Abstract
Background
Glutathione S-transferase P1 is a Phase II cytoprotective and detoxifying enzyme that is widely expressed in human airways. The glutathione S-transferase P1 Ile105Val polymorphism has been linked with atopic disorders and asthma. Yet, little is known about the regulation of allergic inflammation by glutathione S-transferase P1 in human asthmatics.
Objective
To establish the effect of the glutathione S-transferase P1 Ile105Val polymorphism on allergen-induced airway inflammation and oxidant stress, and nonspecific bronchial hyperresponsiveness to methacholine and reactivity to specific allergen in mild human atopic asthmatics in vivo.
Methods
Five Val105/Val105, twelve Val105/Ile105, and twenty Ile105/Ile105 mild atopic asthmatics underwent methacholine challenge, inhaled allergen challenge, and endobronchial allergen provocation through a bronchoscope. A panel of inflammatory cytokines and chemokines, F2-isoprostanes and isofuranes, markers of oxidative stress, thromboxane B2, and immunoglobulin E were measured in bronchoalveolar lavage fluid at baseline and 24 hrs after allergen instillation.
Results
Asthmatics with glutathione S-transferase P1 Val105/Val105 compared to asthmatics with the glutathione S-transferase P1 Val105/Ile105 and Ile105/Ile105 had greater generation of acute phase cytokines (TNF-α, IL-6, CXCL8), IL-12, CCL11, thromboxane B2, and immunoglobulin E at 24 hrs after local allergen challenge. The GSTP1 genotype had no effect on airway hyperresponsiveness to methacholine and the reactivity to specific allergen.
Conclusion
The glutathione S-transferase P1 Ile105Val polymorphism markedly modifies allergen-provoked airway inflammation in atopic asthmatics in vivo. Modulation of the biochemical milieu in response to allergen provides a mechanistic explanation for regulatory effects of glutathione S-transferase P1 polymorphism on airway pathophysiology, and may guide improvement of future therapeutic methods in human atopic asthmatics. These findings must me confirmed in a larger study population of asthmatics with various ethnicities.
Keywords: Glutathione S-transferase P1, Ile105Val polymorphism, atopic asthma, allergen challenge, methacholine challenge, allergic inflammation, oxidant stress
Introduction
Glutathione S-transferase P1 (GSTP1) is a Phase II cytoprotective and detoxifying enzyme belonging to a large family of GSTs [1]. GSTP1 is widely expressed in human airways, predominantly in alveolar macrophages and the epithelial cells and, and provides the majority of GST activity in the lung [1,2]. The major physiological role of GSTP1 is elimination of electrophiles by conjugating them with glutathione [1]. However, GSTP1 has also antioxidant properties due to selenium-independent peroxidase activity [1] and modulates major cellular signalling and gene transcription pathways including c-Jun N-terminal kinase (JNK) [3], tumour necrosis factor (TNF)-receptor-associated factor 2 (TRAF2) [4], and peroxiredoxin-1 [5].
Asthma is a heterogeneous airway disorder linked to airway inflammation and oxidative stress [6,7]. The loss of GSTP1 activity by gene deletion results in an exaggerated response to allergic sensitization and challenge in a mouse model of asthma [8]. Paradoxically, antigen-induced inflammation inhibits expression of GSTP1 in the lungs of OVA-sensitized mice [9]. Moreover, decreased expression of GSTP1 in nasal epithelium has also been found in asthmatic children [7].
A large number of GST genes have been recognized as polymorphic and the relevance of some genetic variants of GST to human pathology has been documented [1]. A single nucleotide polymorphism at position 313 in GSTP1 that converts an adenine to a guanine (A→G) is both functional and frequently associated with various diseases. GSTP1variants containing Val105 have a higher efficiency for diol epoxides of polycyclic aromatic hydrocarbons but are less effective for 1-chloro-2,4-dinitrobenzene than Ile105 containing GSTP1 [10]. The Ile to Val substitution in codon 5 of exon 5 (Ile105→Val105) lies in close proximity to the catalytic centre of the enzyme and presumably accounts for the change in activity of the enzyme [1].
