Abstract
Background/Aims
Alanine aminotransferase (ALT) and γ-glutamyltransferase (GGT) are widely used markers of liver disease. Several population-based cohort studies have found associations of these liver enzymes with all-cause mortality. None of these studies controlled for genetic variation as well as fetal and early life exposure, whether environmental or genetic.
Methods
We studied the associations of ALT and GGT with all-cause mortality using data for 686 twins (73–94 years old) included in the Longitudinal Study of Aging Danish Twins.
Results
An increase in 1 logged U/L of GGT was associated with a 15% increase in the hazard ratio (HR) for mortality [95% confidence interval (CI) 0.99, 1.32] but there was no strong evidence of an association of ALT with all-cause mortality (HR = 1.07, 95% CI 0.82, 1.40) when controlling for potential confounders. In this analysis, the study population was treated as individuals, with similarities between twins accounted for by using robust standard errors. However, an intrapair analysis in which the proportion of twin pairs in which the twin with the higher level of ALT or GGT died first was compared with 50% (expected under the null hypothesis), found no strong evidence that higher ALT or GGT was associated with earlier death within twin pairs; the results were consistent in both monozygotic and dizygotic twins.
Conclusions
γ-glutamyltransferase but not ALT predicts mortality among older Danish twins when using traditional methods for controlling for potential confounders and existing diabetes and cardiovascular disease. Environmental developmental origins may explain the association, but larger twin studies are required to replicate our findings.
Keywords: alanine aminotransferase, γ glutamyltransferase, mortality, twin studies
Alanine aminotransferase (ALT) and γ-glutamyltransferase (GGT) are widely used markers of liver disease. Limited evidence suggests that ALT is the liver enzyme most closely associated with liver fat content and non-alcoholic fatty liver disease (NAFLD) (1). γ-Glutamyltransferase has long been considered a marker of excessive alcohol intake and more recently has been suggested to reflect oxidative stress (2) and exposure to environmental pollutants (3), as well as NAFLD. Consistent evidence shows that both ALT and GGT are positively associated with the metabolic syndrome (4) and predict incident diabetes (5). γ-Glutamyltransferase also predicts incident coronary heart disease (CHD) and stroke above and beyond established risk factors (6); fewer studies have assessed ALT as a determinant of incident CHD and stroke than GGT, but current evidence suggests a weaker association for ALT than for GGT with CHD and stroke risk. As cardiovascular diseases are a leading cause of mortality, it would be reasonable to hypothesize that liver enzymes predict all-cause mortality.
Several population-based cohort studies have examined the association of these liver enzymes with all-cause mortality. Recent analysis of the NHANES-III found that the hazard ratio (HR) among participants with unexplained elevation of ALT (attributed to NAFLD) was 1.37 [95% confidence interval (CI) 0.98, 1.91] compared with other participants (7). Alanine aminotransferase also predicted mortality among participants with a body mass index (BMI) < median (22.7 kg/m2) in a Japanese cohort, but not among participants with a BMI greater than the median (8). Two additional cohort studies found that GGT, but not ALT, predicts all-cause mortality (9, 10). Finally, associations of GGT with all-cause mortality have been found in several prospective cohort studies (11–13). None of these studies, however, controlled for genetic variation as well as fetal and early life exposure, whether genetic or environmental. This may be of consequence because evidence suggests that both genetics (14) and exposures in utero (15), as reflected by birth weight and pre-pubertal exposures, as reflected by leg length (16), are associated with adults levels of ALT and GGT.
Here, we studied the associations of ALT and GGT with all-cause mortality using data for twins included in the Danish Twin Registry. The advantage of using twin data is that intra-uterine exposures (genetic and environmental) and shared environmental exposures in early life are inherently controlled for in monozygotic twins, and partially in dizygotic twins (for genetic exposures). We also control for adult lifestyle factors and examine whether potential associations of liver enzymes with mortality can be attributed to pre-existing diabetes and/or cardiovascular diseases.
