Abstract
We have developed a ten-week curriculum for molecular biology that uses 16S ribosomal RNA genes to characterize and compare novel bacteria from hot spring communities in Yellowstone National Park. The 16S rRNA approach bypasses selective culture-based methods. Our molecular biology course offered the opportunity for students to learn broadly applicable methods while contributing to a long-term research project. Specifically, students isolated and characterized clones that contained novel 16S rRNA inserts using restriction enzyme, DNA sequencing, and computer-based phylogenetic methods. In both classes, students retrieved novel bacterial 16S rRNA genes, several of which were most similar to Green Nonsulfur bacterial isolates. During class, we evaluated student performance and mastery of skills and concepts using quizzes, formal lab notebooks, and a broad project assignment. For this report, we also assessed student performance alongside data quality and discussed the significance, our goal being to improve both research and teaching methods.
Molecular biology courses typically include laboratory components that enable students to learn methods in DNA analysis. Many curricula utilize defined materials that lead students through the process of verifying previous results. One such text-based curriculum involves cloning and transferring lux genes from Vibrio fischeri to Escherichia coli(12). Given the immense microbial diversity in most environments and the simplicity of molecular procedures afforded by straight-forward kits and equipment, we implemented a research-driven curriculum for our molecular biology course that is based on analyzing 16S rRNA genes (13). We contend that this research-based approach can be modified to any sample source to elucidate microbial diversity that relates to medical, applied, or environmental issues. DNA-based methods are even more appropriate because traditional culture-based approaches have been estimated to detect as little as 1% of bacteria from many environments (1, 9). Applications using rRNA information include inferring the identity of novel sequences, predicting metabolic lifestyles for organisms that are not amenable to pure culture, and improving media development (7, 9).
The goal of our particular research project is to improve our understanding of unusual and as yet uncultured red filamentous Green Nonsulfur (GNS) phototrophs from hot springs in Yellowstone National Park. Based on the approach of Woese, students analyzed and compared novel bacterial 16S rRNA genes from red bacterial communities against available DNA databases to make predictions about microbial identity, diversity, and metabolism (13). They described novel sequences, expanding our collective understanding of microbial diversity. Student involvement in this project has led to research publications, presentations, and funding opportunities that have supported the acquisition of major course equipment. Reciprocally, these outcomes have fostered an increase in biology majors earning research-oriented molecular biology positions following graduation, most of which have been contingent on having completed this particular course.
Our ten-week course in molecular biology (Biology 475), offered annually (6 to 7 students per year), comprises two of the four class credits; the remaining two credits involve two one-hour weekly lectures that cover broad comparative concepts in molecular biology. As part of a rural, public, midsized liberal arts university (enrollment ? 4,500), our department offers undergraduate degrees in general biology, biology education, and molecular biology. Biology 475 provides elective credit for the former two options and is required for the latter. Students who take this course have taken a 200-level introductory course in biology and a 300-level General Microbiology course, both from the principal lab instructor (Boomer).
METHODS
General methods overview. This curriculum was divided into five, two-week units (Table 1). Sequentially, these were: Plasmid Isolation, Restriction Enzymes, DNA Sequence Analysis, Bioinformatics and Phylogenetics, and PCR-Based Cloning. Protocols describing these methods are widely available, diverse, and often equipment- or project-specific. A complete or comparative summary of these methods is beyond the scope of this presentation. The purpose of this section is to highlight specific reagents and equipment that we have found useful for 16S rRNA cloning projects in the classroom setting. Some protocols and reagents were purchased commercially. While straightforward, we recommend that instructors carefully separate kit reagents into aliquots appropriate for efficient and individual student use. Other protocols were based on standard procedures described in Molecular Protocols(2) and The Manual of Environmental Microbiology(8). These sources have been invaluable for general troubleshooting. Precise information about our protocols can be found in our recent research publication (3) and on our course website (http://www.wou.edu/las/natsci_math/biology/boomer/boomer.html).
TABLE 1.