The link between human asthma and the GSTP1 polymorphism was originally reported by British investigators in a case-controlled study that showed a decreased risk of asthma and atopy among individuals with the GSTP1 Val105/Val105 genotype as compared to GSTP1 Ile105/Ile105 individuals [11]. Subjects with GSTP1 Ile105/Val105 variant had an intermediate risk of asthma [11]. A recent meta-analysis of 17 investigations on GSTP1 Ile105Val and asthma, although suggested a weak protective effect of the Val105, showed a tremendous heterogeneity of the data [12]. In fact, some studies suggest deteriorating effect of GSTP1 Val105 on asthma [13,14].
We hypothesized that the Ile105→Val105 polymorphism of GSTP1 modulates airway inflammation and reactivity in human atopic asthmatics. The aim of our study was to determine the effect of the Ile105→Val105 polymorphism of GSTP1 on allergen-induced airway inflammation and oxidant stress, and nonspecific bronchial hyperresponsiveness to methacholine and reactivity to specific allergen in human atopic asthmatics in vivo. We employed segmental and inhaled allergen challenges that are established models of exacerbation in atopic asthmatics and provide important tools for studying the pathophysiology of allergic asthma in vivo [15].
Methods
Patients
Thirty seven mild atopic asthmatics by National Asthma Education and Prevention Program guidelines [16] were recruited from the Middle Tennessee area. None of the volunteers had a history of tobacco smoking, other lung disorders or a respiratory infection in the 6 weeks preceding the study. Patients had positive skin test with aeroallergens, methacholine challenge, and inhaled allergen provocation. All medications were discontinued at least 48 hrs prior to procedures. Pregnant women were excluded by urine HCG testing. Volunteers consented verbally and in writing to the protocol that was approved by the Vanderbilt University Committee for the Protection of Human Subjects (IRB number: 051158). An Investigational New Drug agreement with the U.S. Food and Drug Administration for the use of allergens for inhaled and bronchoscopic provocations in humans was obtained.
Study protocol
Volunteers underwent bronchoscopy with baseline (before allergen challenge) bronchoalveolar lavage (BAL) in the lingua of the left upper lobe followed by segmental allergen challenge (SAC) in a subsegment of the right middle lobe. The allergen challenged subsegment was lavaged 24 hrs later. At least 3 weeks after the bronchoscopy studies asthmatics underwent methacholine challenge followed by an inhaled allergen provocation the next day. The same allergen was used for both the inhaled and segmental allergen challenge.
Allergy skin test
Prick skin testing was performed with diluent and histamine controls in parallel with a set of standardized aeroallergens (Dermatophagoides pteronyssinus, Dermatophagoides farinae, cat hair, Bermuda grass, Kentucky bluegrass, fescue meadow grass, orchard grass, redtop grass, ryegrass, sweet vernal grass, timothy grass, short ragweed) (Greer Laboratories, Lenoir, NC) according to practice guidelines guidelines [17].
Bronchoscopy studies
Subjects were fasted overnight. Midazolam 1–2 mg and fentanyl 50–100 mcg were administered intravenously if patients wished conscious sedation. Topical lidocaine (≤ 7 mg/kg) was used for airway anaesthesia. BAL was performed using three 50 ml aliquots of normal saline and immediately suctioned back. SAC was done using two incremental doses of the allergen to which the volunteers had positive skin test, each dose in a 5 ml aliquot of normal saline. The first dose of the allergen was 10-fold higher than the concentration that gave 2+ skin reaction on intradermal test (5–8 mm wheal, 20–30 mm erythema). The subsegment was watched for 2 minutes. None of the volunteers developed visible local inflammatory reaction after the first dose of allergen. Thus, in each subject the second dose of the same allergen was instilled at concentration 100-fold higher than the 2+ skin reaction, and the bronchoscope was then removed [18].
Methacholine challenge
The methacholine (Provocholine, Methapharm Inc., Brantford, Ontario, Canada) challenge was performed according to the American Thoracic Society guidelines [19] using a Salter dosimeter (Salter Labs, Arvin, CA) and Flow Screen computerized spirometer (VIASYS Healthcare GmbH, Hoechberg, Germany).
Inhaled allergen challenge
Allergen inhalation challenge was accomplished as described by Cockcroft and colleagues [20]. The greatest fall in FEV1 between 0–3 hrs post allergen was taken as the early airway response (EAR), and the maximum FEV1 fall at 3–8 hrs was the late airway response (LAR). Allergen aerosols were generated using a Wright nebulizer connected to a 2-way Hans Rudolph valve mouthpiece (Roxon, Montreal, Canada) attached to a wall oxygen source at 50 psi. Spirometry was performed using Flow Screen computerized spirometer.
