Abstract
Many people infected with the human immunodeficiency virus type-1 (HIV) exhibit mild or severe neurological problems, termed HIV-associated neurocognitive disorder (HAND), even when receiving antiretroviral therapy. Thus, novel adjunctive therapies must be developed to overcome the neurotoxic effect of HIV. New therapies require a better understanding of the molecular and cellular mechanisms of HIV-induced neurotoxicity and the risk factors that, besides inflammation and T cell depletion and drugs of abuse, render the central nervous system (CNS) a target of HIV-induced neurotoxicity. HIV appears to impair neuronal plasticity, which refers to the innate ability of the CNS to respond to injury and promote recovery of function. The availability of brain-derived neurotrophic factor (BDNF), a potent neurotrophic factor that is present in abundance in the adult brain, is essential for neuronal plasticity. BDNF acts through a receptor system composed of Trk and p75NTR. Here we present experimental evidence that some of the clinical features of HIV-mediated neurological impairment could result from altered BDNF/TrkB/p75NTR regulation and function.
Keywords: apoptosis, drug abuse, gp120, neuroAIDS, p75NTR, proBDNF
Introduction
Human immunodeficiency virus type-1 (HIV) infection is a global health problem. HIV promotes a progressive depletion of T cells, causing acquired immunodeficiency syndrome (AIDS) and 2 million AIDS-related deaths per year [1]. The introduction of aggressive treatment with highly active antiretroviral therapy (HAART), consisting of a cocktail of drugs that inhibit viral replication, has been shown to improve immune recovery, delay progression to AIDS and reduce mortality among HIV-infected subjects [2]. However, HIV-infected individuals must continue treatment with antiretroviral therapy for their entire lives, as the virus almost invariably re-emerges when the drugs are withdrawn.
An individual’s susceptibility to the viral infection and subsequent disease severity and clinical manifestations are influenced by a variety of factors. These include use of illicit drugs which decreases the immune response [3], as well as host genetic variability. For instance, Caucasian individuals deficient in CCR5, one of the main chemokine co-receptors for HIV entry into macrophages [4], are resistant to HIV infection [5–7]. Moreover, genetic epidemiological cohort studies have shown polymorphisms in genes such as cytokines and their receptors and several human leukocyte antigen (HLA) alleles [8] which influence HIV progression to AIDS.
HIV infecs the brain despite antiretroviral therapy
HIV also infects the central nervous system (CNS) [9]. HIV infection of the CNS (often referred to as neuroAIDS) promotes neurological signs termed HIV-associated neurocognitive disorder (HAND) in more than 50% of patients not receiving any form of antiretroviral therapy [10]. HAND symptoms may include asymptomatic neurocognitive impairment, minor cognitive disorders or, in its more severe form of HIV-associated dementia (HAD), profound motor and behavioural/psychosocial abnormalities that disrupt work or other activities of daily living [11]. HAND pathology includes loss of both synaptic connections and neuronal differentiation [12]. In addition, HIV promotes neuronal apoptosis, especially in children [13, 14]. HAART has decreased the severity of neurological signs [12, 15]. Indeed, recent estimates since the advent of HAART indicate that HAD is present in 1% to 2% of subjects with AIDS. Yet, HAART has not eliminated mild neurocognitive deficits and asymptomatic neurocognitive impairments [16]. Moreover, by controlling the viral load, HAART allows an individual with HIV to typically live longer with milder medical symptoms. Age can then play a role in HAND because age-associated medical comorbidities, including cardiovascular disease, are significant risk modifiers for cognitive loss in HIV-positive subjects [17, 18].
The reason why HAND persists among HAART-treated individuals is still under debate. Some antiretroviral drugs are present at such low concentrations in lymph nodes that they may not stop HIV replication completely [19]. In addition, other components of HAART may not reduce HIV infection in the CNS because they poorly penetrate the blood–brain barrier (BBB) or because the virus develops drug resistance. It is noteworthy that neurologically impaired individuals have a higher viral load in the cerebrospinal fluid (CSF) than in plasma. Indeed, clinical evidence has shown that the CNS of these individuals acts as an HIV sanctuary site unless a highly CNS-penetrating antiretroviral regimen is initiated [20]. Thus, there is great need for a better understanding of how the virus causes neurological problems.
Neurobiology of HIV
HIV impairs synaptic plasticity
Infection of the CNS occurs very early after seroconversion [21]. HIV is carried through the BBB by infected monocytes and perivascular macrophages. Although HIV does not infect neurons, postmortem brains of subjects with HAND have shown a decrease in neuronal plasticity/function at several levels. In this review, the term plasticity refers to the ability of mature CNS to undergo changes in neuronal processes, including spine formation and reorganization of altered synaptic network, in response to an injurious stimulus. Impaired neuronal plasticity can be seen both at cellular and systemic levels. At the cellular level, subjects with HAND exhibit synaptodendritic damage and decreased synaptic and dendritic density [12] that can lead to interruption of the neural network and ultimately to caspase-3-dependent neuronal apoptosis [22]. This, in turn, manifests at the system level as grey and white matter atrophy [20, 23] in both cortical and subcortical regions as demonstrated at autopsy. The basal ganglia are particularly affected [24, 25]. The fact that HAART can reverse, although partially, HAND symptoms is consistent with the notion that synaptodendritic injury, and not neuronal loss, is the main cause of impaired neuronal function. Thus, the degree of neuronal pathology in HAND is more in line with lack or loss of synaptic plasticity than frank injury as it occurs in trauma, stroke or ischaemia. Synaptic plasticity may vary considerably between individuals. Moreover, a complicating factor for neuroAIDS is the fact that a number of HIV-positive individuals also abuse various illicit drugs. Heroin abuse is a major transmission route for HIV, while abuse of stimulants, such as cocaine and methamphetamine, has become a primary risk factor for HIV infection. Other drugs of abuse such as alcohol, on the other hand, have been shown to increase oxidative stress and to cause brain tissue atrophy and poorer performance on a variety of neurocognitive assessments [26]. HIV may potentiate the effects of drugs of abuse in lowering neuronal plasticity at the cellular level. Thus, an ideal therapeutic compound for neuroAIDS should be able to promote neuronal plasticity even in the presence of illicit drugs and provide a gradual restoration of neuronal function by re-establishing the altered neural network.
