Abstract
Background
Identification of risk factors for reduced asthma control could improve the understanding and treatment of asthma. A promoter polymorphism in the 5-lipoxygenase gene affects gene expression and response to asthma therapy but its impact on disease control remains unclear.
Objective
We sought to determine if the ALOX5 promoter SP1 tandem repeat polymorphism was associated with changes in cysteinyl leukotriene production, lung function, airway inflammation and asthma control score.
Methods
We analyzed 270 children 6-17 years old with poorly controlled asthma enrolled in a 6-month clinical trial (NCT00604851). In secondary analysis, we associated the ALOX5 promoter SP1 tandem repeat polymorphism genotype (rs59439148) with asthma outcomes using both additive and recessive genetic models. We evaluated FEV1 percent predicted, symptom control, exhaled nitric oxide and urinary LTE4 levels.
Results
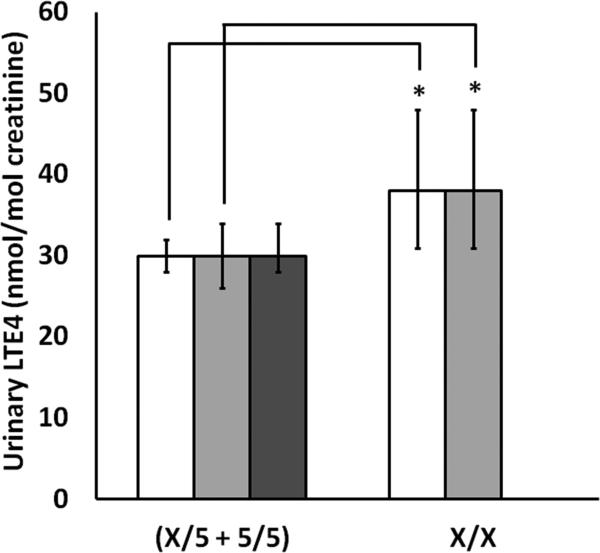
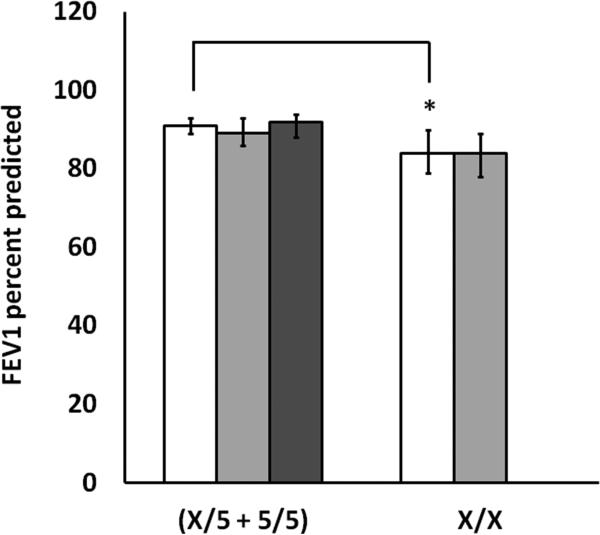
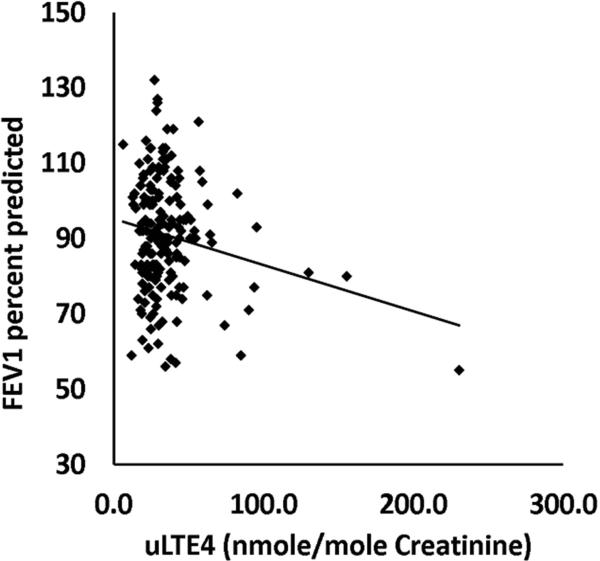
14.8% (40/270) of all children (and 28% (38/135) of African Americans) carried 2 non-5 repeat variant alleles of rs59439148. Children who were homozygous for variant alleles had significantly higher urinary LTE4 levels (38 versus 30 nmol/mol creatinine, p=.0134), significantly worse FEV1% predicted (84 versus 91, p=.017), and a trend toward worse asthma control. FEV1% predicted values were significantly negatively correlated with urinary LTE4 (r = -0.192, p=.009).
Conclusion and Clinical Relevance
Carrying two copies of a minor variant ALOX5 promoter SP1 tandem repeat allele contributes to increased cysLT exposure as determined by urinary LTE4 levels, reduced lung function, and potentially worse asthma control. ALOX5 promoter SP1 tandem repeat genotype may be a risk factor for worse asthma outcomes.
Keywords: Asthma, Children, ALOX5, 5-Lipoxygenase, Leukotriene, Asthma Control, FEV1, Exhaled Nitric Oxide, Genetic Association
INTRODUCTION
Asthma is one of the most common chronic diseases of childhood. The lack of adequate symptom control continues to be a major public health problem. Asthma symptom exacerbations constitute a leading cause of missed school days, impaired quality of life, emergency department visits and hospitalizations in children[1]. African Americans, for unknown reasons, have a greater prevalence and severity of asthma than Whites[2]. Factors that promote impaired symptom control and loss of lung function must be identified to improve the understanding of asthma pathogenesis and therapeutic control.
Leukotrienes (LTs) are naturally occurring eicosanoid lipids that are important inflammatory mediators in the pathogenesis of asthma. The cysteinyl LTs (LTC4, LTD4 and LTE4) bind to and activate two G-protein coupled receptors, cysLTR1 and cysLTR2[3], contributing to several deleterious airway processes including influx of eosinophils, hyperreactivity, smooth muscle hypertrophy, mucosal edema and mucus hypersecretion[4]. The cysteinyl leukotrienes (cysLTs) and LTB4 are formed from arachidonic acid, which is released from cell-membrane phospholipids by the action of cytosolic phospholipase A2. Arachidonic acid is converted to LTA4 by membrane-bound 5-lipoxygenase (5-LO) and 5-lipoxygenase activating protein (FLAP)[5].