Laboratory methods
Genomic DNA was extracted from blood leukocytes with a Wizard Genomic DNA Purification Kit (Promega, Madison, WI). Probes used for the ABI assay (TaqMan® SNP Genotyping Assay, Applied Biosystems by Life Technologies, Carlsbad, CA) were labelled at their 5′ end with either VIC® or 6-carboxy-fluorescine reporter dyes. The reaction components include: 2.5μl Taqman Universal PCR Master Mix and 0.125μl or 0.250μl assay mix (Applied Biosystems), and approximately 5ng of genomic DNA in a total volume of 10μl per single tube reaction. PCR amplification was performed in a thermal cycler (Techne TC-412, Burlington, NJ) with an initial step of 95°C for 10 min followed by 50 cycles of 92°C for 15 sec and 60°C for 1 min. After amplification, the fluorescence of each sample was read on the ABI 7900HT (DNA Resources Core, Vanderbilt University) and analyzed with the Sequence Detection Software (Applied Biosystems).
BAL cells were counted in Reichert Brightline Metallized Phase Hemacytometer. F2-isoprostanes, isofuranes, and thromboxane B2 (TXB2) were analyzed by stable isotope dilution method in conjunction with gas chromatography/negative ion chemical ionization/mass spectrometry as previously described [21]. Interleukin(IL)-4, IL-5, IL-10, IL-12p70, IL-13, interferon (IFN)-γ, Tumour Necrosis Factor (TNF)-α, and IL-8 (CXCL8) were measured using human Plex Multi-Spot assay (Meso Scale Discovery, Gaithersburg, MD). IL-3, IL-6, CCL5 (RANTES), CCL11 (eotaxin), and immunoglobulin E (IgE) were analyzed by Cytometric Bead Array (BD Biosciences, San Jose, CA).
Statistical analysis
Patients’ baseline characteristics were compared by using the chi-square test for categorical variables, and the Mann-Whitney U test for continuous variables. Data are presented as proportions for categorical variables and mean ± standard deviation (SD) for continuous variables. There was no difference in percentages of cells, concentrations of mediators and markers of oxidative stress between Val105/Ile105 and Ile105/Ile105 both before and after allergen challenge, therefore a recessive model was used to compare GSTP1 genotype Val105/Val105 with Val105/Ile105 and Ile105/Ile105 combined. In addition, percentages of cells, concentrations of mediators and markers of oxidative stress between genotypes were compared at baseline and after allergen challenge separately by using Mann-Whitney U tests. The effect of GSTP1 genotype was further assessed by comparing the change in outcome variables after allergen challenge between groups using general linear models (GLM). Baseline value of outcome variable was adjusted as a model covariate as well as other baseline covariates including age, gender, and race. Residuals were assessed graphically for normality and transformation on the dependent variable was done to correct non-normal residuals if needed. Significance was accepted when p < 0.05. R-software version 2.11.1 and SPSS version 19 (SPSS Inc., Chicago, Il, USA) were used for data analyses.
Results
Subjects
The study population consisted of 37 mild atopic asthmatics (25 women and 12 men). Thirty three patients were Caucasians and 4 African Americans. All patients had a normal baseline spirometry and the vast majority of them did not use any daily asthma medications. Four patients provided history of past inhaled corticosteroid use. Only two individuals took daily 100mg Fluticasone/50mg Salmeterol by inhalation. Discontinuation of the inhaled corticosteroids and beta-agonist prior to provocative challenges was not associated with any adverse consequences as judged by subjective and objective (spirometric) criteria. Volunteers were asked to continue the same treatment regimen throughout the study that typically consisted of an inhaled short acting beta-agonist as needed, during the entire period of the study. Table 1 provides additional demographic data concerning the volunteers.
Table 1.