CNS effects due to neuroinflammation
The search for pharmacological compounds that prevent HIV neuropathology requires a better understanding of the molecular and cellular mechanisms of HIV neurotoxicity. Why is plasticity impaired in HAND? Most subjects with HAND suffer from protracted forms of HIV encephalitis, a neuro-inflammatory condition characterized by the presence of HIV-infected microglial cells, formation of microglial nodules, multinucleated giant cells, astrogliosis and myelin loss [27–29]. Activated macrophages/microglia and distressed astrocytes may inhibit plasticity by reducing the uptake of excitotoxic neurotransmitters [30]. These, in turn, reduce the formation of dendritic spines and synapses. In addition, infected glial cells may release inflammatory cytokines such as interleukin-1β and tumour necrosis factor-α [31] and chemokines, including CXCL12 [32], which all impair neuronal survival [33–35]. Thus, glial cells, once compromised, have the ability to promote/exacerbate HIV-induced neurotoxicity by reducing homeostasis-mediated plasticity. Whether inflammatory cytokines reach levels that are harmful to neurons in vivo is uncertain, although in vitro data have shown that cytokines can promote neuronal loss. However, the role of microglia as the leading cause of the neuropathology of HIV remains speculative, as microglia can also be activated by distressed and dying neurons. Moreover, some regions of the forebrain such as the basal ganglia show selective vulnerability to synaptodendritic injury that cannot be explained solely by inflammatory cytokines. Furthermore, atrophy of axons and neuronal processes often precedes the death of the cell body and is seen in other neurodegenerative diseases that do not exhibit an immune response, such as Alzheimer’s disease [36]. These considerations support the hypothesis that HIV promotes the release of a diversity of soluble host cell-derived factors and viral proteins that may cooperate in causing the pathology of synapses.
Direct neurotoxic effect of HIV proteins
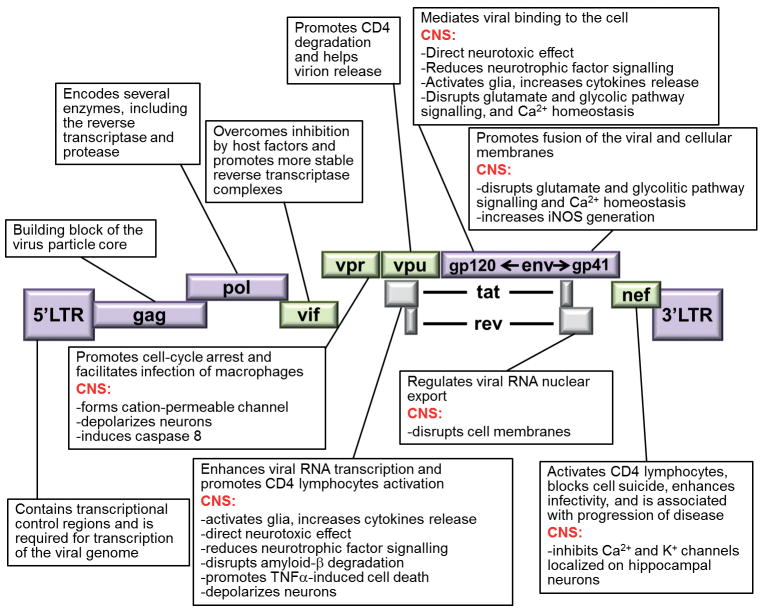
There are at least nine HIV proteins that are known to cause neuronal cell death (Fig. 1). Some of these proteins are shed from the virus or are released by infected cells. One viral protein that can cause neuronal injury is Tat, the transactivator of transcription. Tat is vital for HIV replication by influencing both transcription initiation and elongation [37] through chromatin remodelling at the HIV promoter. Moreover, Tat, which can be released from HIV-infected cells [38] at concentrations lower than those needed to support viral replication, reduces neuronal survival by several indirect and direct mechanisms, such as the production of inflammatory cytokines [39], impairment of mitochondrial function [40] and activation of ionotropic glutamate receptors [41]. The neurotoxic mechanisms of Tat have been reviewed in more detail elsewhere [42]. The accessory proteins Nef, Vif, Vpr and Vpu have also key roles in HIV pathogenesis. These proteins interfere with various host cell functions including cytoskeletal contraction [43] thereby optimizing viral replication or promoting (Vpu) the release of virions from infected cells [44]. These proteins once released from infected macrophages/microglia can cause neuronal apoptosis [45] by a number of mechanisms including activation of caspase-8 (Vpr) and formation (Vpr and Vpu) or direct binding (Nef) to ion channels leading to lethal abnormal membrane depolarization [46].
Fig. 1.
Schematic diagram of HIV proteins and their function. Adapted from [12].
Another viral gene product that causes neuronal apoptosis is the glycoprotein gp120. This protein exerts an important function in the cycle of viral infection. Indeed, gp120 is the envelope protein that binds to chemokine co-receptors CCR5 and CXCR4 and allows the virus to change conformation and enter cells [47]. Even a short exposure of neurons to gp120 can produce neuronal apoptosis by a variety of mechanisms [48, 49]. The viral protein has also been shown to promote axonal degeneration [50] and dendritic injury [51, 52], two key pathological events that may account for the synaptodendritic atrophy observed in HAD [53]. Moreover, gp120 transgenic mice exhibit neuronal loss and dendritic simplification [54], a clear indication that gp120 alone decreases synaptic plasticity. Thus, a new mechanism of neurotoxicity could be proposed in which these proteins interact directly with membrane-associated receptors and activation of signalling pathways to reduce neuronal plasticity and promote cell death.
HIV and neurotrophin brain-derived neurotrophic factor (BDNF)
BDNF and neuronal plasticity
Neuronal plasticity is influenced either directly or indirectly by genetic factors as well as non-genetic factors such as age, experience, mood, exercise and drug abuse [55]. Most importantly, neuronal plasticity is brought about by neurotrophic factors, i.e. naturally occurring diffusible polypeptides that promote survival of a variety of CNS cells and are equally essential for inducing differentiation of surviving neurons into their mature phenotypes [56]. In some animal models, trophic effects on CNS neurons have been demonstrated on their processes rather than on their cell bodies. This is especially true for the neurotrophins, a neurotrophic family of trophic factors that includes nerve growth factor (NGF) [57], BDNF [58] and neurotrophin-3 and -4 [59]. BDNF is one of the most abundant neurotrophic factors in the adult CNS. Neurotrophic effects of the neurotrophins include synaptogenesis and sprouting of central cholinergic neurons [60], modulation of dendritic branching and spines in the cortex, and long-term potentiation in the hippocampus [61, 62]. Through these properties, the neurotrophins, and in particular BDNF, play a critical role in learning and memory and preservation of cortical circuits. Conversely, a reduction in BDNF secretion/activity has been associated with numerous functional deficits including loss of cortical and hippocampal synapses, impairment of spatial learning and memory and disruption of cortical organization in both rodents [63, 64] and humans [65–68].
Preclinical neuroscientists have also placed special emphasis on the functional (‘clinical’) properties of BDNF. Indeed, BDNF is important to many forms of plasticity in relation to chronic neurological conditions that are characterized by loss of specific BDNF-sensitive neuronal populations [69]. For instance, BDNF levels are decreased in nigrostriatal dopamine neurons in Parkinson’s disease [70, 71], and in cortical neurons in both Huntington’s disease [72] and schizophrenia [73]. In Alzheimer’s disease, which is categorized as a neurological disorder with reduced numbers of neurons and connections in the cortex and hippocampus, as well as impaired short-term memory [74], there is a deficiency of BDNF synthesis in the brain [75] or CSF [76]. Ageing, one of the major risk factors for neurodegeneration, is also associated with lower levels of BDNF [77]. Conversely, the delivery of BDNF into the CNS of animal models of ageing reverses neuronal atrophy and improves age-related cognitive impairment [78]. These findings suggest that the plasticity-promoting properties of BDNF could be extended to neuroprotection. Therefore, BDNF could be a potential therapeutic agent for the treatment of these neurological diseases. Most importantly, these observations suggest that an environment characterized by lower levels or activity of BDNF (or other neurotrophic factors) could be a common risk factor for the loss of synapses (Fig. 2) and, consequently, the development of neurological diseases including HAND.