Summary of course units, goals, and on-demand assessment
| Unit | Title | Concepts | Skills | Sample questions | Assessment |
|---|---|---|---|---|---|
| 1 | Plasmid DNA isolation from E. coli harboring 16S clones | Organization of bacterial DNA and plasmids; Structure and function of 16S rRNA; Chemistry of DNA and proteins; Spectrophotometry of nucleic acids | Use of microbiological media; Column separation and elution; Centrifugation; Calculating DNA concentration | Explain what each of the following reagents does and when it is used: SDS, nuclease-free water, resin, EDTA, and ampicillin. After cell lysis, neutralization, and centrifugation, the genomic DNA is in the pellet but the plasmid is not. Why? |
Conceptual Understanding Inquiry Conceptual |
| 2 | Restriction analysis of 16S clones | Origin and function of restriction enzymes; Enzyme optima and buffer Restriction fragment length polymorphisms; Principles of agarose gel electrophoresis | Calculating restriction digests; Graphing DNA mobilities; Mapping unknown plasmids; Computerized gel imaging | Using the provided Gibco catalog, determine how many times PinAI would cut a chromosome of 1 million base pairs? You have decided that your insert can only be cut with PinAI. However, your vector lacks this site. What other options do you have to clone your insert into your vector? |
Performance Performance Understanding Inquiry |
| 3 | DNA sequencing of 16S clones | Principles of DNA replication; Taq polymerases and extremozymes; Chemistry and structure of nucleotides; Principles of acrylamide gel electrophoresis | DNA sequencer operation; PCR operation; Multitasking integrated tasks | Consider the following things that are needed for DNA replication: template, primers, polymerase, and monomers. For each, explain its purpose and compare and contrast what is used for in vitro vs in vivo replication. Compare and contrast gel electrophoresis methods used for restriction analysis with those used for sequence analysis. |
Conceptual Conceptual |
| 4 | Bioinformatics and phylogenetics | Molecular evolution and chronometers; Structure and significance of 16S rRNA; Taxonomy and diversity of bacteria; Phylogenetic trees as “hypotheses”; Levelsof scientific literature | Computer-based DNA editing; Multiple sequence alignment; Internet-based data retrieval; Computational phylogenetics; Statistical analysis | In order for a molecular phylogeny to reflect organismal phylogeny, what four properties must the molecular data possess? Within the context of your phylogenetics lab exercise, what is your ultimate goal? |
Conceptual Understanding Inquiry |
| 5 | DNA isolation and cloning of new 16S community libraries | Topoisomerases and ligases in cloning; The lac operon, genetics and applications; Transformation and heat shock response; Organic extractions; Effects of buffer on PCR | Comparative DNA isolation; PCR operation; RFLP-based library screening | Explain how each of the following steps were achieved during genomic isolation: DNA precipitation, separation of DNA, and cell lysis. Discuss specific reagents. The vector that was used to clone PCR product was called pCRTopo/T-A. How does it achieve ligation so efficiently? |
Conceptual Conceptual |
Generating an inventory of 16S rRNA clones. Prior to implementing this curriculum, we troubleshot most methods and archived a significant inventory of 16S rRNA clones, necessary starting reagents for this class. During Units 1 through 4 (weeks 1 to 8), each student characterized 2 to 3 different 16S rRNA clones from this inventory. During Unit 5 (weeks 9 and 10), they prepared new clones from new samples, replenishing our inventory. This approach was chosen because characterization methods appeared technically more forgiving than PCR-based cloning procedures.
To generate 16S rRNA libraries, we homogenized and lysed frozen mat samples containing unknown bacteria of interest. Total genomic DNA was extracted and purified using standard phenol-chloroform and alcohol-salt precipitation. Genomic DNA was subjected to PCR amplification using either broad bacterial primers (1492RPL and 8FPL (10)) or GNS-specific primers (77FGNS and 953RRED) designed based on data from this project (3). DNA from variable environments was optimally amplified using a suite of PCR buffers that varied MgCl2 concentration. Thus, we combined the MasterAmp PCR Optimization Kit (Epicentre Technologies, Madison, Wis.) with standard Taq polymerase. Amplified product was directly ligated into the vector, pCR 2.1-TOPO (Invitrogen/Life Sciences, Carlsbad, Calif.) and transformed into chemically competent One Shot E. coli TOP10 cells (Invitrogen/Life Sciences). For PCR product-based cloning, these approaches have efficiently replaced older methods, bypassing restriction enzymes, gel isolation, and ligase-mediated recombinant technology.
We have found it useful to perform in-class experimental variations not only for teaching purposes but also because students must consider and compare past class results, an integral part of research science. For example, members of the 2000 class worked with larger red filamentous cells that had been crudely separated from smaller unicells in the mat prior to lysis; members of the 2001 class worked with whole communities, filaments, and unicells. The 2001 class compared PCR amplification products generated with general bacterial primers to products generated with GNS-specific primers; the 2000 class only used broad specificity bacterial primers.
Plasmid isolation—large and small scale. Students performed plasmid isolation procedures at two key points during the term. During Unit 1 (weeks 1 and 2), students isolated large-scale quantities of 2 to 3 assigned starting clones that they would characterize for the next six weeks. They employed the Promega Midi-Prep Kit (Promega, Madison, Wis.), the isolation kit recommended for our DNA sequencing apparatus, a Li-Cor 4200 Gene ReadIR Single Dye system (Li-Cor Inc., Lincoln, Nebr.). During Unit 5, students isolated crude, small-scale quantities of plasmid from ten white colonies from the new library using rapid “boiling miniprep” procedures (3).