Methods
The sample was taken from participants in the Longitudinal Study of Aging Danish Twins (LSADT), a cohort sequential study of elderly Danish twins. Longitudinal Study of Aging Danish Twins began in 1995 with an assessment of all members of same-sex twin pairs born in Denmark before 1920 and thus at least 75 years old at the beginning of 1995. This initial cohort was followed up in 1997, 1999, 2001, 2003 and 2005. Additional cohorts were added in 1997, 1999 and 2001 and subsequently followed at 2-year intervals. The rate of participation has been high over the multiple waves of LSADT, with participation rates generally falling between 70 and 80%. The Danish Civil registration system allows a complete follow-up of all participants (17). During the 1997 survey (14), 689 twins provided full blood samples. Participants were 73–94 years old at baseline. Alanine aminotransferase and GGT were available for 686 participants, of whom 604 comprised 302 complete twin pairs and an additional 82 twins were included without their twin sib.
Alanine aminotransferase and GGT were measured using the Technicon AXON® System (Bayer Diagnostics, Tarrytown, NY, USA) described previously (14). Information on height and weight, used to determine BMI, smoking, alcohol consumption, physical activity, social class and existing diabetes and cardiovascular disease (CVD) were self-reported. Smoking was categorized as never, ex-smoker and current smoker. Alcohol consumption was categorized as abstainers and thirds of the alcohol consumption distribution. Physical activity was categorized as none, participating in light exercise (such as gardening, short walks and bicycle rides) and heavy exercise (such as heavy gardening, long walks, bicycle rides, dancing, sports and gymnastics). Social class was determined using a validated classification tool in which the leading determinant is one’s position within the work environment (18).
Date of death was obtained from the Danish civil registration system, which records the date of death of all Danish persons residing in Denmark. Mortality data were available up to 27 November 2008. This research was approved by the scientific ethical committee for Vejle and Funen counties, and all participants provided written informed consent.
Statistical analysis
The distributions of ALT and GGT were skewed and were subsequently log transformed. The associations between thirds of ALT and GGT and potential confounders of their association with mortality were studied using linear and logistic regression, as appropriate. Survival analysis using Cox’s proportional hazards models was conducted in order to study the relation between each liver enzyme and mortality. In these models, participants’ age was the time axis and risk was assessed from the date of the blood test to the date of death or 27 November 2008 in those who survived to the end of this follow-up. We estimated HRs per change in 1 logged unit of ALT and GGT. All twins, regardless of whether their twin sib was included in the study, were included in these analyses. Because observation within twin pairs might be correlated, the analyses were performed using the robust estimator of variance, assuming independence between pairs.
To exploit the use of within-twin comparisons to control for intrauterine and childhood exposures, we computed the proportion of times the co-twin with the higher level of ALT died first and repeated the same analysis for GGT (separately). We compared these proportions with the null hypothesis of equality (50%/50%), using the standard binomial test.15 This analysis was limited to the subgroup of same-sex complete twin pairs in which at least one twin had died (N= 224). It was then repeated for twin pairs with a difference of 10 U/L or more of ALT (N= 61) or GGT (N= 109), separately. All analyses were conducted using STATA version 10.1 (2008; Stata Corporation, College Station, TX, USA).