Demographic findings, history of atopy and asthma, asthma medication use, and allergens employed for challenges (p values calculated using one way ANOVA or Fisher’s exact test for interval and nominal values, respectively).
| Val105/Val105 | Val105/Ile105 | Ile105/Ile105 | p | |
|---|---|---|---|---|
| Count or mean±SD, range | ||||
| Gender, M/F | 2/3 | 3/9 | 7/13 | 0.78 |
| Age | 29.7±7.5 (20–37) | 30±7.4 (23–46) | 28±6.6 (20–43) | 0.60 |
| Race | ||||
| White | 4 | 12 | 17 | 0.32 |
| African American | 1 | 0 | 3 | |
| Family history of atopy and asthma | 4 | 10 | 17 | 0.96 |
| Baseline spirometry (%predicted) | ||||
| FVC | 101±15.6 (83–123) | 110.5±11 (92–128) | 101±14 (70–141) | 0.68 |
| FEV1 | 105±9.9 (89–122) | 100±14.5 (79–126) | 92±12.6 (73–116) | 0.80 |
| FEV1/FVC | 91.5±9.6 (79–106) | 90±8.3 (75–104) | 80±9.6 (67–107) | 0.80 |
| Drugs (daily or as needed) | ||||
| Short-acting β2-agonist | 5 | 12 | 20 | NA* |
| Long-acting β2-agonist | 1 | 1 | 0 | 0.18 |
| Inhaled corticosteroids | 1 | 2 | 3 | 0.96 |
| Nasal corticosteroids | 2 | 2 | 0 | 0.03 |
| Leukotriene modifier | 1 | 2 | 2 | 0.78 |
| Antihistamine | 3 | 7 | 6 | 0.21 |
| Allergens (type/dose) used for segmental allergen challenge | ||||
| Dust mite | 1/55 AU | 4/0.55–5.5 AU, mean 3.85 AU | 5/0.55–505 AU, mean 129 AU | 0.59 |
| Cat | 1/55 AU | 6/0.55–550 AU, mean 111.1 AU | 10/0.055–5.5, mean 2.3 AU | |
| Ragweed | 1/0.55 AU | 0 | 2/.055–.0055 AU, mean 0.03 AU | |
| Bermuda | 0 | 1/0.5 AU | 1 | |
| Bluegrass | 1/0.055 AU | 1/550 AU | 0 | |
| Rye grass | 1/0.055 AU | 0 | 1/105 AU | |
| Timothy grass | 0 | 0 | 1/0.055 AU | |
Fisher exact test could not be performed as everyone in the cohort used short-acting beta agonist.
Effect on inflammatory airway cells
Asthmatics with Val105/Val105 had higher percentage of neutrophils in BAL at baseline compared to patients with Val105/Ile105 and Ile105/Ile105 GSTP1 genotypes. The difference was not significant at 24 hrs after allergen provocation. Other cell types were not affected by GSTP1 polymorphism (Table 2). In addition, analysis using a recessive model showed no difference in inflammatory cells between Val105/Val105 and Val105/Ile105 and Ile105/Ile105 GSTP1 genotypes after adjusting for baseline cell percentages, age, gender and race, at 24 hrs after allergen challenge.
Table 2.
Percentages of cells in BAL fluid at baseline (Bas) and after allergen challenge (All) (mean ± SD, range, p values are obtained using recessive models with Val105/Val105 vs. Val105/Ile105 and Ile105/Ile105 combined).
| Variable | Val105/Val105 | Val105/Ile105 + Ile105/Ile105 | p values* | |
|---|---|---|---|---|
| Macrophages | Bas | 76.2±15.9 (56–89) | 86.6±7.7 (64–98) | 0.15 |
| All | 28.2±10.1 (14–42) | 49.5±26.0 (2–98) | 0.09 | |
| Eosinophils | Bas | 2.6±3.1 (0–8) | 1.7±2.7 (0–11) | 0.31 |
| All | 30.8±31.9 (3–69) | 13.9±19.6 (1–80) | 0.18 | |
| Neutrophils | Bas | 5.6±2.7 (2–9) | 2.0±2.3 (0–11) | 0.008 |
| All | 35.4±27.5 (5–68) | 27.9±25.7 (0–75) | 0.65 | |
| Lymphocytes | Bas | 13.0±13.6 (4–37) | 7.9±7.8 (0–28) | 0.75 |
| All | 11.8±7.6 (5–21) | 7.9±7.2 (0–32) | 0.21 | |
P values were obtained with non parametric Mann-Whitney U test
Effect on allergen-induced inflammatory mediators, oxidant stress, and IgE
Asthmatics with all three GSTP1 SNP combinations at position 105 had similar baseline concentrations of cytokines and TXB2 in BAL. However, instillation of specific allergen provoked a greater production of IL-6 (p=0.03), IL-12 (p=0.02), CXCL8 (p=0.03), and TNF-α (p=0.01) in the airways of asthmatics with the Val105/Val105 compared to asthmatics with Val105/Ile105 and Ile105/Ile105 GSTP1 genotypes (Figure 1). GSTP1 polymorphism had no effect on allergen-induced generation of other Th2 cytokines and CCL5 (Table 1 online supplement).