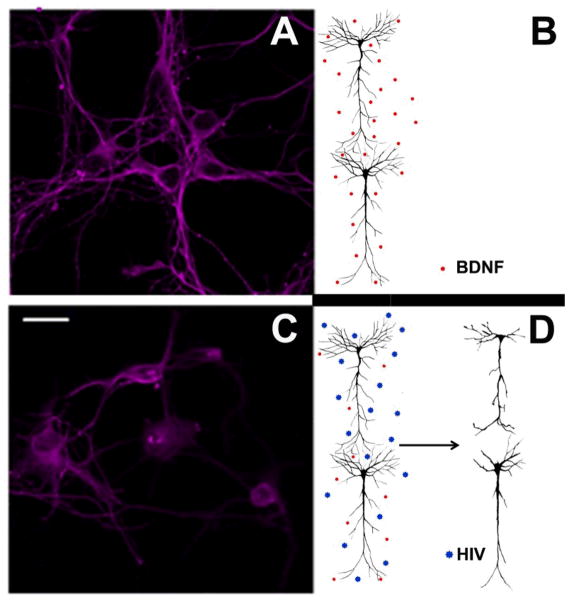
Fig. 2.
HIV induces synaptic simplification through gp120. A) Cortical neurons exposed to a physiological environment (B) in which BDNF and other neurotrophic factors, released in an activity-dependent manner, maintain an intact neuronal architecture. C) Image of neurons exposed to HIV or gp120 in which (D) the lack of trophic support evokes short processes and elimination or pruning of processes that eventually culminate in apoptosis. Scale bar, 10 μm.
HIV reduces BDNF expression
New insights into the relationship between BDNF and HAND have been provided by the findings of a number of experimental studies. For example, HIV-positive subjects have lower concentrations of serum BDNF, but not of NGF, than HIV-negative individuals [79]. The decrease in BDNF is not linked to drug abuse or a particular allelic variant of BDNF (Val66Met) [79] which was previously shown to reduce the release of BDNF [65]. In addition, HIV decreases BDNF mRNA in T cells [79], suggesting a direct effect of HIV on BDNF expression. Given the well-known anti-apoptotic effect of the neurotrophins in T cells [80, 81], it is possible that a decrease in BDNF could be among the mechanisms employed by HIV to induce apoptosis of T cells. On the other hand, HIV also decreases BDNF in postmortem human brain, particularly in the cortex and striatum [82]. Moreover, the decrease in BDNF correlates with impaired cognitive and motor function, suggesting that loss of BDNF synthesis could be a risk factor for neurological complications associated with HIV infection. Overall, these results suggest that an altered expression of neurotrophins may contribute to the immune dysregulation of AIDS, and constitute one of the causes of the pre-HAND condition leading to synaptic simplification.
The effect of HIV on BDNF is most probably mediated by gp120 because exposure of rodent neurons to the envelope protein both in vivo [83] and in vitro [84] decreases BDNF. Moreover, BDNF +/− mice which undergo premature loss of cortical synapses [85] are also more sensitive to gp120-mediated neuronal apoptosis [83]. Conversely, the apoptotic effect of gp120 in neurons is inhibited by exposure to BDNF prior to gp120 [86] or by delivering BDNF into the brain [87]. Thus, in line with the hypothesis that neurotrophic factors are essential for synaptic plasticity and recovery of function [88], it is tempting to suggest that HIV, through gp120, reduces the trophic environment or minimizes the benefit of endogenous neurotrophic factors (Fig. 2).
Experimental evidence to support BDNF as a therapy for HAND
The potential use of BDNF or other trophic factors as therapeutic agents for neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases, is supported mainly by evidence from animal models [89] in which disease progress and delivery of BDNF can be better controlled than in clinical studies. However, as described above, clinical studies have shown that decreased BDNF levels in the brain correlate with functional neurological deficits, further supporting the notion that BDNF could be a useful therapeutic agent in various neurological diseases, including HAND. Support for BDNF as a potential therapeutic option for HAND comes from the important discovery of several cellular and molecular mechanisms of neuroprotection against the neurotoxic action of viral proteins. For example, BDNF blocks the neurotoxic effect of HIV proteins Tat and gp120 by the activation of anti-apoptotic genes including Bcl-2 [90] or the reduction of pro-apoptotic caspase-3 [86]. This is not surprising as BDNF has been shown to reduce apoptosis [91, 92]. Nevertheless, BDNF can inhibit HIV-mediated neuronal loss by other mechanisms. Indeed, BDNF has been shown to downregulate the chemokine receptor CXCR4 [33, 86] to which gp120 binds and initiates the apoptotic cascade [93, 94]. Moreover, BDNF promotes adult neurogenesis [95] which is impaired in HAND [96]. There is concern about the efficiency of BDNF to cross the BBB; nevertheless, the interplay between BDNF and synaptic plasticity suggests that this neurotrophin could be used in combination with HAART to prevent HIV-mediated synaptic simplification and neuronal apoptosis.
Neurotrophin receptors
The cell death receptor p75NTR
Neurotrophins bind to two entirely distinct classes of receptors, p75NTR and Trks. p75NTR, a member of the tumour necrosis factor receptor family, was initially cloned and characterized as a low-affinity receptor for NGF with an apparent molecular weight of 75 kDa [97, 98]; however, this receptor binds to all neurotrophins with similar affinity [99] and therefore has been termed p75NTR (i.e. p75 neurotrophin receptor) [100, 101]. P75NTR does not contain a catalytic motif. Thus, upon activation, p75NTR recruits adaptor protein complexes that relay signals important for regulating neuronal cell survival, differentiation and synaptic plasticity. However, the structure of p75NTR also contains a death domain [102] which mediates cell death. Indeed, when activation of p75NTR occurs without a concomitant activation of Trk, p75NTR promotes death of oligodendrocytes [103] as well as axonal degeneration in the peripheral nervous system [104] and CNS [105]. P75NTR-mediated cell death appears to occur through activation of c-Jun N-terminal kinase (JNK) [106, 107], and inhibition of anti-apoptotic proteins Bcl-2 or Bcl-xL [108]. It is intriguing that gp120 also activates JNK and other kinases upstream of JNK that play a role in apoptosis [109]. Therefore, these pro-apoptotic molecules may be augmented by p75NTR activation in HIV-positive individuals, thus lowering the threshold for HIV-induced neurotoxicity.