Results
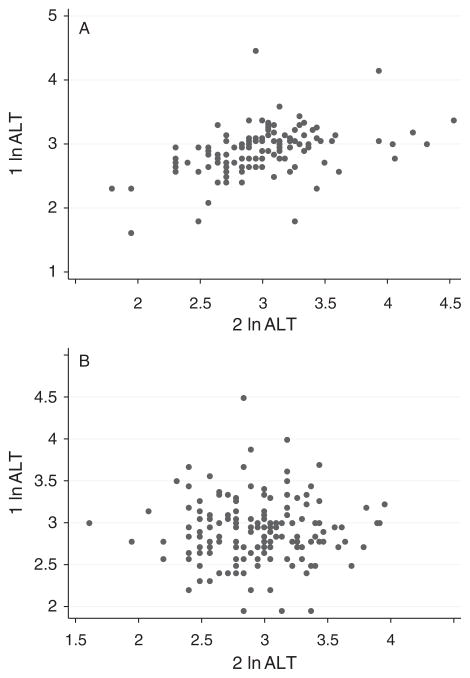
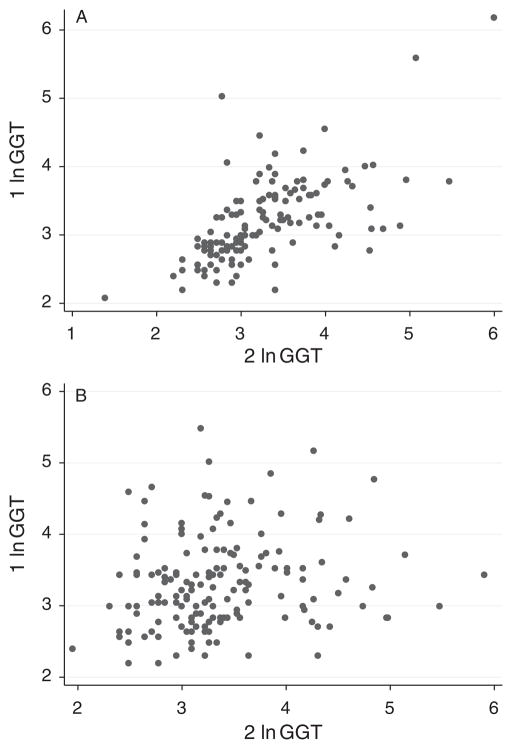
The characteristics of the 686 twins included in this study by sex are presented in Table 1. Of the 686 twins, 604 comprised 302 complete twin pairs, of whom 289 twin pairs were same-sex twin pairs (130 monozygotic and 159 dizygotic). The scatter plots of ALT and GGT (separately) within same-sex twin pairs for monozygotic and dizygotic twins are presented in Figures 1 and 2 respectively. Both ALT and GGT showed higher but still modest correlations among monozygotic twins (ALT r = 0.44, GGT r = 0.62) compared with dizygotic twins (ALT r = 0.02, GGT r = 0.15).
Table 1.
Participants’ characteristics by sex
| Men (n = 232) | Women (n = 454) | |
|---|---|---|
| Age, mean (SD) | 78.8 (3.9) | 79.4 (4.6) |
| ALT (U/L), geometric mean (IQR) | 19.3 (16.0–34.0) | 17.9 (14.0–23.0) |
| GGT (U/L), geometric mean (IQR) | 32.5 (22.0–44.0) | 25.2 (16.0–34.0) |
| Never smoked (%) | 18.2 | 48.0 |
| Low social class (%) | 19.4 | 15.1 |
| Most physically active (%) | 42.7 | 36.8 |
| Current alcohol consumers (%) | 90.9 | 81.5 |
| Lifelong abstainers from alcohol (%) | 1.3 | 2.4 |
| BMI (kg/m2), mean (SD) | 25.3 (3.0) | 23.7 (4.0) |
| Diabetes (%) | 8.1 | 4.6 |
| CVD (%) | 33.8 | 26.2 |
ALT, alanine aminotransferase; CVD, cardiovascular disease; GGT, γ-glutamyltransferase; IQR, inter-quartile range; SD, standard deviation.
Fig. 1.
Scatterplot of alanine aminotransferase for monzygotic (A) and dizygotoic (B) same-sex twin pairs: monozygotic twin pairs and dizygotic twin pairs.
Fig. 2.
Scatterplot of γ-glutamyltransferase for monzygotic (A) and dizygotoic (B) same-sex twin pairs: monozygotic twin pairs and dizygotic twin pairs.
The age-adjusted means or prevalences of participants’ characteristics across thirds of the ALT and GGT distributions are presented in Table 2. There was evidence that age and the likelyhood of belonging to a low social class decreased across increasing thirds of the ALT distribution. Body mass index, the probability of belonging to the highest category of physical activity and of not abstaining from alcohol increased across the ALT distribution. There was no strong evidence of associations of ALT with sex, smoking, lifelong abstinence from alcohol, the prevalence of diabetes or CVD in this population. The proportion of females and the prevalence of never smokers decreased across increasing thirds of the GGT distribution. Body mass index, and the prevalence of diabetes and CVD increased across increasing thirds of the GGT distribution. There was no strong evidence of associations of GGT with age, social class, physical activity and alcohol consumption.
Table 2.