Restriction enzymes. Restriction enzymes were utilized in the lab for three lab exercises. During Unit 2 (weeks 3 and 4), students used three different and informative restriction enzymes (EcoRI, HhaI, and PstI) to assess genetic diversity among assigned project clones. During Unit 5 (weeks 9 and 10), students screened 16S rRNA libraries for the presence of insert using an insert-flanking EcoRI site specific to this vector. Images of agarose gels were digitally captured and analyzed using a Fotodyne Investigator Analyst workstation (Fotodyne Inc., Hartland, Wis.).
DNA sequence analysis. DNA sequencing methods were based on standard chain-termination procedures (11) and performed using the SequiTherm EXCEL II DNA Sequencing Kit-LC (Epicentre Technologies). During the PCR amplification step, students set up acrylamide gels based on Li-Cor-specific protocols and equipment (Li-Cor Inc.).
Bioinformatics and phylogenetics. Using Base ImageIR software (Li-Cor Inc.), students edited obtained sequences. They submitted each to the Basic Local Alignment Search Tool (BLAST) on the National Center for Biotechnology Information website (http://www4.ncbi.nlm.nih.gov) in order to determine similarity to sequences in the GenBank database. Each student aligned his/her sequences to a dataset that contained representative 16S rRNA sequences from major lineages of bacteria and additional sequences chosen and retrieved based on BLAST results. Sequences were compiled into the biosequence editor program SeqPup version 0.9 (D. G. Gilbert) and aligned with ClustalW version 1.7 (J. Thompson, T. Gibson, and D. Higgins). Finally, students performed phylogenetic analysis using maximum parsimony methods with bootstrap resampling using PAUP 4.0b8 (Swofford, D. L., Sinauer Associates Inc., Sunderland, Mass.).
Assessment objectives. For each unit described above, we defined specific objectives for mastering biological and chemical concepts and related skills with an emphasis on microbial systematics and applications (Table 1). To encourage mastery of concepts and skills, we used a combination of lab notebook assignments (43% of total lab grade), quizzes (32% of total lab grade), and a phylogenetics report for evaluation (25% of total lab grade).
Assessment strategies and procedures employed follow those discussed in the National Research Council’s Inquiry and the National Science Education Standards(5). Our formats ranged from “on-demand” constructed responses (e.g., quizzes) to increasingly prolonged responses in “over time” projects (e.g., lab notebooks and the phylogenetics report). Specific examples of assessment objectives and on-demand quiz questions are provided in Table 1. Summative assessments were designed to evaluate the inquiry components of our curriculum, specifically: conceptual understandings in science (“conceptual” in Table 1), ability to perform scientific inquiry (“performance” in Table 1), and understandings about inquiry (“understanding inquiry” in Table 1). Over time assessments we employed are discussed in more detail below.
Lab teaching methods and assignments. For Units 1 to 3 (weeks 1 to 6) and Unit 5 (weeks 9 and 10), each unit consisted of a pair of in-lab exercises. The first of each pair was completed “side-by-side”; students worked with the primary instructor to master and understand skills, reagents, and equipment. Students worked with protocols in hand, discussing major points and questions as they completed procedures and took optional notes. At the end of the lab, students were required to record only the location and amount of all final products in their formal notebooks (10% of each Unit grade).
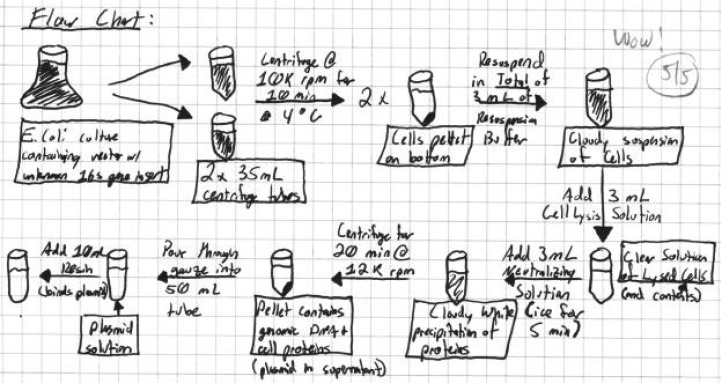
The second lab of each pair was deemed “independent”; students entered the lab with formally prepared prelab notebook exercises, executed the lab, and managed time with virtually no assistance. The prelab (56% of each Unit grade) evaluated whether students were organized, prepared, and understood concepts and skills. Prelab elements included a flow chart, a purpose-oriented reagent list, and procedures. An original student flow chart detailing large-scale plasmid isolation (Unit 1) is shown in Fig. 1. The in-lab portion of the notebook (34% of each Unit grade) consisted of observations gathered during the lab, data analyses, and a discussion of the results.