5-Lipoxygenase is encoded by ALOX5, which is located on chromosome 10q11.21. Early studies identified tandem hexanucleotide repeat variants in the core promoter of ALOX5 that were associated with diminished reporter gene activity in tissue culture[6]. The promoter polymorphism can include 3 to 7 copies of the Sp1-binding motif GGGCGG. The 5 repeat allele is the major allele among various populations, including both African Americans and whites, and is thus referred to as the major repeat variant. Compared to the common 5 repeat allele, variants with non-5 repeats have been associated with variable response to leukotriene inhibitors and leukotriene receptor antagonists [7, 8], supporting the notion that the major repeat variant is associated with increased production of LTs. In a separate study, patients who were homozygous for minor repeat variants had reduced response to montelukast, a leukotriene receptor antagonist (LTRA), which is also consistent with the major repeat variant being associated with increased LT production[9]. In contrast, gene expression studies in primary leukocytes from healthy individuals homozygous for the 3 and 4 repeat alleles had higher 5-LO, FLAP, and LTA4H expression compared with homozygous carriers of the major repeat variant[10]. Other studies[11], including a pharmacogenetic study of montelukast, also suggest that minor repeat variants may be associated with increased production of LTs[8].
The biologically active cysLTs, LTC4 and LTD4, are rapidly converted to LTE4, a fraction of which is excreted in the urine. Urinary LTE4 (uLTE4) concentration is a sensitive measure of whole body cysLT production[12]. In the present study, we tested the hypotheses that the ALOX5 promoter SP1 tandem repeat polymorphism (rs59439148) is associated with urinary leukotriene levels, FEV1 percent predicted (FEV1%pred), Asthma Control Questionnaire (ACQ) scores[13] and exhaled nitric oxide (ENO). We evaluated these associations among 270 well characterized children with asthma whose symptoms were poorly controlled on ICS, age 6 to 17 years, who participated in a randomized clinical trial conducted by the American Lung Association Asthma Clinical Research Centers.
METHODS
The design and results of the parent study have been published[14]. All participants gave written informed consent. The parent study (Study of Acid-Reflux in Childhood Asthma (SARCA)) was registered with ClinicalTrials.gov (NCT00604851) and was approved by the Nemours Florida IRB (82404-31) and by all other IRBs of the American Lung Association – Asthma Clinical Research Centers (ALA-ACRC). SARCA was designed to assess the efficacy of daily oral lansoprazole versus placebo for the treatment of persistent asthma in a 24-week multicenter randomized clinical trial. All participants had to be between 6 and 17 years of age with asthma that was inadequately controlled by the participant's baseline therapeutic regimen which included ICS. Inadequate control was defined as a screening ACQ>1.25, two or more unscheduled healthcare visits or steroid bursts in the previous twelve months, weekly asthma-related nocturnal awakening, or the need for rescue bronchodilator use more than once per week over the previous month. The current study includes only participants who consented to genetic analysis.
Data Collected
Baseline characteristics were recorded at the screening visit (V1). Participants were screened to ensure a population with inadequate asthma control on inhaled corticosteroids. Following a 2-week run-in period, participants submitted a saliva sample for DNA isolation at the randomization visit (V2). Following the 24-week intervention, consenting participants also submitted a urine sample for urinary leukotriene at the final visit (V9). Asthma control was assessed using the 7-item ACQ[13] that was also recorded at the final visit. The ACQ considers FEV1 plus six measures of symptom control from the previous seven days. ACQ score ranges from 0 to 6, with a higher score indicating worse asthma control; the minimal clinically important difference (MID) is 0.5. A pre-bronchodilator FEV1 was determined by spirometry adhering to ATS/ERS-defined procedures[15].
DNA Preparation and Genotyping
Patient genomic DNA was prepared from buccal cells in saliva samples collected using Oragene DNA sample collection kits (DNA Genotek Inc., Kanata, Ontario, Canada) following the manufactures guidelines. The ALOX5 promoter SP1 tandem repeat polymorphism (marker rs59439148) was genotyped as previously described[16, 17]. (See online repository for examples of representative genotypes).
Briefly, the genomic region encompassing the promoter repeats was PCR-amplified using 25ng of genomic DNA with the following primers: Forward: 5' FAM-labeled ACAGACACCTCGCTGAGGAGAGAC 3'; Reverse: 5' GAGCAGCGAGCGCCGGGAGCC 3'. Reactions conditions included 0.4uM of each primer, 1.5mM MgCl2, 2% DMSO, 2mM dATP, 2mM dCTP, 2mM dTTP, 0.5mM dGTP, and 1.5mM deaza dGTP (Roche Diagnostics, Mannheim, Germany), 1.25U of Platinum Taq (Invitrogen, Carlsbad, CA), in a final volume of 25ul. Thermocycling conditions were as follows: an initial denaturation step at 95° C for 12min; 10 cycles at 95° C for 1min and 68° C for 2min; and an 25 additional cycles at 94° C for 30sec, 60° C for 30sec, and 72° C for 45sec. PCR products were diluted with formamide and run on an Applied Biosystems (ABI) 3100 Genetic Analyzer. Genotypes were assigned using ABI's fragment analysis software.
Measurement of Urinary LTE4
uLTE4 concentrations were determined by LC-MS/MS analysis (Kronos Science, Phoenix, Arizona) using an API 4000 LC/MS/MS System (Applied Biosystems/MDS SCIEX) in conjunction with an Agilent 1100 Series HPLC system (Agilent, Santa Clara, California). The method's accuracy ranges from 100% to 114%. The intra and inter-precision ranges are from 4% to 6% daily and 13 to 19% over a three year period. Normalized values were expressed as the ratio of LTE4 (nmol) to creatinine (mol) ratio. The method has a limit of quantitation of 91 fmole/mL.
Measurement of Exhaled Nitric Oxide
Fractional exhaled Nitric Oxide (ENO) was measured in all patients at the final visit of the trial using on-line breath collection consistent with the ATS expert panel procedures[18]. Participants inhaled nitric oxide-free gas through a scrubbing filter to total lung capacity and slowly exhaled to residual volume at a near constant flow-rate. ENO was analyzed using a chemiluminescence analyzer that is calibrated daily (Sievers NOA 280-I or the Aerocrine NIOX®).