Participant characteristics (mean or prevalence and 95% confidence interval) by thirds of alanine aminotransferase and γ-glutamyltransferase
| 1st third | 2nd third | 3rd third | P linear trend | |
|---|---|---|---|---|
| ALT | 5–16 U/L (n = 272) | 17–21 U/L (n = 216) | 22–93 U/L (n = 199) | |
| Age, mean | 79.7 (79.1, 80.3) | 79.2 (78.5, 79.8) | 78.5 (77.9, 79.0) | < 0.01 |
| Female (%) | 69.9 (63.2, 75.7) | 63.4 (55.9, 70.3) | 63.8 (55.8, 71.1) | 0.17 |
| Never smoked (%) | 37.9 (32.1, 44.0) | 36.3 (29.9, 43.2) | 39.7 (32.9, 46.9) | 0.74 |
| Low social class (%) | 19.8 (15.3, 25.2) | 15.9 (11.3, 21.8) | 12.6 (8.5, 18.2) | 0.04 |
| Most physically active (%) | 32.1 (26.7, 38.0) | 41.7 (35.0, 48.6) | 44.2 (37.3, 51.4) | < 0.01 |
| Current alcohol consumers (%) | 79.6 (74.0, 84.2) | 88.0 (82.3, 92.0) | 87.9 (82.6, 91.8) | 0.01 |
| Lifelong abstainers from alcohol (%) | 2.2 (0.1, 0.5) | 1.9 (0.1, 5.9) | 2.0 (0.1, 5.2) | 0.86 |
| BMI (kg/m2), mean | 23.8 (23.3, 24.3) | 24.1 (23.5, 24.6) | 25.2 (24.7, 25.8) | < 0.01 |
| Diabetes (%) | 4.4 (2.5, 7.6) | 7.4 (4.5, 12.0) | 6.0 (3.5, 10.3) | 0.36 |
| CVD (%) | 27.6 (21.7, 33.2) | 31.5 (25.6, 38.1) | 27.6 (21.7, 34.5) | 0.85 |
| GGT | 4–20 U/L (n = 260) | 21–32 U/L (n = 203) | 33–484 U/L (n = 223) | |
| Age (mean) | 79.6 (78.9, 80.3) | 78.7 (78.1, 79.3) | 79.1 (78.4, 79.7) | 0.21 |
| Female (%) | 80.8 (74.6, 85.7) | 59.6 (51.7, 67.0) | 55.2 (47.3, 62.8) | < 0.01 |
| Never smoked (%) | 46.5 (39.9, 53.3) | 31.8 (25.5, 39.0) | 33.0 (26.8, 40.0) | < 0.01 |
| Low social class (%) | 14.7 (10.7, 19.9) | 20.9 (15.5, 27.6) | 14.5 (10.5, 19.6) | 0.99 |
| Most physically active (%) | 40.0 (33.8, 46.5) | 41.4 (34.7, 48.4) | 34.2 (28.2, 40.9) | 0.22 |
| Current alcohol consumers (%) | 85.4 (79.9, 89.5) | 82.7 (76.8, 87.3) | 85.5 (79.6, 89.9) | 1.00 |
| Lifelong abstainers from alcohol (%) | 1.9 (0.1, 5.3) | 2.0 (0.1, 5.2) | 2.3 (0.1, 6.2) | 0.83 |
| BMI (kg/m2), mean | 23.7 (23.2, 24.2) | 24.5 (23.9, 25.0) | 24.7 (24.2, 25.2) | < 0.01 |
| Diabetes (%) | 4.2 (2.4, 7.5) | 5.4 (3.0, 9.5) | 8.1 (5.0, 12.7) | 0.09 |
| CVD (%) | 22.4 (17.3, 28.2) | 31.5 (25.4, 38.4) | 33.6 (27.6, 40.3) | < 0.01 |
ALT, alanine aminotransferase; BMI, body mass index; CVD, cardiovascular disease; GGT, γ-glutamyltransferase.