Figure 1.
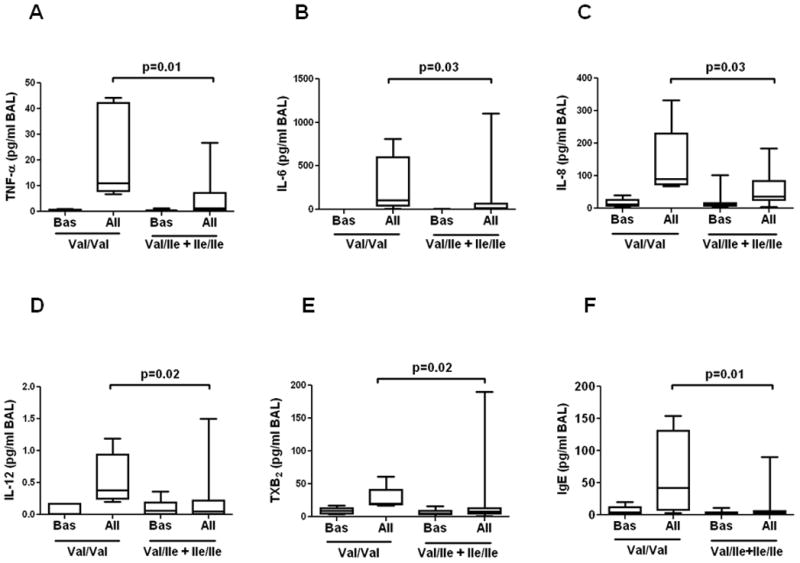
Box plots comparing baseline (Bas) and post-allergen (All) BAL concentrations of TNF-α (A), IL-6 (B), IL-8 (C), IL-12 (D), TXB2 (E) and IgE (E) in atopic asthmatics with GSTP1 Val/Val vs. combined group of patients with Val/Ile and Ile/Ile (Mann-Whitney U test).
In addition, Val105/Val105 asthmatics compared to Val105/Ile105 and Ile105/Ile105 patients generated greater amounts of the cyclooxygenase metabolite, TXB2 after instillation of specific allergen (p=0.02) (Figure 1), although this effect disappeared after adjusting for baseline TXB2 level, age, gender and race.
Levels of F2-isoprostanes and isofuranes, specific markers of oxidative stress in vivo [21], were also higher in asthmatics with Val105/Val105 compared to patients with GSTP1 Val105/Ile105 and Ile105/Ile105 although the difference was not quite statistically significant (Table 1 online supplement).
The concentrations of total IgE in BAL fluid following allergen challenge were significantly higher in patients with GSTP1 Val105/Val105 compared to Val105/Ile105 and Ile105/Ile105 asthmatics (Figure 1).
Except of TXB2, the effect of GSTP1 polymorphism on allergen-induced generation of the measured mediators and IgE was essentially consistent after adjusting for baseline concentrations and the age, gender, and ethnicity of volunteers.
Effect on airway hyperresponsiveness to methacholine and reactivity to specific allergen
The threshold dose of methacholine causing 20% decrease in FEV1 (Met-PC20) did not differ among asthmatics with various GSTP1 genotypes. Likewise, the concentrations of allergen provoking 20% decrease in FEV1 during the early asthmatic response (All-PD20) were similar among the groups (Table 3). The study did not show a relationship between the GSTP1 polymorphism and LAR.
Table 3.
Provocative concentrations of methacholine and allergen (dilution fold of the allergen stock solution) causing 20% decrease in FEV1 (mean ± SD, range, p values Val105/Val105 vs. Val105/Ile105 + Ile105/Ile105).
| Val105/Val105 | Val105/Ile105 + Ile105/Ile105 | p value | |
|---|---|---|---|
| Methacholine PC20 | 4.8±5.9 (0.24–12.4) | 3.35±4.3 (0.09–20) | 0.9 |
| Allergen PC20 | 132.8±122 (4–256) | 69.8±113.1 (4–512) | 0.3 |
Discussion
Our study demonstrates greater generation of acute phase cytokines (TNF-α, IL-6, CXCL8), IL-12, and TXB2 at 24 hrs after allergen challenge in atopic asthmatics with the homozygous GSTP1 Val105/Val105 genotype compared to asthmatics with the GSTP1 Val105/Ile105 and Ile105/Ile105 genotypes. Allergen-induced levels of F2-isoprostanes and isofuranes, markers of oxidative stress [21], were also higher in individuals with GSTP Val105/Val105 although the difference narrowly missed statistical significance. To our knowledge these data are novel.
