Abstract
Aims
Although aberrant Ca2+ release (i.e. Ca2+ ‘leak’) from the sarcoplasmic reticulum (SR) through cardiac ryanodine receptors (RyR2) is linked to heart failure (HF), it remains unknown whether and under what conditions SR-derived Ca2+ can actually cause HF. We tested the hypothesis that combining dysregulated RyR2 function with facilitated Ca2+ uptake into SR will exacerbate abnormal SR Ca2+ release and induce HF. We also examined the mechanisms for these alterations.
Methods and results
We crossbred mice deficient in expression of cardiac calsequestrin (CASQ2) with mice overexpressing the skeletal muscle isoform of SR Ca2+ATPase (SERCA1a). The new double-mutant strains displayed early mortality, congestive HF with left ventricular dilated hypertrophy, and decreased ejection fraction. Intact right ventricular muscle preparations from double-mutant mice preserved normal systolic contractile force but were susceptible to spontaneous contractions. Double-mutant cardiomyocytes while preserving normal amplitude of systolic Ca2+ transients displayed marked disturbances in diastolic Ca2+ handling in the form of multiple, periodic Ca2+ waves and wavelets. Dysregulated myocyte Ca2+ handling and structural and functional cardiac pathology in double-mutant mice were associated with increased rate of apoptotic cell death. Qualitatively similar results were obtained in a hybrid strain created by crossing CASQ2 knockout mice with mice deficient in phospholamban.
Conclusion
We demonstrate that enhanced SR Ca2+ uptake combined with dysregulated RyR2s results in sustained diastolic Ca2+ release causing apoptosis, dilated cardiomyopathy, and early mortality. Our data also suggest that up-regulation of SERCA activity must be advocated with caution as a therapy for HF in the context of abnormal RyR2 function.
Keywords: Calsequestrin, SERCA, CPVT, Heart failure, Apoptosis
1. Introduction
Abnormal intracellular calcium (Ca2+) handling, and in particular, dysregulated Ca2+ release from the sarcoplasmic reticulum (SR), has been implicated in a range of cardiac diseases, including arrhythmias and systolic heart failure (HF).1 In cardiac muscle, Ca2+-induced Ca2+ release (CICR) from the SR via ryanodine receptor channels (RyR2s) during the action potential results in systolic contraction. The SR Ca2+-binding protein calsequestrin (CASQ2) plays an important role in the regulation of SR Ca2+ release by both buffering Ca2+ in the SR and facilitating RyR2 closure during the diastolic phase.2 Re-uptake of Ca2+ by the SR Ca2+ ATPase (SERCA2a) pump initiates diastolic relaxation.
Mutations in RyR2 and CASQ2 have been linked to catecholaminergic polymorphic ventricular tachycardia (CPVT), a cardiac rhythm disorder characterized by episodes of ventricular tachycardia following exercise or infusion of catecholamines in patients with otherwise functionally and anatomically normal hearts.3 At myocyte level, the mutations have been shown to destabilize RyR2 activity thus causing premature Ca2+ release in the form of spontaneous Ca2+ waves, followed by oscillations of membrane potential, known as delayed afterdepolarizations, and extra-systolic action potentials.4,5 However, the mechanisms underlying the adverse effects of adrenergic stimulation in CPVT remain to be fully elucidated.
It has also been suggested that excessive diastolic SR Ca2+ release causes HF,6–8 a disease state determined by compromised systolic and/or diastolic myocardial function and hypertrophy. SR-derived Ca2+ could also activate Ca2+-dependent signalling cascades resulting in pathological remodelling and/or myocyte death, thus contributing to HF development.9,10 Although dysregulated RyR2 activity and diastolic SR Ca2+ release are characteristic features of advanced HF in both humans and animal models,6–8,11,12 it remains unclear whether abnormal RyR2-mediated Ca2+ signalling plays a causal role in HF development. Indeed, while >100 mutations in RyR2 and 12 in CASQ2 (http://www.fsm.it/cardmoc) are implicated in CPVT, none of these mutations has been reliably linked to HF. Conceivably, RyR2 dysfunction is too mild or it can cause HF only in combination with other factors absent in hearts expressing these RyR2 (CASQ2) mutants.