Data Analysis
Baseline values stratified by genotype were compared by Fisher's Exact Test (categorical variables) or by independent sample Student's T-test (continuous variables). uLTE4 levels, FEV1%pred, ACQ score and ENO (all obtained following 24 weeks of treatment) were compared according to genotype at marker rs59439148 using independent sample Student's T-tests. uLTE4 and ENO levels were log transformed prior to analysis as they were positively skewed. ACQ scores were square root transformed prior to analysis as they had a moderate degree of positive skewing, and contained zero values. FEV1%pred values were normally distributed. Both recessive (5/5 + 5/X versus X/X) and additive (5/5 versus 5/X versus X/X) general linear models were tested, however the recessive model more accurately described the distribution of the data. The recessive model was also used to model the association between ALOX5 promoter SP1 tandem repeat genotype and asthma phenotypes in a previous pharmacogenetic study conducted by our laboratory[8]. All tests were two-tailed at a level of significance of 0.05. Reported means and 95% confidence intervals are the corresponding back-transformed values. All analyses were performed in IBM SPSS Statistics Version 19 (IBM, Armonk, New York, USA).
RESULTS
Participant Characteristics
Of the 306 participants, 270 gave consent for the current study and included 135 (50%) African American, and 103 (38%) White children (Table 1). Lansoprazole treatment did not affect FEV1, ACQ[14], ENO or uLTE4 (data not shown). Patients were stratified by genotype at marker rs59439148 into two groups: 1) patients having at least one ALOX5 promoter allele with 5 SP1 tandem repeats (X/5 + 5/5; 230 patients, 84%), and 2) patients with all other combinations (X/X; 40 patients, 15%). The baseline characteristics of the two groups were similar (Table 1), with race and current LTRA therapy being the exceptions. Patients with X/X genotype were more likely to be taking montelukast for asthma control (p=.024), though adherence to montelukast during the intervention period did not differ by genotype (data not shown). Variant allele (X, non-5) frequency was greater in African Americans than in other races, particularly the 3 repeat variant, which has an allele frequency of 31% in African Americans versus 0.5% in Whites (Table 2). The 5 repeat allele is the most common allele for all races with a frequency of 48% and 82% in African Americans and Whites, respectively (Table 2). In African Americans, the most common genotypes were 3/5 (24%), 5/5 (24%), and 4/5 (21%). In Whites, 5/5 (64%), and 4/5 (31%) were the most common genotypes, while few other variant combinations were present (Table 2). The study was under powered to assess the prevalence of the polymorphism within groups of different ethnicity.
Table 1.
Baseline Demographic Characteristics by ALOX5 Promoter Genotype
| Demographic Characteristics | All Patients | X/X | 5/5 + 5/X | n | P-Value |
|---|---|---|---|---|---|
| n | 270 | 40 | 230 | 270 | |
| Female, n (%) | 104 (38.5) | 18 (45) | 86 (37) | 270 | 0.382 |
| Race, n (%) | 270 | 0.001 | |||
| White | 103 (38.1) | 0 (0) | 103 (45) | ||
| African American | 135 (50.0) | 38 (95) | 97 (42) | ||
| Other | 32 (11.9) | 1 (3) | 26 (11) | ||
| Hispanic/Latino, n (%) | 47 (17.4) | 0 (0) | 47 (20) | 270 | 0.001 |
| Age in years, mean (SD) | 11 (3) | 11 (3) | 11 (3) | 270 | 0.328 |
| Age of asthma onset, mean (SD) | 3 (3) | 4 (4) | 3 (3) | 270 | 0.057 |
| Weight (kg), mean (SD) | 50 (20) | 50 (40) | 50 (20) | 270 | 0.845 |
| BMI (kg/cm2), mean (SD) | 23 (6) | 23 (6) | 22 (6) | 270 | 0.458 |
| Treatment assignment | 270 | 0.016 | |||
| Lansoprazole, n (%) | 132 (48.9) | 27 (68) | 105 (46) | 132 | |
| Placebo, n (%) | 138 (51.1) | 13 (33) | 125 (54) | 138 | |
| Pre-bd FVC | 2.8 (1.0) | 2.4 (0.70) | 2.9 (1.0) | 270 | 0.011 |
| Pre-bd FEV1 | 2.2 (0.78) | 1.9 (0.61) | 2.3 (0.79) | 270 | 0.0033 |
| %pred pre-bd FEV1 | 90 (15) | 85 (15) | 93 (15) | 270 | 0.0040 |
| ACQ | 1.2 (0.77) | 1.4 (0.86) | 1.2 (0.76) | 270 | 0.16 |
| ACT | 19 (4.0) | 19 (4.0) | 19 (4.1) | 270 | 0.63 |
| ER visits (in 12 mo) | 3 (4) | 3.7 (3.2) | 2.8 (4.3) | 270 | 0.18 |
| Oral steroids (in 12 mo) | 1.8 (0.12) | 2.4 (2.2) | 1.7 (2.0) | 270 | 0.062 |
| Leukotriene inhibitor, n (%) | 153 (56.7) | 29 (73) | 120 (52) | 270 | 0.024 |
| ICS-LABA, n (%) | 155 (57.4) | 23 (58) | 129 (56) | 270 | 1.00 |
| ETS reported, n (%) | 80 (29.6) | 15 (38) | 65 (28) | 270 | 0.262 |
| Allergies make asthma worse, n (%) | 214 (79.3) | 34 (85) | 180 (78) | 270 | 0.403 |
| Atopy, n (%) | 228 (84.4) | 37 (93) | 191 (83) | 270 | 0.159 |
| Family history of asthma, n (%) | 221 (81.9) | 34 (85) | 187 (81) | 270 | 0.663 |
| Past history of Sleep apnea, n (%) | 11 (4.1) | 1 (3) | 10 (4) | 270 | 1.00 |
| Low birth-weight (<2.5kg), n (%) | 17 (6.3) | 3 (8) | 14 (6) | 270 | 0.724 |
BMI – body mass index, SD – standard deviation, FVC – forced vital capacity, FEV1 – forced Expiratory volume, 1 second, ACQ – Asthma Control Questionnaire, ACT – Asthma Control Test, ER – emergency room, ICS – inhaled corticosteroid, LABA – long-acting beta agonist, ETS – environmental tobacco smoke.
Table 2.