Our findings are likely to be relevant to the pathomechanism of human asthma. GSTP1 is mainly expressed in alveolar macrophages and airway epithelial cells that greatly contribute to airway pathobiology in asthma [2]. TNF-α, CXCL8, and IL-6 enhance allergic inflammation and airway remodelling [6]. IL-12 affects expression of the atopic phenotype by supporting differentiation of naïve CD4+ helper T cells toward the Th1 subset [6]. TXB2 is a potent bronchoconstrictor as well as a modulator of inflammation and remodelling [22]. Oxidant stress plays a major pathophysiological role in asthma [7], and evidence of greater airway oxidant stress has been previously reported in asthmatic children with GSTP1 Val105/Val105 compared to GSTP1 Ile105/Ile105 [23]. Similarly to our study in atopic asthma, an increased production of IgE has been found in diisocyanate-induced asthmatics carrying the GSTP1 Val105/Val105 genotype [24].
GSTP1 polymorphism was not associated with nonspecific airway hyperresponsiveness to methacholine or airway reactivity to specific allergen. Our data concur with Imboden and colleagues who found no relationship between GSTP1 genotypes and sensitivity to methacholine [25] but disagree with Fryer and colleagues who have reported a protective role of GSTP1 Val105/Val105 on nonspecific bronchial hyperresponsiveness [11]. Importantly, all our volunteers were only mild atopic asthmatics with well controlled disease whereas patients in the latter study were phenotypically inhomogeneous. Nevertheless, studying larger sample size might be unnecessary to reveal modifying effect of the GSTP1 polymorphism on airway reactivity in atopic asthma.
The mechanism underlying the regulation of allergen-provoked airway inflammation in atopic asthma is unknown. Polymorphism in codon 5 alters conjugation of the protein with xenobiotics which may affect detoxifying functions of GSTP1 [1]. However, GSTP1 is also capable of interacting with several proteins involved in cell signalling and gene expression such as. JNK1 [3], and mitogen-activated protein (MAP0 [26]. Nonetheless, the hypothesis that GSTP1 regulates signalling and gene expression in atopic asthma needs further investigation.
Currently, the pathophysiologic role of GSTP1 polymorphism in atopic asthma is not completely understood. Although mainly attributed to regulation of oxidant stress and detoxification of xenobiotics [1], our study clearly demonstrates more complex modulatory function of GSTP1 on allergen-induced airway inflammation in atopic asthmatics in vivo. Clinical and epidemiological data concerning regulation of asthma by the GSTP1 variants in humans are conflicting and support both protective and deteriorating role of the Val105 allele on the disease [12], perhaps due to inconsistent study populations that included patients with various asthma phenotypes. Our patients represented exclusively carefully characterized atopic asthmatics. Interestingly, Kamada and colleagues found a link between the homozygous Val105 genotype and asthma among atopic children [14]. In addition to allergens, GSTP1 regulates airway response to a variety of non-specific environmental factors affecting asthma such as ozone, nitrogen dioxide, tobacco smoke, and air pollution [12, 27]. Thus, future studies should demonstrate how GSTP1 modulates airway response to allergen accompanied by diverse environmental factors because complex exposures are more likely in natural surroundings. For example, Gilliland and colleagues showed augmentation of ragweed-induced generation of IgE, histamine and IL-4 by second-hand smoke [28] and diesel exhaust particles [29] in atopics with Ile105/Ile105 compared to Val105/Ile105 after nasal challenge, although the study population did not include any subjects with the Val105/Val105. However, there are also studies showing detrimental role of GSTP1 Val105/Val105 rather than Ile105/Ile105 on allergen sensitization and asthma especially in children [26,30].
A major limitation of our investigation is a small number of participants, especially asthmatics with GSTP1 Val105/Val105 who predominantly represented the White ethnicity, although a pooled and race-stratified analysis revealed similar outcomes in our study. Nonetheless, the possibility that regulation of the allergen-induced airway inflammation by GSTP1 in atopic asthma is race-dependent should be addressed in future studies.