FIG. 1.
Student-generated flow chart depicting large-scale plasmid isolation procedures, Unit 1. This component of the prelab assignment comprised 10% of each Unit Lab grade. Evaluation notes in upper right corner are instructors additions.
During Unit 4 (weeks 7 and 8), we deviated from the repeated format above. Based on collected and combined sequence data, students formally assembled, analyzed, and discussed phylogenetic trees. Stated results requirements for this report were: (i) a summary of BLAST data emphasizing microbial diversity and origin, (ii) aligned data annotated in terms of three known 16S rRNA structural elements, and (iii) a maximum parsimony tree with bootstrap resampling. Our stated discussion requirements were: (i) a comparison of BLAST and tree-derived results, (ii) an assessment of the alignment based on known 16S rRNA stem loops, and (iii) a quantitative and qualitative evaluation of the significance of the tree in the context of BLAST data and the research project.
RESULTS
Research results. During Unit 1, students isolated between 0.18 and 1.48 μg/μl plasmid based on A260 values (Table 2). Students in the 2000 class, on average, isolated more plasmid (mean = 0.89 ± 0.32) than members of the 2001 class (mean = 0.77 ± 0.42). The purity of both class’ plasmid DNA was 1.60 to 2.21, based on A260/A280 values (Table 2). Predicted concentrations were corroborated by restriction analyses performed during Unit 2 (data not shown).
TABLE 2.
Summary of DNA isolation, purity, and sequence output
| Student | Site-clone | Amount aμg/l | Purityb (A260/A280) | Number of bases readc,d |
|---|---|---|---|---|
| 2000 Class | ||||
| Student 1 | Hillside-30 | 0.71 | 1.88 | 557 |
| Hillside-55 | 1.00 | 1.87 | 890 | |
| Student 2 | Hillside-27 | 0.99 | 1.89 | 513 |
| Hillside-43 | 0.82 | 1.86 | 975 | |
| Student 3 | Hillside-3 | 1.29 | 1.84 | 592 |
| Hillside-10 | 0.99 | 2.12 | 695 | |
| Student 4 | Hillside-1 | 0.83 | 1.88 | 692 |
| Hillside-25 | 0.70 | 2.21 | 831 | |
| Student 5 | Hillside-31 | 0.59 | 1.86 | 673 |
| Hillside-53 | 1.18 | 1.87 | 530 | |
| Student 6 | Hillside-8 | 0.49 | 1.87 | 651 |
| Hillside-36 | 0.40 | 1.68 | <200 | |
| Student 7 | Hillside-26 | 0.91 | 1.88 | 576 |
| Hillside-48 | 1.56 | 1.93 | 563 | |
| 2001 Class | ||||
| Student 8 | Fairy-1e | 0.92 | 1.92 | 746 |
| Fairy-9 | 1.07 | 1.92 | 430 | |
| Fairy-22 | 1.48 | 1.96 | 681 | |
| Student 9 | Fairy-6e | 0.43 | 1.89 | 461 |
| Fairy-57 | 0.18 | 1.70 | <200 | |
| Fairy-60 | 0.16 | 1.60 | <200 | |
| Student 10 | Fairy-3e | 0.71 | 1.94 | 343 |
| Fairy-7 | 1.13 | 1.96 | 241 | |
| Fairy-12 | 0.83 | 1.95 | <200 | |
| Student 11 | Fairy-2 | 1.06 | 1.94 | 321 |
| Fairy-11 | 1.22 | 1.81 | <200 | |
| Fairy-20 | 1.06 | 1.94 | <200 | |
| Student 12 | Fairy-5e | 0.27 | 1.90 | 481 |
| Fairy-52 | 0.18 | 1.71 | <200 | |
| Fairy-54 | 0.16 | 1.88 | <200 | |
| Student 13 | Fairy-33 | 0.97 | 1.91 | 647 |
| Fairy-13 | 0.95 | 1.90 | <200 | |
| Fairy-4 | 1.01 | 1.90 | <200 |
2000 class average was 0.89 (±0.32); 2001 class average was 0.77 (±0.42).
2000 class average was 1.90 (±0.13); 2001 class average was 1.87 (±0.10).
2000 class average was 672.15 (±145); 2001 class average was 483.44 (±174).