Allele and Genotype Frequencies of Sp1 Binding Motifs in the ALOX5 Promoter
| Allele, n (%) | African American | White | Other | Total |
| 3 | 85 (31) | 1 (.5) | 9 (14) | 95 (18) |
| 4 | 49 (18) | 32 (16) | 11 (17) | 92 (17) |
| 5 | 129 (48) | 169 (82) | 42 (66) | 340 (63) |
| 6 | 7 (3) | 4 (2) | 2 (3) | 13 (2) |
| totals | 270 | 206 | 64 | 540 |
| Genotype, n (%) | ||||
| 3 / 3 | 17 (13) | 0 (0) | 0 (0) | 17 (6) |
| 3 / 4 | 16 (12) | 0 (0) | 1 (3) | 17 (6) |
| 3 / 5 | 33 (24) | 1 (1) | 8 (25) | 42 (16) |
| 3 / 6 | 2 (1) | 0 (0) | 0 (0) | 2 (1) |
| 4 / 4 | 2 (1) | 0 (0) | 0 (0) | 2 (1) |
| 4 / 5 | 28 (21) | 32 (31) | 9 (28) | 69 (22) |
| 4 / 6 | 1 (1) | 0 (0) | 1 (3) | 2 (1) |
| 5 / 5 | 32 (24) | 66 (64) | 12 (38) | 110 (41) |
| 5 / 6 | 4 (3) | 4 (4) | 1 (3) | 9 (3) |
| totals | 135 | 103 | 32 | 270 |
| Combined, n (%) | ||||
| 5 / 5 | 32 (24) | 66 (64) | 12 (38) | 110 (41) |
| 5 / X | 65 (48) | 37 (36) | 18 (56) | 120 (44) |
| X / X | 38 (28) | 0 (0) | 2 (6) | 40 (15) |
| totals | 135 | 103 | 32 | 270 |
ALOX5 promotor genotype and urinary LTE4
We examined the association between levels of uLTE4 and genotype at marker rs59439148 at the end of the 24-week trial. We found that the geometric mean of uLTE4 levels was significantly higher in the X/X cohort than in the X/5 + 5/5 cohort (38 versus 30 nmole/mole creatinine, pvalue = 0.013, 95% CIs [31,48], and [28,32], respectively) (Figure 1). This association was driven primarily by African American patients since they contributed 86% of the X/X variants, however it was apparently not due to other factors associated with race since there was no significant difference in uLTE4 levels between African Americans and Whites with the X/5 or 5/5 genotype (30 versus 30 nmole/mole creatinine, pvalue = 0.943, 95% CIs [29,31], and [29,31], respectively). Binary logistic regression modeling suggests that genotype at rs59439148 is not a predictor of uLTE4 strata (defined as >50 nmole/mole creatinine or <= 50 nmole/mole creatinine).
Figure 1. Urinary LTE4 Levels by ALOX5 Promoter Genotype.
Urinary LTE4 by genotype for marker rs59439148 in all patients (white bars), in African American patients (light gray bars) and in White patients (dark gray bars). None of the White participants had x/x genotype. Bars represent geometric mean with error bars representing 95% confidence limits. Urine samples were collected at week 24 of the study. Values that differ significantly by genotype are indicated with ‘*’ (p-value < 0.05).
ALOX5 promoter genotype and lung function
We examined FEV1%pred by genotype following the 24 week intervention. Mean FEV1%pred was significantly lower for patients with X/X genotype than for patients with X/5 or 5/5 genotypes (84 versus 91%pred, pvalue = 0.017, 95% CIs [79, 90], and [89, 93], respectively, Figure 2). Unexpectedly, when stratified by race, the association was weaker in African Americans, but exhibited the same trend (84 versus 89%, pvalue = 0.093, 95% CIs [86, 93], and [78, 89], respectively). For comparison, FEV1%pred in African Americans and Whites with the X/5 or 5/5 genotype was 89 versus 92%, pvalue = 0.221, 95% CIs [88.97], and [91,100], respectively. We were interested to determine if elevated uLTE was associated with lung function. In the 183 patients for which we had both uLTE4 levels and FEV1%pred data from the final visit, we found that FEV1%pred was inversely related to uLTE4 (r = -0.192, pvalue = 0.009, Figure 3). Analysis of the residuals suggested that a linear model was appropriate (Kolmogorov-Smirnova test for normality p=0.200, Shapiro-Wilk test for normality p=0.805, skewness = 0.01, kurtosis = -0.093). Binary logistic regression modeling suggests that 5/5 + 5/X individuals are 2.7 times more likely to have an FEV1%pred <= 80 % than X/X individuals (p-value=0.012, 95% CIs [1.2, 5.9]).
Figure 2. Forced Expiratory Volume in 1 Second by ALOX5 Promoter Genotype.
Mean FEV1%pred genotype for marker rs59439148 in all patients (white bars), in African American patients (light gray bars) and in White patients (dark gray bars). None of the White participants had x/x genotype. Bars represent mean with error bars representing 95% confidence limits. Lung function data were collected at week 24 of the study. Values that differ significantly by genotype are indicated with ‘*’ (p-value < 0.05).
Figure 3. FEV1 percent predicted is plotted as a function of uLTE4.
Data is taken from all asthmatic patients (n=183) with both a valid FEV1%pred value and uLTE4 level at the final visit. Zero suppression is present on the y-axis. r = -0.192, p = .009.
Recently, Dal Negro et al. measured urinary LTE4 levels in normal healthy individuals[19]. For the age range corresponding to the current study, they found that the mean urinary LTE4 level was 17.3±8.6 nmol/mol creatinine. We expected that the correlation between uLTE4 and FEV1 percent predicted would be driven primarily by patients with elevated uLTE4. When we included only children with elevated uLTE4 levels (≥3 standard deviations above the mean value defined by Dal Negro[19]), the negative association was strengthened (r = -.508, p=0.002, Supplemental Figure 1).
ALOX5 promoter genotype, asthma symptoms and exhaled nitric oxide
For patients with the X/X genotype, the square root mean asthma symptom control score was higher (suggesting worse asthma control) than for patients with X/5 + 5/5 genotypes (1.1 versus 0.81, pvalue=0.09, 95% CIs [0.8,1.13], and [0.70,0.92], respectively), however the difference did not reach statistical significance (See supplemental Figure 2). Similarly, there was no significant association between genotype at rs59439148 and levels of eNO (data not shown). Binary logistic regression modeling suggests that genotype at rs59439148 is not a predictor of ACQ strata (defined as >= 1.2 nmole/mole creatinine or < 1.2 nmole/mole creatinine).