Trk receptors
The other component of the neurotrophin receptor complex is the proto-oncogene Trk. This is a receptor tyrosine kinase which, like other tyrosine kinase receptors, is activated by ligand-induced formation of non-covalently associated receptor dimers [110]. The neurotrophins cause dimerization of Trk, resulting in activation via transphosphorylation of the cytoplasmic domain kinases. This, in turn, activates major signalling pathways including the phosphoinositide 3-kinase-Akt, mitogen-activated protein kinase and phospholipase C-γ [111]. There are three structurally related Trks (TrkA, B and C) which show selective binding to the neurotrophins: BDNF binds to TrkB, NGF to TrkA and NT-3 to TrkC [112], although at high concentrations BDNF can also bind to TrkC [113]. Trk gene deletion in experimental animals produces loss of neurons and severe neurological impairment. However, Trk alone cannot discriminate between neurotrophins; both Trk and p75NTR are necessary to confer high-affinity binding and ligand specificity to the neurotrophins and to influence most actions of neurotrophins on neuronal differentiation and survival. Thus, p75NTR modulates Trk receptor function by promoting ligand binding as well as accessibility to neurotrophins through promotion of axon growth and target innervation. In addition, p75NTR can promote endocytosis and retrograde transport of Trk which facilitates neurotrophin signalling [114].
In addition to Trk, p75NTR can form a complex with the so-called truncated Trk (Trk.T1), an isoform of Trk generated from an alternative splicing which does not contain the tyrosine kinase domain. In certain neuronal populations, TrkB.T1 or TrkC.T1 can function as inhibitors of full-length Trk through a dominant-negative mechanism [91]. Nevertheless, Trk isoforms and p75NTR also exhibit some neurotrophic properties such as neuronal crest proliferation and differentiation and regulation of neuronal branching as well as BDNF signalling [115].
Toxic effects of HIV on CNS neurons
HIV and proBDNF
p75NTR can also bind the larger precursor (pro) neurotrophin proteins (proneurotrophins) including proBDNF [116]. Proneurotrophins are abundant in the mature brain [117] and can be released from neurons [118]. Proneurotrophins can be cleaved in the endoplasmic reticulum by the proconvertase furin [119] or extracellularly by proteases such as plasmin and matrix metalloproteinases [120], and thus converted to mature neurotrophins (Fig. 3A). Conversion of proneurotrophin to mature neurotrophin is an important process for synaptic plasticity. In fact, proNGF and proBDNF have the opposite effects to the mature neurotrophins, including neuronal apoptosis [121], axonal degeneration in the developing as well as mature nervous system [105, 122, 123], presynaptic terminal retraction [124] and long-term depression [125]. Furthermore, proneurotrophins have no affinity for Trk (Fig. 3B). Elegant studies have demonstrated that proneurotrophins bind to a dual receptor system formed by p75NTR and sortilin, a type I transmembrane protein [126–128] that is structurally unrelated to both Trk and p75NTR. Thus, neuronal survival/death depends on whether proBDNF rather than mature BDNF is released, and on the ability of extracellular proBDNF to bind to p75NTR. It is interesting that p75NTR generates ceramide through sphingomyelin hydrolysis [129, 130]. Ceramide, a sphingolipid that is significantly increased in HIV-infected cells [131] as well as in the brain and CSF of subjects with HAD [132], promotes apoptosis of various cells [133], including neurons [134].
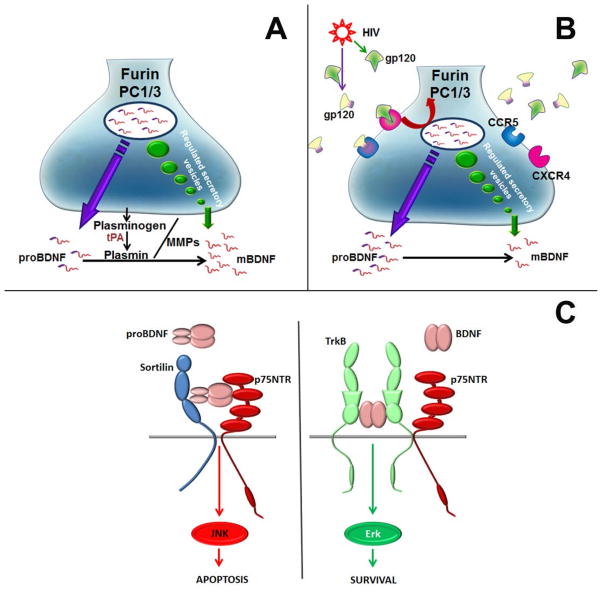
Fig. 3.
Proposed mechanisms of HIV-mediated synaptic simplification. (A) HIV-negative synapse. Under physiological conditions, proBDNF is released in an activity-dependent manner and is processed to mature BDNF either intracellularly by furin or extracellularly by plasmin and matrix metalloproteinases (MMPs). Plasmin is generated from plasminogen by released tissue plasminogen (tPA). (B) HIV-positive synapse. Gp120, shed from HIV, binds to chemokine receptors. This leads to reduced intracellular processing of proBDNF which appears to occur through a decrease in furin and/or prohormone convertase (PC1/3) synthesis/activity [82]. (C) Extracellular BDNF/proBDNF. Mature BDNF binds with high affinity to its cognate receptor TrkB and low affinity to p75NTR. This binding promotes synaptic plasticity through activation of ERK and/or PI kinase. By contrast, released proBDNF binds with high affinity to p75NTR/sortilin which, in turn, activates degeneration of p75NTR-positive neurons through a c-Jun N-terminal kinase (JNK)-mediated mechanism.
Why is proBDNF important in HAND? The severity of cognitive impairment in HIV-positive subjects correlates with synaptodendritic degeneration [12], a phenomenon that can be evoked by lack of trophic support. However, as discussed above, proBDNF is a negative regulator through p75NTR of synaptic plasticity and axonal growth. Thus axonal degeneration can also be initiated by proBDNF. HIV has been shown to alter metalloproteinases within the CNS [135]; therefore, HIV might decrease the processing of proBDNF to mature BDNF (Fig. 3B) and, consequently, promote an environment that is conducive to the activation of p75NTR (Fig. 3C). This hypothesis is supported by recent data showing that the pro-apoptotic effect (including synaptic simplification) of HIV and gp120 in rodents is blocked by a p75NTR antagonist [82]. Moreover, lysates from the frontal cortex or striatum from subjects with HAND contain more proBDNF than those from HIV subjects without dementia [82]. Although conclusively demonstrating activation of p75NTR (or another receptor) in vivo in humans presents a technical challenge, a conclusion may be drawn about HIV toxicity and p75NTR activation from the fact that the levels of proBDNF were higher in HAND than HIV subjects without dementia. Because both the frontal cortex and striatum are part of the cognitive circuitry, it is possible that altered levels of proBDNF could be a risk factor for synaptic degeneration in subjects with HIV and therefore could be used as a biomarker for HAND.