The results of the Cox proportional hazard models examining ALT and GGT as determinants of survival are presented in Table 3. There were 19 incident cases of diabetes during the follow-up period (till 31 December 2003; median follow-up: 8.8 years) and 186 of CVD. There was no strong evidence of an association of ALT with mortality. In comparison, GGT was positively associated with all-cause mortality even after controlling for potential confounders (Table 3, models 1–5).
Table 3.
Hazard ratios (95% confidence interval) for mortality per 1 logged unit of alanine aminotransferase or γ-glutamyltransferase
| Model 1 (n = 686) | Model 2 (n = 677) | Model 3 (n = 677) | Model 4 (n = 671) | Model 5 (n = 671) | |
|---|---|---|---|---|---|
| ALT | 0.88 (0.66, 1.16) | 1.09 (0.84, 1.42) | 1.08 (0.83, 1.41) | 1.10 (0.84, 1.45) | 1.07 (0.82, 1.40) |
| GGT | 1.20 (1.03, 1.39) | 1.19 (1.04, 1.38) | 1.19 (1.03, 1.37) | 1.21 (1.04, 1.40) | 1.15 (0.99, 1.32) |
Model 1, age, sex adjusted; Model 2, as in 1 plus smoking, socio-economic position and physical activity; Model 3, as in 2 plus alcohol consumption (abstainers and thirds of the alcohol consumption distribution); Model 4, as in 3 plus BMI; Model 5, as in 4 plus pre-existing diabetes and CVD. ALT, alanine aminotransferase; GGT, γ-glutamyltransferase.
We also examined whether twins with higher ALT and GGT (separately) had a shorter life span compared with their twin sib. The results of the intrapair analysis are presented in Table 4. Overall, we found no strong evidence that the twin with the higher ALT or GGT measurement died before their twin sib in all same-sex twin pairs or when stratifying for zygosity. When data were examined separately for monozygotic and dizygotic twins both sets of results were consistent with the null hypothesis (Table 4). When analyses were limited to twin pairs with a 10 U/L difference in ALT and GGT (separately), the results were not substantially different from those presented in Table 4, but sample sizes were small (N= 61 and 103 for ALT and GGT respectively).
Table 4.
Intrapair comparisons
| Proportion (N) in which the twin with higher GGT/ALT died first | 95% confidence interval | N of twin pairs | P-value | |
|---|---|---|---|---|
| All same-sex twin pairs | ||||
| ALT | 0.53 (122) | 0.47, 0.59 | 230 | 0.36 |
| GGT | 0.53 (123) | 0.47, 0.60 | 230 | 0.29 |
| Monozygotic twin pairs | ||||
| ALT | 0.53 (55) | 0.44, 0.63 | 103 | 0.49 |
| GGT | 0.50 (52) | 0.41, 0.60 | 103 | 0.92 |
| Dizygotic, same sex twin pairs | ||||
| ALT | 0.53 (67) | 0.44, 0.61 | 127 | 0.53 |
| GGT | 0.56 (71) | 0.47, 0.65 | 127 | 0.18 |
ALT, alanine aminotransferase; GGT, γ-glutamyltransferase.
Finally, we repeated the survival analysis (reported in Table 3) using data only for twin pairs who contributed to the intrapair analysis. The results were not substantially altered from those presented in Table 3, although confidence intervals were wider due to the reduced sample size.
Discussion
In this study of elderly Danish twins, we found strong evidence that an increase in 1 logged U/L of GGT was associated with a 15% increase in the HR for mortality (95% CI 0.99, 1.32) but there was no strong evidence of an association of ALT with all-cause mortality (HR = 1.07, 95% CI 0.82, 1.40) when controlling for a wide range of potential confounders. Similar findings have been reported previously (9, 10). In an intrapair analysis, we found no strong evidence that higher ALT or GGT was associated with earlier death within both monozygotic and dizygotic twin pairs.
One potential explanation for these findings is that the association of GGT with all-cause mortality (as presented in Table 3) is driven by characteristics that are shared by twin sibs such as genotype or early life environmental characteristics. These characteristics are more fully controlled for in the intrapair analysis (presented in Table 4) and this may explain why the association between GGT and mortality was not noted in the intrapair analysis.