Information obtained from a single sequencing run.
Indicates a sequence that was vector only.
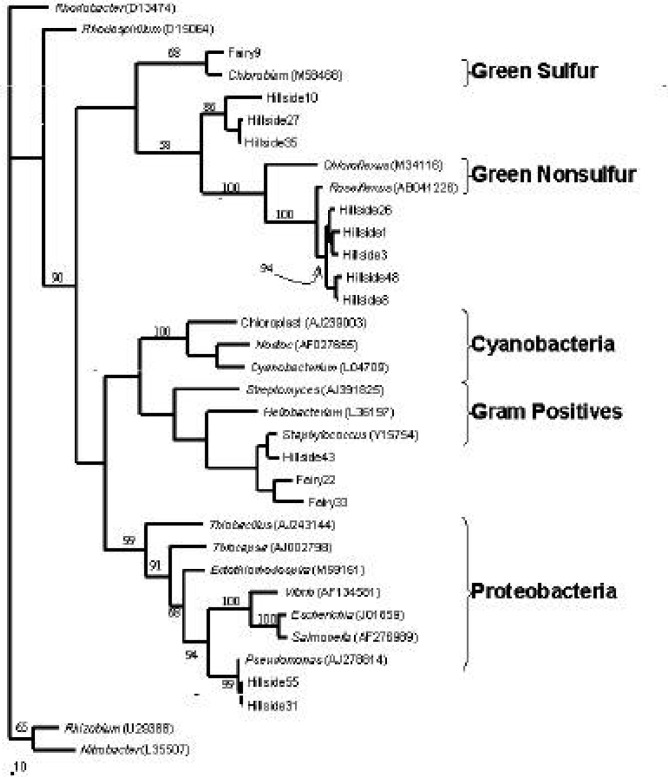
During Units 3 and 4, most students in the 2000 class generated adequate sequence data (at least 200 base pairs) for subsequent computational analyses. Over half of the 2000 class isolated GNS-like sequences from Hillside Spring (Table 2). However, less than half of the 2001 class generated adequate sequence data from the Yellowstone Fairy Spring clones. BLAST analysis demonstrated that all clones for which adequate sequence data were generated contained novel bacterial 16S rRNA genes (Tables 2 and 3). The remaining clones appeared to contain no insert (Table 2). Figure 2 depicts a phylogenetic tree from combined student data that was useable. Phylogenetic analysis served to support BLAST similarity results. For example, Hillside Spring clones 31 and 55, both similar to cultured Pseudomonas genera based on BLAST results, grouped likewise on the tree (99% bootstrap support). In some cases, phylogenetic trees improved classification of unknowns. For example, Fairy Spring clones 9 and 33, similar only to other “uncultured” 16S rRNA isolates based on BLAST results, grouped with gram positives on the tree, albeit with poor bootstrap support (less than 50%). That students addressed such strengths and weaknesses of the data was an essential component of the phylogenetic report. Observed genetic diversity could also be correlated with restriction fragment length polymorphisms (RFLPs) observed during Unit 2 (data not shown).
TABLE 3.
Summary of BLAST results
| Site | Clone(s) | Top two blast hits (accession) | Origin | Inferred lineage |
|---|---|---|---|---|
| Hillside | 1, 3, 8, 10, 25, 26 27, 30, 48, 53 | Roseiflexus castenholzii (AB041226) | Hot spring, Japan | Green nonsulfur |
| Uncultured (M62775) | Hot spring, Yellowstone | Gram positive | ||
| Hillside | 55 | Pseudomonas sp. (PSP297354) | Hospital sewage | Proteobacteria |
| Pseudomonas sp. (PSP297353) | Freshwater fish farm | Proteobacteria | ||
| Hillside | 43 | Bacillus psychrotolerans (AJ277983) | Not reported | Gram positive |
| Bacillus psychrophilus (X54969) | Not reported | Gram positive | ||
| Hillside | 31 | Uncultured (AF320337) | Hot spring, Yellowstone | Proteobacteria |
| Pseudomonas sp. (PSP297354) | Hospital sewage | Proteobacteria | ||
| Fairy | 9 | Uncultured (AF047635) | Iron Mountain pyrite sample | Not inferred |
| Uncultured (AF234699) | Nitrifying sludge sample | Not inferred | ||
| Fairy | 22 | Uncultured (U68674) | Deforested soil, East Amazonia | Not inferred |
| Uncultured (U81652) | Anaerobic wine distillery | Not inferred | ||
| Fairy | 7 | Uncultured (AF316769) | Crater Lake community | Not inferred |
| Helicobacter heilmanii (AF058770) | Feline gastrointestinal contents | Proteobacteria | ||
| Fairy | 2 | Uncultured (AF254393) | Bioremediation consortium | Proteobacteria |
| Bdellovibrio sp. (AF084863) | Not reported | Proteobacteria | ||
| Fairy | 33 | Uncultured (L22045) | Hot spring, Yellowstone | Gram positive |
| Uncultured (AJ302943) | Metal-polluted ground water | Not inferred |
FIG. 2.
