D2-like receptor antagonist haloperidol did not affect decision-making in either sham-operated or lesioned rats.
These data suggest that the deficits in medial PFC function and concomitant changes in dopamine signaling may be contributing to the decision-making deficits observed in a number of psychiatric illnesses. Indeed, schizophrenia [38], ADHD [2], substance abuse [3], and Parkinson’s Diseases [5] have all be associated with abnormalities in cortical functioning and dopamine signaling. Thus, treatments that address these pathologies may help to normalize the suboptimal decision-making strategies seen amongst psychiatric populations.
Highlights.
Lesions of the medial prefrontal cortex impaired stable decision-making
The D1 receptor antagonist SCH23390 reversed the lesion-induced decision-making deficit.
The D2-like receptor antagonist haloperidol did not affect decision-making.
Acknowledgments
Funded by a NARSAD Young Investigator Award and NIH R15MH098246 to TAP. The authors would like to thank Cassie Burley, Abigail Lofchie, Avery O’Hara and Kristina Welch for their technical assistance.
5.0 References
- 1.Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cognitive, Affective and Behavioral Neuroscience. 2008;8:375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- 2.Sonuga-Barke EJ, Fairchild G. Neuroeconomics of attention-deficit/hyperactivity disorder: differential influences of medial, dorsal, and ventral prefrontal brain networks on suboptimal decision making? Biol Psychiatry. 2012;72:126–133. doi: 10.1016/j.biopsych.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher DA, O’Sullivan SS, Evans AH, Lees AJ, Schrag A. Pathological gambling in Parkinson’s Disease: risk factors and differences from dopamine dysregulation. An analysis of published case series. Movement Disorders. 2007;22:1757–1763. doi: 10.1002/mds.21611. [DOI] [PubMed] [Google Scholar]
- 5.Voon V, Mehta AR, Hallet M. Impulse control disorders in Parkinson’s disease: recent advances. Current Opinion in Neurology. 2011;24:324–330. doi: 10.1097/WCO.0b013e3283489687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray MJ, Miyasaki JM, Zurowski M, Ko JH, Cho SS, Pellecchia G, Antonelli F, Houle S, Lang AE, Strafella AP. Extrastriatal dopaminergic abnormalities of DA homeostasis in Parkinson’s patients with medication-indcued pathological gambling: a [11C] FLB-457 and PET study. Neurobiology of Disease. 2012;48:519–525. doi: 10.1016/j.nbd.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand M, Kalbe E, Labudda K, Fujiwara E, Kessler J, Markowitsch HJ. Decision-making impairments in patients with pathological gambling. Psychiatry Res. 2005;133:91–99. doi: 10.1016/j.psychres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Cavedini P, Riboldi G, Keller R, D’Annucci A, Bellodi L. Frontal lobe dysfunction in pathological gambling patients. Biol Psychiatry. 2002;51:334–341. doi: 10.1016/s0006-3223(01)01227-6. [DOI] [PubMed] [Google Scholar]
- 9.Struglia F, Stratta P, Gianfelice D, Pacifico R, Riccardi I, Rossi A. Decision-making impairment in schizophrenia: Relationships with positive symptomatology. Neuroscience Letters. 2011;502:80–83. doi: 10.1016/j.neulet.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Ritter LM, Meador-Woodruff JH, Dalack GW. Neurocognitive measures of prefrontal cortical dysfunction in schizophrenia. Schizophrenia Research. 2004;68:65–73. doi: 10.1016/S0920-9964(03)00086-0. [DOI] [PubMed] [Google Scholar]
- 11.Beninger RJ, Wasserman J, Zanibbi K, Charbonneau D, Mangels J, Beninger BV. Typical and atypical antipsychotic medications differentially affect two nondeclarative memory tasks in schizophrenic patients: a double dissociation. Schizophrenia Research. 2003;61:281–292. doi: 10.1016/s0920-9964(02)00315-8. [DOI] [PubMed] [Google Scholar]
- 12.Yip SW, Sacco KA, George TP, Potenza MN. Risk/reward decision-making in schizophrenia: a preliminary examination of the influence of tobacco smoking and relationship to Wisconsin Card Sorting Task performance. Schizophrenia Research. 2009;110:156–164. doi: 10.1016/j.schres.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cerebral Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- 15.Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34:2329–2343. doi: 10.1038/npp.2009.62. [DOI] [PubMed] [Google Scholar]