A previous study of the same study population of elderly Danish twins estimated that substantial proportions (33 and 61%) of the variance of ALT and GGT could be explained by genetic factors (14). Recent genomewide association studies have identified genotypes associated with plasma levels of liver enzymes (19) and NAFLD (20), a leading determinant of ALT and GGT levels. While the null association within both monozygotic and dizygotic twins could be explained by genetic variation with pleiotropic effects that influence liver enzymes and survival, they argue somewhat more towards intrauterine and a shared early family environment as an explanation. This is because within dizygotic twin pair analyses, we do not fully control for genetic variation (dizygotic twins share 50% of their genetic variation) and therefore if this explained the association, we would expect the within monozygotic twin pairs estimate to be consistent with the null but the within dizygotic twin pairs estimate to be greater than the null value of 0.50. However, our sample size is small for these separate analyses and the most appropriate interpretation of our overall results would be to suggest that either genetic or environmental developmental origins explain the association of GGT with survival.
In a prospective cohort of older (60–79 years old) British women, birth weight and leg length, which are biomarkers of exposures affecting intra-uterine and pre pubertal growth, respectively, were inversely associated with adult levels of ALT and GGT (13, 18). These biomarkers and exposures could reflect influences that may be genetic or environmental in nature. Bathum et al. (14) estimated that non-shared environmental factors explain (the remaining) 67 and 39% of the ALT and GGT variance. Thus, it is plausible that both genetic and early life environmental characteristics result in elevated liver enzymes and an increased risk of premature mortality.
We believe that our results are probably relevant to the general population and not limited to twins alone. The geometric means of ALT and GGT in the British Women’s Heart and Health Study (older British women 60–70 years of age) were 12.8 and 22.8 U/L; the corresponding values in the Caerphilly Prospective Study (older Welsh men 55–65 years of age) were 22.0 and 31.9 U/L, comparable to the population means in this study (see Table 1).
The main strength of this study lies in its ability to adjust for common factors, whether genetic or early life environmental, in intrapair analyses. This approach allows for strong control of these early life characteristics, even when no data have been collected on them. However, due to the limited sample size and statistical power, we cannot rule out chance as an explanation for our findings in the intrapair analysis and studies of larger twin populations could shed further light on this matter. Yet, repeating the survival analysis while limiting the sample to those twins who contributed to the intrapair analysis did not substantially alter the results compared with those obtained for the larger sample. This suggests that the reduced sample size is not the reason for the null finding in the intrapair analysis. Further limitations are that information on prevalent disease at baseline was self-reported and did not include liver disease; that results may not be generalizable to younger populations; and that ALT and GGT were ascertained at a late age. Earlier measures may have greater predictive value. Finally, unfortunately, information on cause of death was not available and therefore associations of ALT and GGT with deaths from liver-related causes and CVD could not be studied, although both are major causes of mortality in the Danish population (21).
In conclusion, GGT but not ALT predicts mortality among older Danish twins when using traditional methods for controlling for potential confounders and existing diabetes and CVD. However, the association of GGT with mortality was not observed in an intrapair analysis but larger twin studies are required to replicate our findings.
Acknowledgments
We would like to thank Inge Petersen and Axel Skytthe (University of Southern Denmark) for their assistance with compiling the data. The study was supported by the US National Institute on Aging research grant, NIA-PO1-AG08761, and the VELUX Foundation. Abigail Fraser is funded by a UK Medical Research Council Fellowship, and Debbie Lawlor works in a Centre that receives UK Medical Research Council funding. The views expressed in this paper are those of the authors and not necessarily any funding body.
Footnotes
Conflict of interests: None to declare.