DISCUSSION
This study showed that among children with asthma that is poorly controlled on inhaled steroids, carrying two copies of a minor variant of the ALOX5 promoter SP1 tandem repeat polymorphism (rs59439148) is associated with increased cysteinyl LT production and impaired lung function. The associations were only observed in individuals carrying two minor variant alleles suggesting that uLTE4 and FEV1%pred phenotypes are expressed in a recessive manner. Individuals homozygous for the minor variant also exhibited a trend toward higher ACQ scores (suggesting worse asthma symptom control), which is consistent with our other findings of elevated cysLT production and lower lung function. Not surprisingly, these same patients were more likely to be prescribed anti-leukotriene medications. Taken together, these results suggest that the genotype of the ALOX5 promoter SP1 tandem repeat polymorphism influences ALOX5 activity, which in turn is an important factor in determining leukotriene production and FEV1.
This observation should be viewed cautiously since it is driven primarily by the African American participants in whom the frequency of variant allele exceeds 50%. As a group, African Americans are known to have lower FEV1 values than Whites[2]. Given our finding that FEV1 is inversely related to uLTE4, it is tempting to speculate that elevated urinary LT, which is driven by the increased frequency of variant ALOX5 alleles, contributes to reduced FEV1 among African American with asthma compared to Whites. This hypothesis is supported by our finding that mean FEV1 is no different between African Americans and Whites who are of the 5/5 + 5/X genotypes.
The ALOX5 gene resides on chromosome 10q.11.21 and encodes an enzyme expressed in bone marrow-derived inflammatory cells that catalyzes conversion of arachidonic acid to LTA4 which is subsequently converted to LTB4 and the cysLTs (LTC4, LTD4, LTE4) by other enzymes in the LT pathway. 5-lipoxygenase function has been associated with several inflammatory diseases including asthma, atherosclerosis and pulmonary hypertension[20]. Others have investigated the importance of ALOX5 in determining both asthma phenotype and response to asthma medications. Drazen and colleagues were the first to show that the ALOX5 promoter SP1 tandem repeat variants associated with 5-lipoxygenase expression and response to an anti-5-lipoxygenase medication[6, 7, 17]. While there are normally five SP1 binding motifs in tandem, they found that a deletion or addition of one or two Sp1 binding sites resulted in significantly less reporter gene activity[6]. Since Drazen's pioneering work, the story of ALOX5 has proven to be more complex. Although variant alleles of the ALOX5 promoter SP1 tandem repeat appear to be associated with reduced transcription in vitro, it is not clear how these variants influence asthma phenotypes. Among patients with the aspirin-sensitive asthma phenotype, minor allele variants led to greater - rather than reduced - airway reactivity compared with homozygous major allele variants[11]. Furthermore, Kalayci and colleagues found that patients homozygous for the minor allele were more likely to have moderate-to-severe asthma and have worse exercise-related bronchial reactivity compared to patients with the homozygous major allele genotype[21].
Allayee et. al, investigated the association of ALOX5 genotype with atherosclerosis and concluded that intima-media thickness was significantly increased by among carriers of two variant alleles (X/X) relative to carriers of the common (5/X + 5/5) allele. They speculated that this may be due to increased production of leukotrienes in homozygous carriers of variant alleles[16]. In conclusion, there is evidence from past studies and the present study that the minor allele variant of ALOX5 promoter SP1 tandem repeat may worsen asthma.
Patients with elevated 5-lipoxygenase activity and leukotriene-driven disease would be expected to have enhanced response to drugs that target the leukotriene pathway. Both Drazen[7] and Telleria[9], separately found that anti-5-lipoxygenase treatment and leukotriene receptor antagonists, respectively, work better in patients with the 5-repeat allele of ALOX5. These findings are what would be expected if variant alleles lead to reduced 5-lipoxygenase expression. However, other authors, including our group (Lima et al.[8]) found the opposite. Lima and colleagues previously found that among patients receiving montelukast, a LT receptor antagonist, individuals who were carriers of a minor allele variant had a 73% reduced risk of one or more exacerbations compared with individuals who were homozygous for the 5-repeat allele, which would be consistent with the variant alleles having increased 5-lipoxygenase expression and cysLT levels. Vikman and colleagues also recently found that lymphocytes from healthy individuals who are homozygous for the shorter 3 and 4 repeat alleles had higher 5-LO expression[10]. Collectively, these past findings suggest that the effect of variants in the ALOX5 promoter SP1 tandem repeat polymorphism may depend on other modifying factors such as specific non5 variant, asthma phenotype and environmental triggers.
Most of the previous studies have primarily involved adults and whites. The population in the current study (51% African Americans and 38% Whites), provides new data and supports and extends our previous work. Our past work showed that primarily among white adults, the minor allele associated with improved response to montelukast, a leukotriene receptor antagonist. One possible explanation for this is that homozygote carriers of the variant alleles have increased leukotriene production, and therefore benefit more from antileukotriene therapy. Though we previously did not measure leukotriene pathway activity, the current study shows among primarily African American children an association between the minor allele and both elevated uLTE4 and reduced lung function. Urinary LTE4 is a stable metabolite of cysLTs, and has been shown to correlate acutely with lung function[22]. Though we did not directly measure 5-lipoxygenase gene expression, our findings are consistent with up-regulation of the leukotriene pathway in minor allele homozygotes. Importantly, these finding are not consistent with expression studies first performed by Drazen[7] and later by Kalayci[21].
Several groups have evaluated the effect of ALOX5 promoter genotype on asthma phenotype. Despite showing reduced ALOX5 mRNA and LTC4 expression in minor allele homozygotes, Kalayci found that minor allele homozygotes had greater airway reactivity and increased likelihood for greater asthma severity. Additionally, Kim and colleagues found that carriers of minor allele variants had greater methacholine-induced airway reactivity[11]. Kalayci proposed that though patients with deletional non-5 variants may have lower constitutive leukotriene expression, their greater airway reactivity might be explained by heightened leukotriene sensitivity during triggered production.