Phylogenetic tree using representative class data. The tree was generated using maximum parsimony methods against a dataset of known bacteria (indicated in italics with accession number in parentheses). The bar indicates 10 nucleotide changes. Branch numbers indicate percent support for that branch. Bacterial lineages are indicated in brackets.
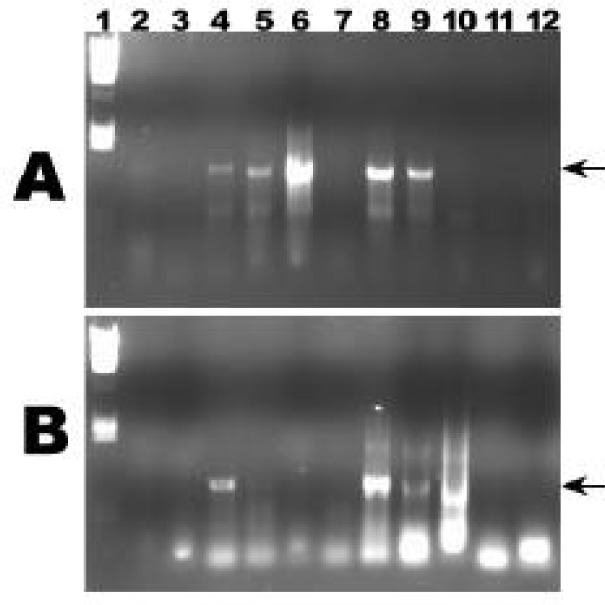
Figure 3 depicts representative 2001 data from Unit 5 which compares PCR product generated using buffers with varying MgCl2 concentrations (lanes 2 to 12, both panels) and broad bacterial versus GNS-specific primers (gel A versus gel B, respectively). The former amplified the larger (1,500 base pair) product; the latter amplified the smaller (900 base pair) product. Subsequent cloning and sequence analysis of this GNS product supports these findings (data not known).
FIG. 3.
Representative PCR data. Panel A was generated using general bacterial 16S primers. Panel B was generated with GNS-specific primers. Lane 1 in both A and B is marker standards (Lambda/HindIII), and lanes 2 through 12 were representative products using different PCR buffers. The arrows at the right indicate the target fragment. PCR product was separated using 1% agarose with standard TAE running buffer.
Evaluation results. We have summarized student performance in Table 4. Students earned 80 to 83% averages on quizzes and 84 to 88% on in-lab assignments. More disparate results were seen on phylogenetics reports, with 2000 class members averaging 89.1% and 2001 members averaging 81.2%. Of thirteen students who have completed this program, six sought and earned positions that utilize molecular biology in academics or private industry. Of three continuing students, one plans to pursue a career in biotechnology and one plans to pursue a career in DNA-oriented forensic science.
TABLE 4.
Summary of student performance and post-course pursuits
| Quiz (%)a | Prelab (%)b | In-lab (%)c | Report (%)d | Post-course pursuits | |
|---|---|---|---|---|---|
| 2000 Class | |||||
| Student 1 | 94 | 99 | 94 | 100 | Research and academics |
| Student 2 | 72 | 93 | 96 | 83 | Optometry school |
| Student 3 | 70 | 75 | 75 | 85 | Biotechnology and industry |
| Student 4 | 96 | 93 | 91 | 90 | Medical school |
| Student 5 | 72 | 93 | 95 | 93 | Dental school |
| Student 6 | 86 | 69 | 72 | 90 | Research and academics |
| Student 7 | 76 | 94 | 95 | 83 | Radiology school |
| 2001 Class | |||||
| Student 8 | 97 | 100 | 100 | 82 | Research and academics |
| Student 9 | 88 | 88 | 83 | 77 | Biotechnology and industry |
| Student 10 | 88 | 95 | 94 | 90 | Molecular Ph.D. program |
| Student 11 | 74 | 65 | 60 | 68 | Continuinge (biotechnology) |
| Student 12 | 76 | 66 | 78 | 97 | Continuinge (education) |
| Student 13 | 75 | 93 | 100 | 73 | Continuinge (forensics) |
2000 class average was 80.9; 2001 class average was 83.0.
2000 class average was 88.0; 2001 class average was 84.5.
