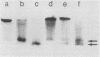
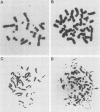
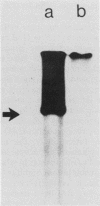
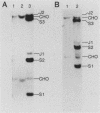
Abstract
Recent experiments have shown that gene amplification can be mediated by submicroscopic, autonomously replicating, circular extrachromosomal molecules. We refer to those molecules as episomes (S. Carroll, P. Gaudray, M. L. DeRose, J. F. Emery, J. L. Meinkoth, E. Nakkim, M. Subler, D. D. Von Hoff, and G. M. Wahl, Mol. Cell. Biol. 7:1740-1750, 1987). The experiments reported in this paper explore the way episomes are formed and their fate in the cell over time. The data reveal that in our system the episomes are initially 250 kilobases, but gradually enlarge until they become double minute chromosomes. In addition, we show that episomes or double minute chromosomes can integrate into chromosomes. Our results also suggest that episomes can be produced by deletion of the corresponding sequences from the chromosome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair G. M., Stallings R. L., Nairn R. S., Siciliano M. J. High-frequency structural gene deletion as the basis for functional hemizygosity of the adenine phosphoribosyltransferase locus in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5961–5964. doi: 10.1073/pnas.80.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo K., Schwab M. Oncogene amplification in tumor cells. Adv Cancer Res. 1986;47:235–281. doi: 10.1016/s0065-230x(08)60201-8. [DOI] [PubMed] [Google Scholar]
- Carroll S. M., Gaudray P., De Rose M. L., Emery J. F., Meinkoth J. L., Nakkim E., Subler M., Von Hoff D. D., Wahl G. M. Characterization of an episome produced in hamster cells that amplify a transfected CAD gene at high frequency: functional evidence for a mammalian replication origin. Mol Cell Biol. 1987 May;7(5):1740–1750. doi: 10.1128/mcb.7.5.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Cowell J. K. Double minutes and homogeneously staining regions: gene amplification in mammalian cells. Annu Rev Genet. 1982;16:21–59. doi: 10.1146/annurev.ge.16.120182.000321. [DOI] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Gardella T., Medveczky P., Sairenji T., Mulder C. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J Virol. 1984 Apr;50(1):248–254. doi: 10.1128/jvi.50.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D. L., Powers V. E. Amplified DNA sequences in Y1 mouse adrenal tumor cells: association with double minutes and localization to a homogeneously staining chromosomal region. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1597–1601. doi: 10.1073/pnas.79.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin B. E., Björck E., Bjursell G., Lindahl T. Sequence complexity of circular Epstein-Bar virus DNA in transformed cells. J Virol. 1981 Oct;40(1):11–19. doi: 10.1128/jvi.40.1.11-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn P., Kapp L. N., Morgan W. F., Painter R. B. Chromosomal changes without DNA overproduction in hydroxyurea-treated mammalian cells: implications for gene amplification. Cancer Res. 1986 Sep;46(9):4607–4612. [PubMed] [Google Scholar]
- Hamkalo B. A., Farnham P. J., Johnston R., Schimke R. T. Ultrastructural features of minute chromosomes in a methotrexate-resistant mouse 3T3 cell line. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1126–1130. doi: 10.1073/pnas.82.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J. L., Milbrandt J. D., Heintz N. H., Azizkhan J. C. DNA sequence amplification in mammalian cells. Int Rev Cytol. 1984;90:31–82. doi: 10.1016/s0074-7696(08)61487-4. [DOI] [PubMed] [Google Scholar]
- Howell N., Belli T. A., Zaczkiewicz L. T., Belli J. A. High-level, unstable adriamycin resistance in a Chinese hamster mutant cell line with double minute chromosomes. Cancer Res. 1984 Sep;44(9):4023–4029. [PubMed] [Google Scholar]