Conclusions
The cellular mechanisms of HIV-mediated synaptic degeneration are currently incompletely understood. HIV may evoke neuronal injury through neurotoxins released by infected or immune-stimulated, inflammatory microglia and macrophages; it is also possible that microglia may monitor synaptic function and be involved in elimination of and scavenging synapses. On the other hand, loss of neurons and their branches underlies the pathophysiology of many neurodegenerative conditions that exhibit inflammation only at the end stage of disease. In these diseases there is evidence for ‘active’ mechanisms in which host signals trigger degeneration by means of pro-apoptotic receptors, including p75NTR. It has been suggested that this receptor causes loss of neurons in a number of neurological disorders including Alzheimer’s [136] and Huntington’s diseases [137], seizures [138, 139] and retinal degeneration [140]. Moreover, p75NTR expression is upregulated in diseases that promote grey matter apoptosis [138, 141, 142]. p75NTR is activated by proBDNF whose levels are increased in subjects with HAD or in gp120-treated neurons. Extracellular proBDNF mediates biological effects that are opposite to those of mature BDNF, including neuronal apoptosis, long-term depression and presynaptic terminal retraction. These findings support a new model of HIV-induced neurotoxicity in which abnormal production of proBDNF by HIV activates p75NTR leading to the pathological changes of synapses and their connections in HAND (Fig. 3). Discovering how HIV evokes an accumulation/release of proBDNF should help clinicians identify new adjunctive therapies that may reverse HIV-mediated neuronal dysfunction.
Acknowledgments
This work was supported by National Institute of Health grants 1R01 DA026174, 1R01 NS079172 and 1R01 NS079172.
Footnotes
Conflict of interest statement
None of the authors has any financial or other conflicts of interest.
References
- 1.http://www.unaids.org/en/Resources/.
- 2.Lucas S. Causes of death in the HAART era. Curr Opin Infect Dis. 2012;25:36–41. doi: 10.1097/QCO.0b013e32834ef5c4. [DOI] [PubMed] [Google Scholar]
- 3.Green TC, McGowan SK, Yokell MA, Pouget ER, Rich JD. HIV infection and risk of overdose: a systematic review and meta-analysis. Aids. 2012;26:403–17. doi: 10.1097/QAD.0b013e32834f19b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scarlatti G, Tresoldi E, Bjorndal A, et al. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3:1259–65. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 5.Dean M, Carrington M, Winkler C, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–62. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 6.Paxton WA, Martin SR, Tse D, et al. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposure. Nat Med. 1996;2:412–7. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 7.Samson M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–5. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 8.Martin MP, Gao X, Lee JH, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–34. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 9.Bissel SJ, Wiley CA. Human immunodeficiency virus infection of the brain: pitfalls in evaluating infected/affected cell populations. Brain Pathol. 2004;14:97–108. doi: 10.1111/j.1750-3639.2004.tb00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 12.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 13.Garden GA, Budd SL, Tsai E, et al. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 2002;22:4015–24. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelbard HA, Epstein LG. HIV-1 encephalopathy in children. Curr Opin Pediatr. 1995;7:655–62. doi: 10.1097/00008480-199512000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Joska JA, Gouse H, Paul RH, Stein DJ, Flisher AJ. Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol. 2010;16:101–14. doi: 10.3109/13550281003682513. [DOI] [PubMed] [Google Scholar]
- 16.McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–21. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- 17.Becker JT, Maruca V, Kingsley LA, et al. Factors affecting brain structure in men with HIV disease in the post-HAART era. Neuroradiology. 2012;54:113–21. doi: 10.1007/s00234-011-0854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherner M, Ellis RJ, Lazzaretto D, et al. Effects of HIV-1 infection and aging on neurobehavioral functioning: preliminary findings. Aids. 2004;18 (Suppl 1):S27–34. [PubMed] [Google Scholar]
- 19.Baheti G, King JR, Acosta EP, Fletcher CV. Age-related differences in plasma and intracellular tenofovir concentrations in HIV-1-infected children, adolescents and adults. Aids. 2013;27:221–5. doi: 10.1097/QAD.0b013e32835a9a2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masliah E, DeTeresa RM, Mallory ME, Hansen LA. Changes in pathological findings at autopsy in AIDS cases for the last 15 years. Aids. 2000;14:69–74. doi: 10.1097/00002030-200001070-00008. [DOI] [PubMed] [Google Scholar]
- 21.Resnick L, Berger JR, Shapshak P, Tourtellotte WW. Early penetration of the blood-brain-barrier by HIV. Neurology. 1988;38:9–14. doi: 10.1212/wnl.38.1.9. [DOI] [PubMed] [Google Scholar]
- 22.James HJ, Sharer LR, Zhang Q, Wang HG, Epstein LG, Reed JC, Gelbard HA. Expression of caspase-3 in brains from paediatric patients with HIV-1 encephalitis. Neuropathol Appl Neurobiol. 1999;25:380–6. doi: 10.1046/j.1365-2990.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- 23.Gray F, Adle-Biassette H, Chretien F, Lorin de la Grandmaison G, Force G, Keohane C. Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin Neuropathol. 2001;20:146–55. [PubMed] [Google Scholar]
- 24.Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, Pearlson GD. Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology. 1993;43:2099–104. doi: 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- 25.Berger JR, Arendt G. HIV dementia: the role of the basal ganglia and dopaminergic systems. J Psychopharmacol. 2000;14:214–21. doi: 10.1177/026988110001400304. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Yuan W, Li MD. Genes and pathways co-associated with the exposure to multiple drugs of abuse, including alcohol, amphetamine/methamphetamine, cocaine, marijuana, morphine, and/or nicotine: a review of proteomics analyses. Mol Neurobiol. 2011;44:269–86. doi: 10.1007/s12035-011-8202-4. [DOI] [PubMed] [Google Scholar]
- 27.Anderson E, Zink W, Xiong H, Gendelman HE. HIV-1-associated dementia: a metabolic encephalopathy perpetrated by virus-infected and immune-competent mononuclear phagocytes. J Acquir Immune Defic Syndr. 2002;31 (Suppl 2):S43–54. doi: 10.1097/00126334-200210012-00004. [DOI] [PubMed] [Google Scholar]
- 28.Everall IP, Hansen LA, Masliah E. The shifting patterns of HIV encephalitis neuropathology. Neurotox Res. 2005;8:51–61. doi: 10.1007/BF03033819. [DOI] [PubMed] [Google Scholar]
- 29.Wiley CA, Achim C. Human immunodeficiency virus encephalitis is the pathological correlate of dementia in acquired immunodeficiency syndrome. Ann Neurol. 1994;36:673–6. doi: 10.1002/ana.410360422. [DOI] [PubMed] [Google Scholar]