2000 class average was 88.3; 2001 class average was 85.8.
2000 class average was 89.1; 2001 class average was 81.2.
Student has not graduated. Parentheses indicate student’s expressed post-course interest.
DISCUSSION
We promote undergraduate research and consequently rely on student-generated data from independent study and course-based research. Prior to the implementation of this curriculum, we had accumulated several lines of non-DNA evidence to support our hypothesis that Yellowstone red communities contained novel filamentous GNS bacteria (3, 4). It was thus a pleasant surprise when the 2000 class isolated multiple Hillside-Spring-derived sequences that resembled Roseiflexus, a red GNS bacterium from hot springs in Japan (6). Class results lead to the design of GNS-specific primers that have enabled us to compare red GNS sequences from four additional red-layer communities in Yellowstone (3).
Given the relative success of the 2000 class, we were surprised that the 2001 class obtained more limited data using Fairy Spring isolates. We attributed sequence data deficits to a combination of lower plasmid yields, less organization in setting up reactions, and more problems loading sequencing gels. These anecdotal observations may also reflect the slight differences we observed for notebook and project averages, both assignments of which required analysis, attention to detail, and organization. Equally troubling from a technical standpoint, only about half of the clones that provided adequate sequence information contained insert. Given that clones had been screened for insert prior to archiving, we surmised that clone instability or contamination contributed to this problem.
All insert-containing clones generated and analyzed contained novel 16S rRNA sequences. Consistent with our hypothesis that observed red filaments were GNS bacteria, the 2000 class isolated predominantly GNS-like sequences because they worked with clones from physically separated filaments prior to lysis. In contrast, we were not surprised that the 2001 class isolated moderately diverse bacterial sequences given that no steps to physically separate filaments from cohabiting unicellular bacteria were taken. While our research project has directly benefited from GNS-like sequences, retrieved non-GNS sequences have been diverse, novel, and interesting (Table 3 and Fig. 2). In particular, BLAST results excited students not only with the ubiquity of bacteria from seemingly everywhere, but also with the breadth of microbial research using 16S rRNA methods being performed throughout the world. Both phylogenetic and BLAST results intrigued students and instructors alike because of data that suggested unexpected relationships between microorganisms (e.g., How could a psychrophilic Bacillus be most similar to a hot-spring-derived bacterial sequence?).
Given the above observations, we are considering key changes to our program. For example, until we evaluated combined student data, we did not appreciate how significant the quantity and quality of the initial plasmid isolation was for the rest of the lab. Therefore, we will take stronger measures to emphasize the need for precision even during these relatively forgiving procedures. While we have avoided grading based on explicit data quality, we advocate that it may be wise to assign a percent of each notebook score based on data quality.
We also intend to substantially condense our existing restriction enzyme unit in order to add Southern blot hybridization methods to our curriculum. We have just successfully designed and implemented GNS-specific probes in our research lab and are troubleshooting methods for classroom use (data not shown). Such analyses using these and other lineage-specific probes would add to this course. We also intend to better link computational DNA results to observed RFLP patterns. For example, students could use available software to predict RFLPs based on obtained DNA sequences or database information in order to draw comparisons with previous results, thereby contributing to the over time and understanding inquiry components of class assessment and teaching strategies.
In terms of assessment, we were somewhat surprised that most scores seemed to correlate with obtained data and data quality. For a curriculum that builds progressively, the implications of early-stage problems on later exercises could potentially have serious effects, both in terms of student progress, morale, and interest. It is unclear whether directly scoring data or product quality at each stage will improve or diminish student performance, both at the bench and in terms of being able to analyze more and better data over time. Nevertheless, we were encouraged that both classes demonstrated similar proficiency on quizzes. While this suggested a similar mastery of concepts, we understand that mastery of content for quizzes is a skill common to most coursework in the sciences. Whether simple practice or proposed course changes will improve skills at the bench remain questions that will guide the way we teach this exciting and evolving class.
In addition to improving such summative assessment approaches, we intend to employ formative assessments that objectively survey student perceptions about the course and our summative methods, including proposed product-based scoring. Until now, we have not performed attitudinal surveys because of small class size and the advanced elective nature of the class. Given increasing local biotechnology industry development (e.g., GeneTools, LLC and AVI BioPharma, Inc.), we anticipate increased student interest in this course. Given such trends and our experience advising all these students, we chose to present known career interests and pursuits. With the 2000 class, half the students actively pursued graduate or professional programs and had laid out these plans well in advance of budding biotechnology industry. Of the three who earned research or biotechnology positions, two had been undecided during class but ended up employed in local positions as a direct result of experience or connections from this class. The third actively pursued a distant medical molecular biology research position. With the 2001 class, most of the students earned or now intend to earn local molecular-oriented positions in research, industry, or forensics.