- Jones R. S., Potter S. S. L1 sequences in HeLa extrachromosomal circular DNA: evidence for circularization by homologous recombination. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1989–1993. doi: 10.1073/pnas.82.7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Schimke R. T. Amplification and loss of dihydrofolate reductase genes in a Chinese hamster ovary cell line. Mol Cell Biol. 1981 Dec;1(12):1069–1076. doi: 10.1128/mcb.1.12.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe T. D., Swyryd E. A., Bruist M., Stark G. R. Stable mutants of mammalian cells that overproduce the first three enzymes of pyrimidine nucleotide biosynthesis. Cell. 1976 Dec;9(4 Pt 1):541–550. doi: 10.1016/0092-8674(76)90036-2. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Bigner S. H., Bigner D. D., Trent J. M., Law M. L., O'Brien S. J., Wong A. J., Vogelstein B. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987 Apr 3;236(4797):70–73. doi: 10.1126/science.3563490. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Zehnbauer B. A., Brodeur G. M., Seeger R. C., Trent J. M., Meltzer P. S., Vogelstein B. Amplification units containing human N-myc and c-myc genes. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1031–1035. doi: 10.1073/pnas.83.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl N. E., Kanda N., Schreck R. R., Bruns G., Latt S. A., Gilbert F., Alt F. W. Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell. 1983 Dec;35(2 Pt 1):359–367. doi: 10.1016/0092-8674(83)90169-1. [DOI] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Copeland N. G., Jenkins N. A., Lampkin B. C., Cavenee W. K. Loss of heterozygosity in three embryonal tumours suggests a common pathogenetic mechanism. Nature. 1985 Jul 25;316(6026):330–334. doi: 10.1038/316330a0. [DOI] [PubMed] [Google Scholar]
- Lin C. C., Alitalo K., Schwab M., George D., Varmus H. E., Bishop J. M. Evolution of karyotypic abnormalities and C-MYC oncogene amplification in human colonic carcinoma cell lines. Chromosoma. 1985;92(1):11–15. doi: 10.1007/BF00327240. [DOI] [PubMed] [Google Scholar]
- Mariani B. D., Schimke R. T. Gene amplification in a single cell cycle in Chinese hamster ovary cells. J Biol Chem. 1984 Feb 10;259(3):1901–1910. [PubMed] [Google Scholar]
- Maurer B. J., Lai E., Hamkalo B. A., Hood L., Attardi G. Novel submicroscopic extrachromosomal elements containing amplified genes in human cells. Nature. 1987 Jun 4;327(6121):434–437. doi: 10.1038/327434a0. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Mirkovitch J., Mirault M. E., Laemmli U. K. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984 Nov;39(1):223–232. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- Mouchès C., Pasteur N., Bergé J. B., Hyrien O., Raymond M., de Saint Vincent B. R., de Silvestri M., Georghiou G. P. Amplification of an esterase gene is responsible for insecticide resistance in a California Culex mosquito. Science. 1986 Aug 15;233(4765):778–780. doi: 10.1126/science.3755546. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Schultes N. P., Szostak J. W. Chromosome length controls mitotic chromosome segregation in yeast. Cell. 1986 May 23;45(4):529–536. doi: 10.1016/0092-8674(86)90284-9. [DOI] [PubMed] [Google Scholar]
- Otto E., Young J. E., Maroni G. Structure and expression of a tandem duplication of the Drosophila metallothionein gene. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6025–6029. doi: 10.1073/pnas.83.16.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Wahl G. M., Stark G. R. Structure of the gene for CAD, the multifunctional protein that initiates UMP synthesis in Syrian hamster cells. Mol Cell Biol. 1982 Mar;2(3):293–301. doi: 10.1128/mcb.2.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson D., Carnright D. V. Biochemical genetic analysis of pyrimidine biosynthesis in mammalian cells: I. Isolation of a mutant defective in the early steps of de novo pyrimidine synthesis. Somatic Cell Genet. 1977 Sep;3(5):483–495. doi: 10.1007/BF01539120. [DOI] [PubMed] [Google Scholar]