- 16.Rivalan M, Ahmed SH, Dellu-Hagedorn F. Risk-prone individuals prefer the wrong options on a rat version of the Iowa Gambling Task. Biological Psychiatry. 2009;66:743–749. doi: 10.1016/j.biopsych.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Zeeb FD, Winstanley CA. Lesions of the basolateral amygdala and orbitofrontal cortex differentially affect acquisition and performance of a rodent gambling task. The Journal of Neuroscience. 2011;31:2197–2204. doi: 10.1523/JNEUROSCI.5597-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivalan M, Coutureau E, Fitoussi A, Dellu-Hagedom Inter-individual decision-making differences in the effects of cingulated, orbitofrontal, and prelimbic cortex lesions in a rat gambling task. Frontiers in Behavioral Neuroscience. 2011;5:22. doi: 10.3389/fnbeh.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baardense PJJ, Winstanely CA, Vanderscheren LJMJ. Simultaneous blockade of dopamine and noradrenaline reuptake promotes disadvantageous decision making in a rat gambling task. Psychopharmacology (Berlin) 2012 doi: 10.1007/s00213-012-2857-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaskiw GE, Karoum F, Freed WJ, Phillips I, Kleinman JE, Weinberger DR. Effect of ibotenic acid lesions of the medial prefrontal cortex on amphetamine-induced locomotion and regional brain catecholamine concentrations in the rat. Brain Res. 1990;534:263–272. doi: 10.1016/0006-8993(90)90138-2. [DOI] [PubMed] [Google Scholar]
- 21.Baca SM, Lipska BK, Egan MF, Bachus SE, Ferguson JN, Hyde TM. Effects of prefrontal cortical lesions on neuropeptide and dopamine receptor gene expression in the striatum-accumbens complex. Brain Res. 1998;797:55–64. doi: 10.1016/s0006-8993(98)00343-6. [DOI] [PubMed] [Google Scholar]
- 22.Reibaud M, Blanc G, Studler JM, Glowinski J, Tassin JP. Non-DA prefronto-cortical efferents modulate D1 receptors in the nucleus accumbens. Brain Res. 1984;305:43–50. doi: 10.1016/0006-8993(84)91117-x. [DOI] [PubMed] [Google Scholar]
- 23.National Academy Press. Guide for the care and use of laboratory animals. 8. National Academy Press; Washington, DC: 2011. [Google Scholar]
- 24.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic Press; Orlando, FL: 2009. [DOI] [PubMed] [Google Scholar]
- 25.de Visser L, Baars AM, van’t Klooster J, van den Bos R. Transient inactivation of the medial prefrontal cortex affects both anxiety and decision-making in male Wistar rats. Frontiers in Neuroscience. 2011;5:102. doi: 10.3389/fnins.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 27.Gill TM, Castaneda PJ, Janak PH. Dissociable roles of the medial prefrontal cortex and nucleus accumbens core in goal-directed actions for different reward magnitudes. Cerebral Cortex. 2010;20:2884–2899. doi: 10.1093/cercor/bhq036. [DOI] [PubMed] [Google Scholar]
- 28.St Onge JR, Floresco SB. Prefrontal cortical contribution to risk-based decision making. Cereb Cortex. 2010;20:1816–1828. doi: 10.1093/cercor/bhp250. [DOI] [PubMed] [Google Scholar]
- 29.Jentsch JD, Woods JA, Groman SM, Seu E. Behavioral characteristics and neural mechanisms mediating performance in a rodent version of the Balloon Analog Risk Task. Neuropsychopharmacology. 2010;35:1797–1806. doi: 10.1038/npp.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalley JW, Thomas KL, Howes SR, Tsai TH, Aparicio-Legarza MI, Reynolds GP, Everitt BJ, Robbins TW. Effects of excitotoxic lesions of the rat prefrontal cortex on CREB regulation and presynaptic markers of dopamine and amino acid function in the nucleus accumbens. Eur J Neurosci. 1999;11:1265–1274. doi: 10.1046/j.1460-9568.1999.00532.x. [DOI] [PubMed] [Google Scholar]
- 31.Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34:681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- 33.Meyer ME, Cottrell GA, Van Hartesveldt C, Potter TJ. Effects of dopamine D1 antagonists SCH23390 and SK&F83566 on locomotor activities in rats. Pharmacol Biochem Behav. 1993;44:429–32. doi: 10.1016/0091-3057(93)90486-d. [DOI] [PubMed] [Google Scholar]
- 34.Paine TA, Carlezon WA., Jr Effects of antipsychotic drugs on MK-801-induced attentional and motivational deficits. Neuropharmacology. 2009;56:788–797. doi: 10.1016/j.neuropharm.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perrault G, Depoortere R, Morel E, Sanger DJ, Scatton B. Psychopharmacological profile of amisulpride: an antipsychotic drug with presynaptic D2/D3 dopamine receptor antagonist activity and limbic selectivity. J Pharmacol Exp Ther. 1997;280:73–82. [PubMed] [Google Scholar]
- 36.Martelle JL, Nader MA. A review of the discovery, pharmacological characterization, and behavioral effects of the dopamine D2-like receptor antagonist eticlopride. CNS Neurosci Ther. 2008;14:248–262. doi: 10.1111/j.1755-5949.2008.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seeman P, Van Tol HHM. Dopamine receptor pharmacology. Trends in Pharmacological Sciences. 1994;15:264–270. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 38.Howes OD, Kapur S. The dopamine hypothesis of schizophenia: Version III—The final common pathway. Schizophrenia Bulletin. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]