Taken together, all of these outcomes support the broad applicability of our 16S rRNA project-based curriculum to a variety of educational and career interests. In terms of adapting this approach, though, instructors should plan to carefully research DNA isolation procedures for specific samples as these can vary. Nevertheless, the efficiency and utility of PCR optimization and cloning reagents solved key trouble spots in our methods and enabled us to move forward with this project and curriculum. Finally, we recommend that instructors prepare an adequate archive of clones and sequences because even a research-based course needs structure and “emergency data” to be effective given clearly variable student performance.
Acknowledgments
A large portion of this work was supported by an NSF Microbial Observatories/Research at Undergraduate Institute grant (NSF-MO/RUI 0074452). The Li-Cor DNA sequencer was acquired via an NSF Improved Laboratory Instrumentation Grant (NSF-ILI DUE-9851322). All research trips to Yellowstone were supported by annual Western Oregon University Foundation grants. We greatly appreciate the assistance and support of Yellowstone National Park personnel. We thank Brian Hedlund, John Gosink, and Russ Herwig for technical assistance and advice regarding DNA extraction, 16S rRNA isolation and analysis, and primer design. We appreciate Dick Castenholz and Bev Pierson for their recommendations about red-layer sites. We thank Philip Wade for helpful assistance with assessment. Students who participated in this curriculum included: Ramon Larios, Chris Coverdill, Kevin Larson, Michelle Hase, Nathan Fitzpatrick, Jeremy Taylor, Daniel Lodge (then an undergraduate), Peter Williams, Nicole Mullins, Norm McIntosh, Jeannine Earnest, Sarah Crosky, and Jim Erdman. All samples were collected during field research trips with Western Oregon University senior-level biology majors. Students who participated in Yellowstone survey and sampling included: Ben Stern, Robin Leitch, Daniel Russell, Joseph Hayes, Kody Phillis, Chris Coverdill, Kevin Larson, Rex Addis, Michelle Hase, Nicole Mullins, Eric Stroup, Mandy Ziglinski, Jessica Cameron, and Jeannine Earnest. Finally we appreciate Robert Turner for his contribution to the lecture portion of this class.
REFERENCES
- 1.Amann R, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Short protocols in molecular biology. 3rd ed. John Wiley and Sons, Inc; New York, N.Y.: 1997. [Google Scholar]
- 3.Boomer SM, Lodge DP, Dutton BE, Pierson B. Molecular characterization of novel red green nonsulfur bacteria from five distinct hot spring communities in Yellowstone National Park. Appl Environ Microbiol. 2002;68:346–355. doi: 10.1128/AEM.68.1.346-355.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boomer SM, Pierson BK, Austinhirst R, Castenholz RW. Characterization of novel bacterio-chlorophyll-a-containing red filaments from alkaline hot springs in Yellowstone National Park. Arch Microbiol. 2000;174:152–161. doi: 10.1007/s002030000189. [DOI] [PubMed] [Google Scholar]
- 5.Committee on Development of an Addendum to the National Science Education Standards on Scientific Inquiry . Inquiry and the National Science Education Standards, a guide for teaching and learning. National Academy Press; Washington D.C.: 2000. [Google Scholar]
- 6.Hanada S, Takaichi S, Matsuura K, Nakamura K. Roseiflexus castenholzii gen. nov., sp. nov., a thermophilic, filamentous, photosynthetic bacterium which lacks chlorosomes. Int. J. Syst. Bacteriol. 52:187–93. doi: 10.1099/00207713-52-1-187. [DOI] [PubMed] [Google Scholar]
- 7.Hugenholtz P, Goebel BM, Pace NR. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol. 1998;180:4765–4774. doi: 10.1128/jb.180.18.4765-4774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurst CJ, Crawford RL, Knudsen GR, McInerney MJ, Stetzenbach LD, editors. Manual of environmental microbiology. 2nd ed. ASM Press; Washington D.C.: 2002. [Google Scholar]
- 9.Pace NR. A molecular view of microbial diversity and the biosphere. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- 10.Reysenbach AL, Wickham GS, Pace NR. Phylogenetic analysis of the hyperthermophilic pink filament community in Octopus Spring, Yellowstone National Park. Appl Environ Microbiol. 1994;60:2113–2119. doi: 10.1128/aem.60.6.2113-2119.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winfrey MR, Rott MA, Wortman AT. Unraveling DNA molecular biology for the laboratory. Prentice-Hall, Inc; Upper Saddle River, N.J.: 1997. [Google Scholar]
- 13.Woese CR. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]