- 30.Bezzi P, Domercq M, Brambilla L, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–10. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 31.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 32.Langford D, Sanders VJ, Mallory M, Kaul M, Masliah E. Expression of stromal cell-derived factor 1alpha protein in HIV encephalitis. J Neuroimmunol. 2002;127:115–26. doi: 10.1016/s0165-5728(02)00068-1. [DOI] [PubMed] [Google Scholar]
- 33.Bachis A, Mocchetti I. The chemokine receptor CXCR4 and not the N-methyl-D-aspartate receptor mediates gp120 neurotoxicity in cerebellar granule cells. J Neurosci Res. 2004;75:75–82. doi: 10.1002/jnr.10826. [DOI] [PubMed] [Google Scholar]
- 34.Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:8212–6. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–5. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masliah E. Mechanisms of synaptic dysfunction in Alzheimer’s disease. Histol Histopathol. 1995;10:509–19. [PubMed] [Google Scholar]
- 37.Gatignol A. Transcription of HIV: Tat and cellular chromatin. Adv Pharmacol. 2007;55:137–59. doi: 10.1016/S1054-3589(07)55004-0. [DOI] [PubMed] [Google Scholar]
- 38.Ensoli B, Buonaguro L, Barillari G, et al. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993;67:277–87. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen P, Mayne M, Power C, Nath A. The Tat protein of HIV-1 induces tumor necrosis factor-alpha production. Implications for HIV-1-associated neurological diseases. J Biol Chem. 1997;272:22385–8. doi: 10.1074/jbc.272.36.22385. [DOI] [PubMed] [Google Scholar]
- 40.Kruman I, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–88. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- 41.Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 2001;78:457–67. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 42.Li W, Li G, Steiner J, Nath A. Role of Tat protein in HIV neuropathogenesis. Neurotox Res. 2009;16:205–20. doi: 10.1007/s12640-009-9047-8. [DOI] [PubMed] [Google Scholar]
- 43.Matarrese P, Malorni W. Human immunodeficiency virus (HIV)-1 proteins and cytoskeleton: partners in viral life and host cell death. Cell Death Differ. 2005;12 (Suppl 1):932–41. doi: 10.1038/sj.cdd.4401582. [DOI] [PubMed] [Google Scholar]
- 44.Nomaguchi M, Fujita M, Adachi A. Role of HIV-1 Vpu protein for virus spread and pathogenesis. Microbes Infect. 2008;10:960–7. doi: 10.1016/j.micinf.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Patel CA, Mukhtar M, Pomerantz RJ. Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J Virol. 2000;74:9717–26. doi: 10.1128/jvi.74.20.9717-9726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van de Bovenkamp M, Nottet HS, Pereira CF. Interactions of human immunodeficiency virus-1 proteins with neurons: possible role in the development of human immunodeficiency virus-1-associated dementia. Eur J Clin Invest. 2002;32:619–27. doi: 10.1046/j.1365-2362.2002.01029.x. [DOI] [PubMed] [Google Scholar]
- 47.Dittmar MT, McKnight A, Simmons G, Clapham PR, Weiss RA, Simmonds P. HIV-1 tropism and co-receptor use. Nature. 1997;385:495–6. doi: 10.1038/385495a0. [DOI] [PubMed] [Google Scholar]
- 48.Bagetta G, Corasaniti MT, Berliocchi L, Navarra M, Finazzi-Agro A, Nistico G. HIV-1 gp120 produces DNA fragmentation in the cerebral cortex of rat. Biochem Biophys Res Commun. 1995;211:130–6. doi: 10.1006/bbrc.1995.1787. [DOI] [PubMed] [Google Scholar]
- 49.Biard-Piechaczyk M, Robert-Hebmann V, Richard V, Roland J, Hipskind RA, Devaux C. Caspase-dependent apoptosis of cells expressing the chemokine receptor CXCR4 is induced by cell membrane-associated human immunodeficiency virus type 1 envelope glycoprotein (gp120) Virology. 2000;268:329–44. doi: 10.1006/viro.1999.0151. [DOI] [PubMed] [Google Scholar]
- 50.Melli G, Keswani SC, Fischer A, Chen W, Hoke A. Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain. 2006;129:1330–8. doi: 10.1093/brain/awl058. [DOI] [PubMed] [Google Scholar]
- 51.Everall IP, Bell C, Mallory M, Langford D, Adame A, Rockestein E, Masliah E. Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol Cell Neurosci. 2002;21:493–501. doi: 10.1006/mcne.2002.1196. [DOI] [PubMed] [Google Scholar]
- 52.Iskander S, Walsh KA, Hammond RR. Human CNS cultures exposed to HIV-1 gp120 reproduce dendritic injuries of HIV-1-associated dementia. J Neuroinflammation. 2004;1:7. doi: 10.1186/1742-2094-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masliah E, Heaton RK, Marcotte TD, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–72. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- 54.Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–93. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 55.Pearson-Fuhrhop KM, Burke E, Cramer SC. The influence of genetic factors on brain plasticity and recovery after neural injury. Curr Opin Neurol. 2012;25:682–8. doi: 10.1097/WCO.0b013e32835a360a. [DOI] [PubMed] [Google Scholar]
- 56.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–62. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 58.Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. Embo J. 1990;9:2459–64. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maisonpierre PC, Belluscio L, Squinto S, Ip NY, Furth ME, Lindsay RM, Yancopoulos GD. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990;247:1446–51. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- 60.Garofalo L, Ribeiro-da-Silva A, Cuello AC. Nerve growth factor-induced synaptogenesis and hypertrophy of cortical cholinergic terminals. Proc Natl Acad Sci U S A. 1992;89:2639–43. doi: 10.1073/pnas.89.7.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–45. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 62.Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:975–90. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- 63.Chen ZY, Jing D, Bath KG, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–3. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang ZJ, Kirkwood A, Pizzorusso T, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–55. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 65.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 66.Erickson KI, Prakash RS, Voss MW, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30:5368–75. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Komulainen P, Pedersen M, Hanninen T, et al. BDNF is a novel marker of cognitive function in ageing women: the DR’s EXTRA Study. Neurobiol Learn Mem. 2008;90:596–603. doi: 10.1016/j.nlm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 68.Pezawas L, Verchinski BA, Mattay VS, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311–22. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
- 70.Howells DW, Porritt MJ, Wong JY, Batchelor PE, Kalnins R, Hughes AJ, Donnan GA. Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp Neurol. 2000;166:127–35. doi: 10.1006/exnr.2000.7483. [DOI] [PubMed] [Google Scholar]
- 71.Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Transm Suppl. 2000:277–90. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- 72.Zuccato C, Ciammola A, Rigamonti D, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science. 2001;293:493–8. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- 73.Durany N, Thome J. Neurotrophic factors and the pathophysiology of schizophrenic psychoses. Eur Psychiatry. 2004;19:326–37. doi: 10.1016/j.eurpsy.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 74.Scheff SW, Price DA. Alzheimer’s disease-related alterations in synaptic density: neocortex and hippocampus. J Alzheimers Dis. 2006;9:101–15. doi: 10.3233/jad-2006-9s312. [DOI] [PubMed] [Google Scholar]