This study has several limitations. Our genetic sub study was ancillary to a placebo controlled, randomized clinical trial of lansoprazole, which was not powered as a genetic study. Consequently we may not have had sufficient numbers to address the hypothesis that lung function was worse in patients carrying two copies of the minor variant ALOX5 promoter SP1 tandem repeat allele and that the findings in the present study may represent false positives. We can only speculate that the associations between homozygous minor allele carriers and poorer asthma control was related to increased 5-lipoxygenase activity without corresponding 5-LO and LTB4 data. Additionally, our study only performed genotyping of the ALOX5 promoter. It is very possible that other polymorphisms in ALOX5, or other leukotriene pathway genes such as LTC4S and LTA4H, may affect the influence of ALOX5 Sp1 promoter genotypes effect on leukotriene production and asthma outcomes.
In summary, to our knowledge, our study is the first which evaluates the effect of the ALOX5 promoter SP1 tandem repeat polymorphism among a pediatric cohort with primarily African American ancestry. Among the White participants in this study, the most common allele was 5 GGGCGG tandem repeats (169/206), and none of the 103 Whites were homozygous for minor allele variants. However, among African Americans, the majority of alleles (141/270) were non-5, and a full 76% (103/135) of African American participants carried at least one variant allele variant. In this cohort of children with poorly controlled asthma, ALOX5 genotype is associated with two markers of worsening asthma - heightened leukotriene production and poorer lung function. It is well established that African Americans are at greater risk for incident asthma and exhibit greater disease morbidity. The results of the current study suggest that part of the reason for this stems from the higher minor allele ALOX5 promoter SP1 tandem repeat frequency in African Americans.
Supplementary Material
Supplemental Figure 1. FEV1%pred is plotted as a function of uLTE4 for patients in which uLTE4 is ≥ 3 SD above the mean reference value for normal patients[19]. Both FEV1%pred and uLTE4 data were collected at week 24 of the study. Hash mark denotes zero suppression on the y-axis. r = -.508, p=.002.
Supplemental Figure 2. Asthma Control Scores by ALOX5 Promoter Genotype. Mean values for the Asthma Control Questionnaire (ACQ) are presented by ALOX5 promoter genotype (rs59439148) with error bars denoting 95% confidence limits. The Juniper ACQ score is plotted by genotype in all patients (white bars), in African American patients (light gray bars) and in White patients (dark gray bars). None of the White participants had x/x genotype. ACQ score data were collected at week 24 of the study. ** represents 2-tailed t-test comparing all patients with at least one wildtype allele versus all patients with two mutant alleles, p-value=0.09.
Supplemental Figure 3. Representative Genotypes for the ALOX5 Promoter Repeat Polymorphism. The genotypes of 5 representative study subjects is shown. Genotypes were assigned based on the size of the fragments (given in parentheses) and indicate the number of tandem Sp1 repeats. Individuals with only one peak are homozygous for that allele.
Acknowledgements
The authors would to thank and acknowledge the work of the research coordinators of the ALA-ACRC.
This research was performed by the American Lung Association Asthma Clinical Research Centers (ALA-ACRC). The members of the ALA-ACRC research group for the trial were as follows:
American Lung Association Asthma Clinical Research Centers
Baylor College of Medicine, Houston: N. A. Hanania (principal investigator), M. Sockrider (co-principal investigator), L. Bertrand (principal clinic coordinator), M. Atik, L. Giraldo, B, Flores (coordinators);
Columbia University–New York University Consortium, New York: J. Reibman (principal investigator), E. DiMango, L. Rogers (co-principal investigators), C. Cammarata and K. Carapetyan (clinic coordinators at New York University), J. Sormillon and E. Simpson (clinic coordinators at Columbia University);
Duke University Medical Center, Durham, N.C.: L. Williams (principal investigator), J. Sundy (co-principal investigator), G. Dudek (principal clinic coordinator), R. Newton and A. Dugdale (coordinators);
Emory University School of Medicine, Atlanta: W.G. Teague (principal investigator), Anne Fitzpatrick, Sumita Khatri (co-principal investigators), R. Patel (principal clinic coordinator), J. Peabody, E Hunter, D Whitlock (coordinators);
Illinois Consortium, Chicago: L. Smith (principal investigator), J. Moy, E. Naureckas, A. Prestridge (co-principal investigators), J. Hixon (principal clinic coordinator), A. Brees, J. Judge (coordinators);
Indiana University, Asthma Clinical Research Center, Indianapolis: M. Busk (principal investigator), P. Puntenney (principal clinic coordinator), N. Busk, J. Hutchins (coordinators);
University of Pennsylvania, Philadelphia: F. Leone (principal investigator), M. Hayes-Hampton (principal clinic coordinator);
National Jewish Health, Denver: R. Katial (principal investigator), M. Krawiecz (co-principal investigator), H. Currier (principal clinic coordinator);
Nemours Children's Clinic–University of Florida Consortium, Jacksonville: J. Lima (principal investigator), K. Blake (co-principal investigator), J Lang (co-principal investigator), D Schaeffer (investigator), A Santos (principal coordinator), M McRae (coordinator)
Hofstra University School of Medicine (formerly North Shore–Long Island Jewish Health System), New Hyde Park, N.Y.: J. Karpel (principal investigator), R. Cohen (co-principal investigator), R. Ramdeo (principal clinic coordinator);
Northern New England Consortium (formerly Vermont Lung Center at the University of Vermont), Colchester, Vt.: C.G. Irvin (principal investigator), A.E. Dixon, D.A. Kaminsky (co-principal investigators), R. Colletti (GI consultant), S.M. Burns, L.M. Bourassa, S.E. Lang, L.V. Griffes (coordinators), R. Pratt, K.B. Nakos, K.J. Girard;
The Ohio State University Medical Center/Columbus Children's Hospital, Columbus: J. Mastronarde (principal investigator), K. McCoy (co-principal investigator), J. Parsons (co-investigator), J. Drake (principal clinic coordinator), R. Compton, L. Raterman, D. Cosmar (coordinators);
Maria Fareri Children's Hospital at Westchester Medical Center and New York Medical College: A. Dozor (principal investigator), Sankaran Krishnan, (Co-Investigator),I. Gherson (principal clinic coordinator), and Lisa Monchil, (coordinator);