- Quinn L. A., Moore G. E., Morgan R. T., Woods L. K. Cell lines from human colon carcinoma with unusual cell products, double minutes, and homogeneously staining regions. Cancer Res. 1979 Dec;39(12):4914–4924. [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification, drug resistance, and cancer. Cancer Res. 1984 May;44(5):1735–1742. [PubMed] [Google Scholar]
- Schimke R. T., Sherwood S. W., Hill A. B., Johnston R. N. Overreplication and recombination of DNA in higher eukaryotes: potential consequences and biological implications. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2157–2161. doi: 10.1073/pnas.83.7.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. M., Horsch R. B., Klee H. J., Kishore G. M., Winter J. A., Tumer N. E., Hironaka C. M., Sanders P. R., Gasser C. S., Aykent S., Siegel N. R., Rogers S. G., Fraley R. T. Engineering herbicide tolerance in transgenic plants. Science. 1986 Jul 25;233(4762):478–481. doi: 10.1126/science.233.4762.478. [DOI] [PubMed] [Google Scholar]
- Simon A. E., Taylor M. W. High-frequency mutation at the adenine phosphoribosyltransferase locus in Chinese hamster ovary cells due to deletion of the gene. Proc Natl Acad Sci U S A. 1983 Feb;80(3):810–814. doi: 10.1073/pnas.80.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R. DNA amplification in drug resistant cells and in tumours. Cancer Surv. 1986;5(1):1–23. [PubMed] [Google Scholar]
- Stark G. R., Wahl G. M. Gene amplification. Annu Rev Biochem. 1984;53:447–491. doi: 10.1146/annurev.bi.53.070184.002311. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Introduction of homologous DNA sequences into mammalian cells induces mutations in the cognate gene. Nature. 1986 Nov 6;324(6092):34–38. doi: 10.1038/324034a0. [DOI] [PubMed] [Google Scholar]
- Trent J., Meltzer P., Rosenblum M., Harsh G., Kinzler K., Mashal R., Feinberg A., Vogelstein B. Evidence for rearrangement, amplification, and expression of c-myc in a human glioblastoma. Proc Natl Acad Sci U S A. 1986 Jan;83(2):470–473. doi: 10.1073/pnas.83.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanin E. F., Henthorn P. S., Kioussis D., Grosveld F., Smithies O. Unexpected relationships between four large deletions in the human beta-globin gene cluster. Cell. 1983 Dec;35(3 Pt 2):701–709. doi: 10.1016/0092-8674(83)90103-4. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Pardoll D. M., Coffey D. S. Supercoiled loops and eucaryotic DNA replicaton. Cell. 1980 Nov;22(1 Pt 1):79–85. doi: 10.1016/0092-8674(80)90156-7. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Robert de Saint Vincent B., DeRose M. L. Effect of chromosomal position on amplification of transfected genes in animal cells. Nature. 1984 Feb 9;307(5951):516–520. doi: 10.1038/307516a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Vitto L., Padgett R. A., Stark G. R. Single-copy and amplified CAD genes in Syrian hamster chromosomes localized by a highly sensitive method for in situ hybridization. Mol Cell Biol. 1982 Mar;2(3):308–319. doi: 10.1128/mcb.2.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worton R. G., Ho C. C., Duff C. Chromosome stability in CHO cells. Somatic Cell Genet. 1977 Jan;3(1):27–45. doi: 10.1007/BF01550985. [DOI] [PubMed] [Google Scholar]
- de Saint Vincent B. R., Delbrück S., Eckhart W., Meinkoth J., Vitto L., Wahl G. The cloning and reintroduction into animal cells of a functional CAD gene, a dominant amplifiable genetic marker. Cell. 1981 Dec;27(2 Pt 1):267–277. doi: 10.1016/0092-8674(81)90410-4. [DOI] [PubMed] [Google Scholar]
- den Dunnen J. T., Bakker E., Breteler E. G., Pearson P. L., van Ommen G. J. Direct detection of more than 50% of the Duchenne muscular dystrophy mutations by field inversion gels. Nature. 1987 Oct 15;329(6140):640–642. doi: 10.1038/329640a0. [DOI] [PubMed] [Google Scholar]