- 75.Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- 76.Laske C, Stransky E, Leyhe T, et al. BDNF serum and CSF concentrations in Alzheimer’s disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41:387–94. doi: 10.1016/j.jpsychires.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 77.Driscoll I, Martin B, An Y, Maudsley S, Ferrucci L, Mattson MP, Resnick SM. Plasma BDNF is associated with age-related white matter atrophy but not with cognitive function in older, non-demented adults. PLoS One. 2012;7:e35217. doi: 10.1371/journal.pone.0035217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagahara AH, Merrill DA, Coppola G, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med. 2009;15:331–7. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Avdoshina V, Garzino-Demo A, Bachis A, et al. HIV-1 decreases the levels of neurotrophins in human lymphocytes. Aids. 2011;25:1126–8. doi: 10.1097/QAD.0b013e32834671b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aloe L, Simone MD, Properzi F. Nerve growth factor: a neurotrophin with activity on cells of the immune system. Microsc Res Tech. 1999;45:285–91. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<285::AID-JEMT12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 81.Tessarollo L. Pleiotropic functions of neurotrophins in development. Cytokine Growth Factor Rev. 1998;9:125–37. doi: 10.1016/s1359-6101(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 82.Bachis A, Avdoshina V, Zecca L, Parsadanian M, Mocchetti I. Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J Neurosci. 2012;32:9477–84. doi: 10.1523/JNEUROSCI.0865-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nosheny RL, Bachis A, Acquas E, Mocchetti I. Human immunodeficiency virus type 1 glycoprotein gp120 reduces the levels of brain-derived neurotrophic factor in vivo: potential implication for neuronal cell death. Eur J Neurosci. 2004;20:2857–64. doi: 10.1111/j.1460-9568.2004.03764.x. [DOI] [PubMed] [Google Scholar]
- 84.Bachis A, Cruz MI, Mocchetti I. M-tropic HIV envelope protein gp120 exhibits a different neuropathological profile than T-tropic gp120 in rat striatum. Eur J Neurosci. 2010;32:570–8. doi: 10.1111/j.1460-9568.2010.07325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lyons WE, Mamounas LA, Ricaurte GA, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–44. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bachis A, Major EO, Mocchetti I. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci. 2003;23:5715–22. doi: 10.1523/JNEUROSCI.23-13-05715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nosheny RL, Ahmed F, Yakovlev A, Meyer EM, Ren K, Tessarollo L, Mocchetti I. Brain-derived neurotrophic factor prevents the nigrostriatal degeneration induced by human immunodeficiency virus-1 glycoprotein 120 in vivo. Eur J Neurosci. 2007;25:2275–84. doi: 10.1111/j.1460-9568.2007.05506.x. [DOI] [PubMed] [Google Scholar]
- 88.Koliatsos VEMI. Trophic factors as therapeutic agents for diseases characterized by neuronal death. In: Koliatsos VERRT, NJ, editors. Cell death and diseases of the nervous system. Humana Press Inc; 1999. pp. 545–91. [Google Scholar]
- 89.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209–19. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- 90.Ramirez SH, Sanchez JF, Dimitri CA, Gelbard HA, Dewhurst S, Maggirwar SB. Neurotrophins prevent HIV Tat-induced neuronal apoptosis via a nuclear factor-kappaB (NF-kappaB)-dependent mechanism. J Neurochem. 2001;78:874–89. doi: 10.1046/j.1471-4159.2001.00467.x. [DOI] [PubMed] [Google Scholar]
- 91.Dorsey SG, Renn CL, Carim-Todd L, et al. In vivo restoration of physiological levels of truncated TrkB. T1 receptor rescues neuronal cell death in a trisomic mouse model. Neuron. 2006;51:21–8. doi: 10.1016/j.neuron.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 92.Han BH, D’Costa A, Back SA, et al. BDNF blocks caspase-3 activation in neonatal hypoxia-ischemia. Neurobiol Dis. 2000;7:38–53. doi: 10.1006/nbdi.1999.0275. [DOI] [PubMed] [Google Scholar]
- 93.Herbein G, Mahlknecht U, Batliwalla F, et al. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–94. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 94.Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–8. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- 95.Bergami M, Rimondini R, Santi S, Blum R, Gotz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:15570–5. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tran PB, Miller RJ. HIV-1, chemokines and neurogenesis. Neurotox Res. 2005;8:149–58. doi: 10.1007/BF03033826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chao MV, Bothwell MA, Ross AH, Koprowski H, Lanahan AA, Buck CR, Sehgal A. Gene transfer and molecular cloning of the human NGF receptor. Science. 1986;232:518–21. doi: 10.1126/science.3008331. [DOI] [PubMed] [Google Scholar]
- 98.Ross AH, Grob P, Bothwell M, et al. Characterization of nerve growth factor receptor in neural crest tumors using monoclonal antibodies. Proc Natl Acad Sci U S A. 1984;81:6681–5. doi: 10.1073/pnas.81.21.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rodriguez-Tebar A, Dechant G, Barde YA. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990;4:487–92. doi: 10.1016/0896-6273(90)90107-q. [DOI] [PubMed] [Google Scholar]
- 100.Brennan C, Rivas-Plata K, Landis SC. The p75 neurotrophin receptor influences NT-3 responsiveness of sympathetic neurons in vivo. Nat Neurosci. 1999;2:699–705. doi: 10.1038/11158. [DOI] [PubMed] [Google Scholar]
- 101.Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–83. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- 102.Feinstein E, Kimchi A, Wallach D, Boldin M, Varfolomeev E. The death domain: a module shared by proteins with diverse cellular functions. Trends Biochem Sci. 1995;20:342–4. doi: 10.1016/s0968-0004(00)89070-2. [DOI] [PubMed] [Google Scholar]
- 103.Gu C, Casaccia-Bonnefil P, Srinivasan A, Chao MV. Oligodendrocyte apoptosis mediated by caspase activation. J Neurosci. 1999;19:3043–9. doi: 10.1523/JNEUROSCI.19-08-03043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kenchappa RS, Zampieri N, Chao MV, Barker PA, Teng HK, Hempstead BL, Carter BD. Ligand-dependent cleavage of the P75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron. 2006;50:219–32. doi: 10.1016/j.neuron.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 105.Park KJ, Grosso CA, Aubert I, Kaplan DR, Miller FD. p75NTR-dependent, myelin-mediated axonal degeneration regulates neural connectivity in the adult brain. Nat Neurosci. 2010;13:559–66. doi: 10.1038/nn.2513. [DOI] [PubMed] [Google Scholar]
- 106.Carter BD, Kaltschmidt C, Kaltschmidt B, Offenhauser N, Bohm-Matthaei R, Baeuerle PA, Barde YA. Selective activation of NF-kappa B by nerve growth factor through the neurotrophin receptor p75. Science. 1996;272:542–5. doi: 10.1126/science.272.5261.542. [DOI] [PubMed] [Google Scholar]