University of Alabama at Birmingham, Birmingham: L.B. Gerald (principal investigator), W.C. Bailey, R. Grad (co-principal investigators), S. Erwin (principal clinic coordinator), A. Kelley, D. Laken (coordinators);
University of Miami, Miami–University of South Florida, Tampa: A. Wanner (principal investigator, Miami), R. Lockey (principal investigator, Tampa), E. Mendes (principal clinic coordinator for University of Miami), S. McCullough (principal clinic coordinator for University of South Florida), M. Grandstaff-Singleton, D. Miller (coordinators);
University of Minnesota, Minneapolis: M.N. Blumenthal (principal investigator), G. Brottman, J. Hagen (co-principal investigators), A. Decker, D. Lascewski, S. Kelleher (principal clinic coordinators), K. Bachman, C. Quintard, C. Sherry (coordinators);
University of Missouri, Kansas City School of Medicine, Kansas City: G. Salzman (principal investigator), C. Dinakar, D. Pyszczynski (co-principal investigators), P. Haney (principal clinic coordinator);
St. Louis Asthma Clinical Research Center: Washington University, St. Louis: M. Castro (principal investigator), L. Bacharier, K. Sumino (co-investigators), J. Tarsi (principal coordinator), B. Patterson (coordinator);
University of California San Diego: S. Wasserman (principal investigator), J. Ramsdell (co-principal investigator), P. Ferguson, K. Kinninger, T. Greene (clinic coordinators);
Chairman's Office University of Alabama, Birmingham (formerly at Respiratory Hospital, Winnipeg, Man., Canada): W. Bailey and N. Anthonisen (research group chair);
Data Coordinating Center, Johns Hopkins University Center for Clinical Trials, Baltimore: R. Wise (center director), J. Holbrook (deputy director), E. Brown (principal coordinator), D. Amend-Libercci, K. Barry, M. Daniel, A. Lears, G. Leatherman, C. Levine, D. Nowakowski, N. Prusakowski, S. Rayapudi, S. Roettger, A. Thurman, D. Shade, E. Sugar, C. Wei;
Esophageal pH Probe Quality Control Center, Children's Center for Digestive Healthcare Pediatric Gastroenterology, Hepatology, and Nutrition, (formerly at Emory University School of Medicine): B. Gold (center director);
Data and Safety Monitoring Board: S. Lazarus (chair), W. Calhoun, M. Cloutier, B. McWilliams, A. Rogatko, C. Sorkness;
Project Office, American Lung Association, New York: E. Lancet (project officer), N. Edelman (scientific consultant), S. Rappaport;
Project Office, National Heart Lung and Blood Institute: V. Taggart (project officer), G. Weinmann (DSMB secretary, airway branch chief);
ALA Scientific Advisory Committee: E. N. Schachter (chair), L. A. Baggott (vice-chair) W. C. Bailey, A. L. Brannen II, M. Castro, B. W. Christman, A. Chuang, R. M. Donaldson, C. Holloway, T. A. Mahr, J. A. Neubauer, J. M. Samet, E. R. Swenson, D. J. Upson, D. J. Weiss, R. Wise.
Support: Supported by grants from the James and Esther King Biomedical Research Program/Florida Department of Health [9KN06] (Lang), the National Heart Lung and Blood Institute ([U01HL080433] (Teague) and [1K23HL096838] (Lang)) and American Lung Association. Support provided by Takeda Pharmaceuticals North America, Inc (Lansoprazole and Placebo) and by GlaxoSmithKline (Albuterol HFA).
Abbreviations List
- 5-LO
5-Lipoxygenase
- ACQ
Asthma Control Questionnaire
- ALA-ACRC
American Lung Association Asthma Clinical Research Centers
- ATS
American Thoracic Society
- CI
Confidence Interval
- ENO
Exhaled Nitric Oxide
- FEV1
Forced Expiratory Volume 1 second
- FEV1%pred
Forced Expiratory Volume in 1 second, percent predicted
- FLAP
5-lipoxygenase activating protein
- IRB
Institutional Review Board
- LT
Leukotriene
- LTRA
Leukotriene Receptor Antagonist
- MID
Minimal clinically important difference
- SARCA
Study of Acid-Reflux in Childhood Asthma
- uLTE4
urinary leukotriene E4
Biography
Dr. Mougey has made substantial contributions to conception, design, and acquisition of data for this study. He contributed to the analysis and interpretation of data. He drafted the submitted article. Dr. Mougey is a guarantor of this work and takes responsibility for the integrity of the work as a whole, from inception to publication.
Dr. Lang has made substantial contributions to conception, design, and acquisition of data for this study. He contributed to the analysis and interpretation of data. He drafted the submitted article. Dr. Lang is a guarantor of this work and takes responsibility for the integrity of the work as a whole, from inception to publication.
Dr. Allayee has made substantial contributions to conception, design, and acquisition of data for this study. He contributed to the analysis and interpretation of data. He helped in drafting the submitted article.
Dr. Teague has made substantial contributions to conception, design, and acquisition of data for this study. He contributed to the analysis and interpretation of data. He helped in drafting the submitted article.
Dr. Dozor has made substantial contributions to conception, design, and acquisition of data for this study. He contributed to the analysis and interpretation of data. He helped in drafting the submitted article.
Dr. Wise has made substantial contributions to conception, design, and acquisition of data for this study. He helped in drafting the submitted article.
Dr Lima has made substantial contributions to the conception, design, and acquisition of data for this study. He contributed to the analysis and interpretation of data. He helped draft the submitted article and revised it critically for important intellectual content.
Footnotes
A complete listing of the American Lung Association Asthma Clinical Research Centers (ALA-ACRCs) can be found at the end of this article.
Disclosures/Conflict of Interest:
Dr. Mougey has no conflicts of interest in the subject matter of this manuscript.
Dr. Lang has no conflicts of interest in the subject matter of this manuscript.
Dr. Allayee has no conflicts of interest in the subject matter of this manuscript.
Dr. Teague has no conflicts of interest in the subject matter of this manuscript.
Dr. Dozor has no conflicts of interest in the subject matter of this manuscript.
Dr. Wise has no conflicts of interest in the subject matter of this manuscript.
Dr. Lima has no conflicts of interest in the subject matter of this manuscript.
Role of Individual Authors:
Dr. Mougey and Dr. Lang contributed equally to the conduct and writing of the research and manuscript.