References
- 1.Westerbacka J, Corner A, Tiikkainen M, et al. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47:1360–9. doi: 10.1007/s00125-004-1460-1. [DOI] [PubMed] [Google Scholar]
- 2.Lee DH, Blomhoff R, Jacobs DR., Jr Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res. 2004;38:535–9. doi: 10.1080/10715760410001694026. [DOI] [PubMed] [Google Scholar]
- 3.Lee DH, Steffes M, Jacobs D. Can persistent organic pollutants explain the association between serum gamma-glutamyltransferase and type 2 diabetes? Diabetologia. 2008;51:402–7. doi: 10.1007/s00125-007-0896-5. [DOI] [PubMed] [Google Scholar]
- 4.Miyatake N, Matsumoto S, Makino H, Numata T. Comparison of hepatic enzymes between Japanese men with and without metabolic syndrome. Acta Med Okayama. 2007;61:31–4. doi: 10.18926/AMO/32912. [DOI] [PubMed] [Google Scholar]
- 5.Fraser A, Harris R, Sattar N, et al. Alanine aminotransferase, gamma glutamyltransferase and incident diabetes: the British Women’s Heart and Health Study and meta-analysis. Diabetes Care. 2009;32:741–50. doi: 10.2337/dc08-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser A, Harris R, Sattar N, et al. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British women’s heart and health study and meta-analysis. Arterioscler Thromb Vasc Biol. 2007;27:2729–35. doi: 10.1161/ATVBAHA.107.152298. [DOI] [PubMed] [Google Scholar]
- 7.Dunn W, Xu R, Wingard DL, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol. 2008;103:2263–71. doi: 10.1111/j.1572-0241.2008.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura K, Okamura T, Kanda H, et al. The value of combining serum alanine aminotransferase levels and body mass index to predict mortality and medical costs: a 10-year follow-up study of National Health Insurance in Shiga, Japan. J Epidemiol. 2006;16:15–20. doi: 10.2188/jea.16.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arndt V, Brenner H, Rothenbacher D, et al. Elevated liver enzyme activity in construction workers: prevalence and impact on early retirement and all-cause mortality. Int Arch Occup Environ Health. 1998;71:405–12. doi: 10.1007/s004200050299. [DOI] [PubMed] [Google Scholar]
- 10.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and [gamma]-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136:477–85. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 11.Wannamethee G, Ebrahim S, Shaper AG. Gamma-glutamyltransferase: determinants and association with mortality from ischemic heart disease and all causes. Am J Epidemiol. 1995;142:699–708. doi: 10.1093/oxfordjournals.aje.a117699. [DOI] [PubMed] [Google Scholar]
- 12.Lee DS, Evans JC, Robins SJ, et al. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk. The Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2007;27:127–33. doi: 10.1161/01.ATV.0000251993.20372.40. [DOI] [PubMed] [Google Scholar]
- 13.Brenner H, Rothenbacher D, Arndt V, et al. Distribution, determinants, and prognostic value of [gamma]-glutamyltransferase for all-cause mortality in a cohort of construction workers from Southern Germany. Prev Med. 1997;26:305–10. doi: 10.1006/pmed.1997.0144. [DOI] [PubMed] [Google Scholar]
- 14.Bathum L, Petersen HC, Rosholm JU, et al. Evidence for a substantial genetic influence on biochemical liver function tests: results from a population-based Danish twin study. Clin Chem. 2001;47:81–7. [PubMed] [Google Scholar]
- 15.Fraser A, Ebrahim S, Davey Smith G, Lawlor DA. Birth weight and adult liver damage in British Women’s Heart and Health Study. Paediatr Perinat Epidemio. 2007;22:12–21. [Google Scholar]
- 16.Fraser A, Ebrahim S, Davey Smith G, Lawlor DA. The associations between height components (leg and trunk length) and adult levels of liver enzymes. J Epidemiol Commun Health. 2008;62:48–53. doi: 10.1136/jech.2006.053181. [DOI] [PubMed] [Google Scholar]
- 17.McGue M, Christensen K. Social activity and healthy aging: a study of aging Danish twins. Twin Res Hum Genet. 2007;10:255–65. doi: 10.1375/twin.10.2.255. [DOI] [PubMed] [Google Scholar]
- 18.Hansen EJ. Social groups in Denmark. SFI Study 48. Copenhagen: Socialforskningsinstituttet; 1984. [Google Scholar]
- 19.Yuan X, Waterworth D, Perry JR, et al. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am J Hum Genet. 2008;83:520–8. doi: 10.1016/j.ajhg.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–525. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juel K, Christensen K. Sex mortality differences in Denmark 1840–2005. Women live longer than men, but great changes during the last 50 years [in Danish] Ugeskr Laeger. 2007;169:2398–403. [PubMed] [Google Scholar]