In summary, our study provides new data and a novel perspective on the regulatory role of GSTP1 polymorphism on allergen-induced airway inflammation in human atopic asthmatics in vivo. Our results suggest upregulation of allergen-induced airway inflammation and oxidative stress in adult atopic asthmatics with the homozygous Val105 GSTP1 variant. Better understanding of the relationship between environmental and genetic factors and the pathomechanism of asthma may provide important guidance for improvement of therapeutic methods for atopic asthma in humans.
Supplementary Material
Acknowledgments
The study was supported by grants from the NIH (K23 HL080030, M01 RR-00095, and NIH P30 ES000267). The authors thank Dr. Ginger Milne, PhD, Stephanie Sanchez and Holly Borntrager for assistance with measurement of eicosanoids in BAL fluid.
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no potential conflict of interest or commercial associations as per journal policy.
References
- 1.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 2.Anttila S, Hirvonen A, Vainio H, Husgafvel-Pursiainen K, Hayes JD, Ketterer B. Immunohistochemical localization of glutathione S-Transferases in human lung. Cancer Res. 1993;53:5643–8. [PubMed] [Google Scholar]
- 3.Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, et al. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–34. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y, Fan Y, Xue B, Luo L, Shen J, Zhang S, et al. Human glutathione S-transferase P1-1 interacts with TRAF2 and regulates TRAF2-ASK1 signals. Oncogene. 2006;25:5787–800. doi: 10.1038/sj.onc.1209576. [DOI] [PubMed] [Google Scholar]
- 5.Ralat LA, Manevich Y, Fisher AB, Colman RF. Direct evidence for the formation of a complex between 1-cysteine peroxiredoxin and glutathione S-transferase π with activity changes in both enzymes. Biochemistry. 2006;45:360–72. doi: 10.1021/bi0520737. [DOI] [PubMed] [Google Scholar]
- 6.Holgate ST. Pathophysiology of asthma: what has our current understanding taught us about new therapeutic approaches. J Allergy Clin Immunol. 2011;128:495–505. doi: 10.1016/j.jaci.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 7.Dworski R. Oxidant stress and asthma. Thorax. 2000;55:S51–S53. doi: 10.1136/thorax.55.suppl_2.S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, Wolf CR, Henderson CJ, Cai Y, Board PG, Foster PS, et al. Glutathione transferase P1. An endogenous inhibitor of allergic responses in a mouse model of asthma. Am J Respir Crit Care Med. 2008;178:1202–10. doi: 10.1164/rccm.200801-178OC. [DOI] [PubMed] [Google Scholar]
- 9.Schroer KT, Gibson AM, Sivaprasad U, Bass SA, Ericksen MB, Willis-Karp M, et al. Downregulation of glutathione S-transferase pi in asthma contributes to enhanced oxidative stress. J Allergy Clin Immunol. 2011;128:539–48. doi: 10.1016/j.jaci.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu X, Xia H, Srivastava SK, Herzog C, Awasthi YC, Ji X, et al. Activity of four allelic forms of glutathione S-transferase hGSTP1-1 for diol epoxides of polycyclic aromatic hydrocarbons. Biochem Biophys Res Comm. 1997;238:397–402. doi: 10.1006/bbrc.1997.7311. [DOI] [PubMed] [Google Scholar]
- 11.Fryer AA, Bianco A, Hepple M, Jones PW, Strange RC, Spiteri MA. Polymorphism at the glutathione S-transferase GSTP1 locus. A new marker for bronchial hyperresponsiveness and asthma. Am J Respir Crit Care Med. 2000;161:1437–42. doi: 10.1164/ajrccm.161.5.9903006. [DOI] [PubMed] [Google Scholar]
- 12.Minelli C, Granell R, Newson R, Rose-Zerilli MJ, Torrent M, Ring SM, et al. Glutathione-S-transferase genes and asthma phenotypes: a human epidemiology (HuGE) systemic review and meta-analysis including unpublished data. Int J Epidem. 2010;39:539–62. doi: 10.1093/ije/dyp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroer KT, Myers JMB, Ryan PH, LeMasters GK, Bernstein DI, Villareal M, et al. Associations between multiple environmental exposures and glutathione S-transferase P1 on persistent wheezing in a birth cohort. J Pediatr. 2009;154:401–8. doi: 10.1016/j.jpeds.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamada F, Mashimo Y, Inoue H, Shao C, Hirota T, Doi S, et al. The GSTP1 gene is a susceptibility gene for childhood asthma and the GSTM1 gene is a modifier of the GSTP1 gene. Intern Arch Allergy Immunol. 2007;144:275–86. doi: 10.1159/000106316. [DOI] [PubMed] [Google Scholar]