References
- 1.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009;123(Suppl 3):S131–145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Rhodes JC, Lara M. Racial and ethnic differences in asthma diagnosis among children who wheeze. Pediatrics. 2005;115:1254–1260. doi: 10.1542/peds.2004-0897. [DOI] [PubMed] [Google Scholar]
- 3.Drazen JM, Austen KF. Leukotrienes and airway responses. Am Rev Respir Dis. 1987;136:985–998. doi: 10.1164/ajrccm/136.4.985. [DOI] [PubMed] [Google Scholar]
- 4.Salvi SS, Krishna MT, Sampson AP, Holgate ST. The anti-inflammatory effects of leukotriene-modifying drugs and their use in asthma. Chest. 2001;119:1533–1546. doi: 10.1378/chest.119.5.1533. [DOI] [PubMed] [Google Scholar]
- 5.Woods JW, Evans JF, Ethier D, Scott S, Vickers PJ, Hearn L, Heibein JA, Charleson S. Singer, II, 5-lipoxygenase and 5-lipoxygenase-activating protein are localized in the nuclear envelope of activated human leukocytes. J Exp Med. 1993;178:1935–1946. doi: 10.1084/jem.178.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.In KH, Asano K, Beier D, Grobholz J, Finn PW, Silverman EK, Silverman ES, Collins T, Fischer AR, Keith TP, Serino K, Kim SW, De Sanctis GT, Yandava C, Pillari A, Rubin P, Kemp J, Israel E, Busse W, Ledford D, Murray JJ, Segal A, Tinkleman D, Drazen JM. Naturally occurring mutations in the human 5-lipoxygenase gene promoter that modify transcription factor binding and reporter gene transcription. J Clin Invest. 1997;99:1130–1137. doi: 10.1172/JCI119241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drazen JM, Yandava CN, Dube L, Szczerback N, Hippensteel R, Pillari A, Israel E, Schork N, Silverman ES, Katz DA, Drajesk J. Pharmacogenetic association between ALOX5 promoter genotype and the response to anti-asthma treatment. Nat Genet. 1999;22:168–170. doi: 10.1038/9680. [DOI] [PubMed] [Google Scholar]
- 8.Lima JJ, Zhang S, Grant A, Shao L, Tantisira KG, Allayee H, Wang J, Sylvester J, Holbrook J, Wise R, Weiss ST, Barnes K. Influence of leukotriene pathway polymorphisms on response to montelukast in asthma. Am J Respir Crit Care Med. 2006;173:379–385. doi: 10.1164/rccm.200509-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Telleria JJ, Blanco-Quiros A, Varillas D, Armentia A, Fernandez-Carvajal I, Jesus Alonso M, Diez I. ALOX5 promoter genotype and response to montelukast in moderate persistent asthma. Respir Med. 2008;102:857–861. doi: 10.1016/j.rmed.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Vikman S, Brena RM, Armstrong P, Hartiala J, Stephensen CB, Allayee H. Functional analysis of 5-lipoxygenase promoter repeat variants. Hum Mol Genet. 2009;18:4521–4529. doi: 10.1093/hmg/ddp414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SH, Bae JS, Suh CH, Nahm DH, Holloway JW, Park HS. Polymorphism of tandem repeat in promoter of 5-lipoxygenase in ASA-intolerant asthma: a positive association with airway hyperresponsiveness. Allergy. 2005;60:760–765. doi: 10.1111/j.1398-9995.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- 12.Rabinovitch N. Urinary leukotriene E4. Immunol Allergy Clin North Am. 2007;27:651–664. vii. doi: 10.1016/j.iac.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 14.Holbrook JT, Wise RA, Gold BD, Blake K, Brown ED, Castro M, Dozor AJ, Lima JJ, Mastronarde JG, Sockrider MM, Teague WG. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA. 307:373–381. doi: 10.1001/jama.2011.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Standardization of Spirometry 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 16.Dwyer JH, Allayee H, Dwyer KM, Fan J, Wu H, Mar R, Lusis AJ, Mehrabian M. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 17.In KH, Silverman ES, Asano K, Beier D, Fischer AR, Keith TP, Serino K, Yandava C, De Sanctis GT, Drazen JM. Mutations in the human 5-lipoxygenase gene. Clin Rev Allergy Immunol. 1999;17:59–69. doi: 10.1007/BF02737597. [DOI] [PubMed] [Google Scholar]
- 18.ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 19.Dal Negro RW, Visconti M, Micheletto C, Tognella S, Guerriero M. Reference urinary LTE4 levels in normal individuals: a pilot study. Eur Ann Allergy Clin Immunol. 43:22–28. [PubMed] [Google Scholar]
- 20.De Caterina R, Zampolli A. From asthma to atherosclerosis--5-lipoxygenase, leukotrienes, and inflammation. N Engl J Med. 2004;350:4–7. doi: 10.1056/NEJMp038190. [DOI] [PubMed] [Google Scholar]
- 21.Kalayci O, Birben E, Sackesen C, Keskin O, Tahan F, Wechsler ME, Civelek E, Soyer OU, Adalioglu G, Tuncer A, Israel E, Lilly C. ALOX5 promoter genotype, asthma severity and LTC production by eosinophils. Allergy. 2006;61:97–103. doi: 10.1111/j.1398-9995.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 22.Green SA, Malice MP, Tanaka W, Tozzi CA, Reiss TF. Increase in urinary leukotriene LTE4 levels in acute asthma: correlation with airflow limitation. Thorax. 2004;59:100–104. doi: 10.1136/thorax.2003.006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. FEV1%pred is plotted as a function of uLTE4 for patients in which uLTE4 is ≥ 3 SD above the mean reference value for normal patients[19]. Both FEV1%pred and uLTE4 data were collected at week 24 of the study. Hash mark denotes zero suppression on the y-axis. r = -.508, p=.002.
Supplemental Figure 2. Asthma Control Scores by ALOX5 Promoter Genotype. Mean values for the Asthma Control Questionnaire (ACQ) are presented by ALOX5 promoter genotype (rs59439148) with error bars denoting 95% confidence limits. The Juniper ACQ score is plotted by genotype in all patients (white bars), in African American patients (light gray bars) and in White patients (dark gray bars). None of the White participants had x/x genotype. ACQ score data were collected at week 24 of the study. ** represents 2-tailed t-test comparing all patients with at least one wildtype allele versus all patients with two mutant alleles, p-value=0.09.
Supplemental Figure 3. Representative Genotypes for the ALOX5 Promoter Repeat Polymorphism. The genotypes of 5 representative study subjects is shown. Genotypes were assigned based on the size of the fragments (given in parentheses) and indicate the number of tandem Sp1 repeats. Individuals with only one peak are homozygous for that allele.