- 107.Salehi AH, Xanthoudakis S, Barker PA. NRAGE, a p75 neurotrophin receptor-interacting protein, induces caspase activation and cell death through a JNK-dependent mitochondrial pathway. J Biol Chem. 2002;277:48043–50. doi: 10.1074/jbc.M205324200. [DOI] [PubMed] [Google Scholar]
- 108.Coulson EJ, Reid K, Barrett GL, Bartlett PF. p75 neurotrophin receptor-mediated neuronal death is promoted by Bcl-2 and prevented by Bcl-xL. J Biol Chem. 1999;274:16387–91. doi: 10.1074/jbc.274.23.16387. [DOI] [PubMed] [Google Scholar]
- 109.Hauser KF, El-Hage N, Buch S, Nath A, Tyor WR, Bruce-Keller AJ, Knapp PE. Impact of opiate-HIV-1 interactions on neurotoxic signaling. J Neuroimmune Pharmacol. 2006;1:98–105. doi: 10.1007/s11481-005-9000-4. [DOI] [PubMed] [Google Scholar]
- 110.Kaplan DR, Hempstead BL, Martin-Zanca D, Chao MV, Parada LF. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991;252:554–8. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- 111.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–91. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 112.Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci. 1995;18:321–6. [PubMed] [Google Scholar]
- 113.Klein R, Nanduri V, Jing SA, et al. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 1991;66:395–403. doi: 10.1016/0092-8674(91)90628-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schecterson LC, Bothwell M. Neurotrophin receptors: Old friends with new partners. Dev Neurobiol. 2010;70:332–8. doi: 10.1002/dneu.20767. [DOI] [PubMed] [Google Scholar]
- 115.Carim-Todd L, Bath KG, Fulgenzi G, et al. Endogenous truncated TrkB. T1 receptor regulates neuronal complexity and TrkB kinase receptor function in vivo. J Neurosci. 2009;29:678–85. doi: 10.1523/JNEUROSCI.5060-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hempstead BL. The many faces of p75NTR. Curr Opin Neurobiol. 2002;12:260–7. doi: 10.1016/s0959-4388(02)00321-5. [DOI] [PubMed] [Google Scholar]
- 117.Fahnestock M, Michalski B, Xu B, Coughlin MD. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol Cell Neurosci. 2001;18:210–20. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- 118.Yang J, Siao CJ, Nagappan G, et al. Neuronal release of proBDNF. Nat Neurosci. 2009;12:113–5. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seidah NG, Benjannet S, Pareek S, Chretien M, Murphy RA. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996;379:247–50. doi: 10.1016/0014-5793(95)01520-5. [DOI] [PubMed] [Google Scholar]
- 120.Mowla SJ, Pareek S, Farhadi HF, et al. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J Neurosci. 1999;19:2069–80. doi: 10.1523/JNEUROSCI.19-06-02069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–8. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 122.Kaplan DR, Miller FD. Axon growth inhibition: signals from the p75 neurotrophin receptor. Nat Neurosci. 2003;6:435–6. doi: 10.1038/nn0503-435. [DOI] [PubMed] [Google Scholar]
- 123.Singh KK, Park KJ, Hong EJ, Kramer BM, Greenberg ME, Kaplan DR, Miller FD. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci. 2008;11:649–58. doi: 10.1038/nn.2114. [DOI] [PubMed] [Google Scholar]
- 124.Yang F, Je HS, Ji Y, Nagappan G, Hempstead B, Lu B. Pro-BDNF-induced synaptic depression and retraction at developing neuromuscular synapses. J Cell Biol. 2009;185:727–41. doi: 10.1083/jcb.200811147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Woo NH, Teng HK, Siao CJ, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–77. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 126.Jansen P, Giehl K, Nyengaard JR, et al. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat Neurosci. 2007;10:1449–57. doi: 10.1038/nn2000. [DOI] [PubMed] [Google Scholar]
- 127.Nykjaer A, Lee R, Teng KK, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–8. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- 128.Teng HK, Teng KK, Lee R, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–63. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–9. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- 130.Lievremont JP, Sciorati C, Morandi E, et al. The p75(NTR)-induced apoptotic program develops through a ceramide-caspase pathway negatively regulated by nitric oxide. J Biol Chem. 1999;274:15466–72. doi: 10.1074/jbc.274.22.15466. [DOI] [PubMed] [Google Scholar]
- 131.Van Veldhoven PP, Matthews TJ, Bolognesi DP, Bell RM. Changes in bioactive lipids, alkylacylglycerol and ceramide, occur in HIV-infected cells. Biochem Biophys Res Commun. 1992;187:209–16. doi: 10.1016/s0006-291x(05)81480-9. [DOI] [PubMed] [Google Scholar]
- 132.Haughey NJ, Cutler RG, Tamara A, et al. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55:257–67. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- 133.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–71. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 134.Pelled D, Raveh T, Riebeling C, Fridkin M, Berissi H, Futerman AH, Kimchi A. Death-associated protein (DAP) kinase plays a central role in ceramide-induced apoptosis in cultured hippocampal neurons. J Biol Chem. 2002;277:1957–61. doi: 10.1074/jbc.M104677200. [DOI] [PubMed] [Google Scholar]
- 135.Conant K, McArthur JC, Griffin DE, Sjulson L, Wahl LM, Irani DN. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol. 1999;46:391–8. doi: 10.1002/1531-8249(199909)46:3<391::aid-ana15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 136.Coulson EJ. Does the p75 neurotrophin receptor mediate Abeta-induced toxicity in Alzheimer’s disease? J Neurochem. 2006;98:654–60. doi: 10.1111/j.1471-4159.2006.03905.x. [DOI] [PubMed] [Google Scholar]
- 137.Zuccato C, Marullo M, Conforti P, MacDonald ME, Tartari M, Cattaneo E. Systematic assessment of BDNF and its receptor levels in human cortices affected by Huntington’s disease. Brain Pathol. 2008;18:225–38. doi: 10.1111/j.1750-3639.2007.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roux PP, Colicos MA, Barker PA, Kennedy TE. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J Neurosci. 1999;19:6887–96. doi: 10.1523/JNEUROSCI.19-16-06887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Volosin M, Trotter C, Cragnolini A, et al. Induction of proneurotrophins and activation of p75NTR-mediated apoptosis via neurotrophin receptor-interacting factor in hippocampal neurons after seizures. J Neurosci. 2008;28:9870–9. doi: 10.1523/JNEUROSCI.2841-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lebrun-Julien F, Bertrand MJ, De Backer O, Stellwagen D, Morales CR, Di Polo A, Barker PA. ProNGF induces TNFalpha-dependent death of retinal ganglion cells through a p75NTR non-cell-autonomous signaling pathway. Proc Natl Acad Sci U S A. 2010;107:3817–22. doi: 10.1073/pnas.0909276107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bussmann KA, Sofroniew MV. Re-expression of p75NTR by adult motor neurons after axotomy is triggered by retrograde transport of a positive signal from axons regrowing through damaged or denervated peripheral nerve tissue. Neuroscience. 1999;91:273–81. doi: 10.1016/s0306-4522(98)00562-4. [DOI] [PubMed] [Google Scholar]
- 142.Wu CK, Thal L, Pizzo D, Hansen L, Masliah E, Geula C. Apoptotic signals within the basal forebrain cholinergic neurons in Alzheimer’s disease. Exp Neurol. 2005;195:484–96. doi: 10.1016/j.expneurol.2005.06.020. [DOI] [PubMed] [Google Scholar]