- 15.Busse WW, Wanner A, Adams K, Reynolds HY, Castro M, Chowdhury B, et al. Investigative bronchoprovocation and bronchoscopy in airway diseases. Am J Respir Crit Care Med. 2005;172:807–16. doi: 10.1164/rccm.200407-966WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Asthma Education and Prevention Program. Experts Panel Rapport 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institute of Health, National Heart, Lung, and Blood Institute; 2007. Pub no. 08-5846. [Google Scholar]
- 17.American College of Allergy, Asthma and Immunology, American Academy of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Practice Parameters for Allergy Diagnostic Testing. Ann Allergy Asthma Immunol. 1995;75:543–625. [PubMed] [Google Scholar]
- 18.Dworski R, Simon HU, Hoskins A, Yousefi S. Eosinophil and neutrophil extracellular DNA traps in human allergic asthmatic airways. J Allergy Clin Immunol. 2011;127:1260–6. doi: 10.1016/j.jaci.2010.12.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Thoracic Society. Guidelines for methacholine and exercise challenge testing. Am J Respir Crit Care Med. 2000;161:309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 20.Cockcroft DW, Davis BE, Boulet L-P, Deschesnes F, Gauvreau GM, O’Byrne PM, et al. The links between allergen skin test sensitivity, airway responsiveness and airway response to allergen. Allergy. 2005;60:56–9. doi: 10.1111/j.1398-9995.2004.00612.x. [DOI] [PubMed] [Google Scholar]
- 21.Roberts LJ, Morrow JD. Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–13. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 22.Boyce JA. Eicosanoids in asthma, allergic inflammation, and host defense. Curr Mol Med. 2008;8:335–49. doi: 10.2174/156652408785160989. [DOI] [PubMed] [Google Scholar]
- 23.Ercan H, Birben E, Dizdar EA, Keskin O, Karaasian C, Soyer OU, et al. Oxidative stress and genetic and epidemiologic determinants of oxidant injury in childhood asthma. J Allergy Clin Immunol. 2006;118:1097–104. doi: 10.1016/j.jaci.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Piirila P, Wikman K, Luukkonen R, Kaaria K, Rosenberg C, Nordman H, et al. Glutathione S-transferase genotypes and allergic responses to diisocyanate exposure. Pharmacogenetics. 2001;11:437–45. doi: 10.1097/00008571-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Imboden M, Rochat T, Brutsche M, Schindler C, Downs SH, Gerbase MW, et al. Glutathione S-transferase genotype increases risk of progression from bronchial hyperresponsiveness to asthma in adults. Thorax. 2008;63:322–8. doi: 10.1136/thx.2007.085555. [DOI] [PubMed] [Google Scholar]
- 26.Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)-from inflammation to development. Curr Opinion Cell Biol. 1998;10:205–19. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 27.Melén E, Nyberg F, Lindgren CM, Berglind N, Zucchelli M, Nordling E, et al. Interactions between glutathione S-transferase P1, Tumor necrosis factor, and traffic-related air pollution for development of childhood allergic disease. Environ Health Perspectives. 2008;116:1077–84. doi: 10.1289/ehp.11117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilliland FD, Li Y-F, Gong H, Jr, Diaz-Sanchez D. Glutathione S-Transferase M1 and P1 prevent aggravation of allergic responses by secondhand smoke. Am Rev Respir Crit Care Med. 2006;174:1335–41. doi: 10.1164/rccm.200509-1424OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilliland, Li Y-F, Saxon A, Diaz-Sanchez D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: randomized, placebo-controlled crossover study. Lancet. 2004;363:119–25. doi: 10.1016/S0140-6736(03)15262-2. [DOI] [PubMed] [Google Scholar]
- 30.Lee YL, Lin YC, Wang JY, Hsiue TR, Guo YL. Glutathione S-transferase P1 gene polymorphism and air pollution as interactive factors for childhood asthma. Clin Exp Allergy. 2004;34:1707–13. doi: 10.1111/j.1365-2222.2004.02099.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