In the present study, we sought to investigate the consequences of combining dysregulated RyR2 function with facilitated SR Ca2+ uptake by using genetic mouse models obtained by overexpression of SERCA (or ablation of its negative regulator phospholamban, PLB) in the absence of CASQ2. We crossbred mice deficient in CASQ2 [CASQ2 knockout (KO)] with mice expressing SERCA1a, the fast skeletal muscle isoform of SERCA [SERCA1a transgenic (TG)] or with PLB knockout mice (PLB.KO). While the SERCA1a.TG and PLB.KO mice are characterized by increased SR Ca2+ load,13,14 heterozygous, and homozygous CASQ2 KO mice show increased susceptibility for adrenergically induced arrhythmias but are otherwise asymptomatic.15,16 We found that combining CASQ2 ablation with up-regulation of SERCA results in sustained diastolic SR Ca2+ release, in turn leading to apoptotic myocyte death, dilated cardiomyopathy, and early mortality.
2. Methods
For details regarding methods, refer to the Supplementary material online, Methods.
2.1. Generation of mouse models
Double-mutant mice for CASQ2 and SERCA1a were generated by breeding two previously described mouse models, the SERCA1a TG mouse13 with the CASQ2 KO mouse.16 WT, TG, KO, CASQ2 heterozygous expressing SERCA1a (HETxTG), and KO expressing SERCA1a (KOxTG) mice were used for the study. Double knockout mice (DKO) for CASQ2 and PLB were created by breeding KO mice for CASQ216 with PLB.KO mice.14 Male, age- and littermate-matched control, and experimental animals were used for all the described studies. This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No.85-23, revised 1996) and the National Research Council Guide for the Care and Use of Laboratory Animals: 8th Edition published by The National Academies Press, 2011, Washington, DC. All the animal experimental protocols were approved by the Institutional Animal Care and Use Committee of The Ohio State University.
2.2. Echocardiography and electrocardiographic recordings
Echocardiographic (ECG) examination was performed to assess systolic and diastolic function in lightly anaesthetized mice (1.5% isoflurane), using the Vevo 2100 echocardiograph system (with a 30 MHz probe), following standard techniques, as previously described.
Continuous ECG recordings were obtained from mice anaesthetized with isoflurane, at minimum effective concentration (1–1.5%) before and after catecholaminergic challenge [isoproterenol (ISO) (1.5 mg/kg ip)], as previously described. Anaesthesia of mice was considered sufficient when the paw pinch test was negative.
2.3. Western blotting
Quantitative immunoblotting was done as previously reported.
2.4. Evaluation of apoptosis using TUNEL staining
Paraformaldehyde-fixed whole heart sections were stained for TUNEL-positive nuclei, using a kit (ROCHE) according to the manufacturer's instructions.
2.5. Cardiac muscle preparation
Mice were administered heparin [40 µL, heparin sodium 10 000 U/mL, APP Pharmaceuticals, LLC, Schaumburg, IL, USA (ip)] 10min before receiving anaesthesia (pentobarbital sodium 70 mg/kg, ip, Lundbeck Inc., Deerfield, IL, USA). Anaesthesia of mice was considered sufficient when the paw pinch test was negative. Thin, uniform, non-branched trabeculae, or small papillary muscles from the right ventricle were mounted between a force transducer and a micromanipulator and stretched to an optimal length as previously described. All experiments were performed at 37°C. The developed tension was recorded with the LabView software and normalized to the cross-sectional area of the muscle.
2.6. Mouse cardiomyocyte isolation and confocal Ca2+ measurements
Ventricular myocytes were isolated from mouse hearts by Collagenase (Worthington) digestion as previously reported. Confocal Ca2+ imaging was performed using a Nikon A1 laser scanning confocal microscope equipped with a ×60 1.4 NA oil objective.
2.7. Statistical methods
Data are presented as mean ± SEM and tested with Student's two-tailed t-test. Kaplan–Meier survival curves were plotted and tested with the SPSS software [Log Rank (Mantel-Cox) test]. ECG data were analysed by cross tabulations with χ2 and one-way analyses of variance were performed, as appropriate. P-values <0.05 were considered significant.
3. Results
3.1. Mice deficient in CASQ2 and expressing SERCA1a display premature mortality with signs of cardiac enlargement and pulmonary congestion
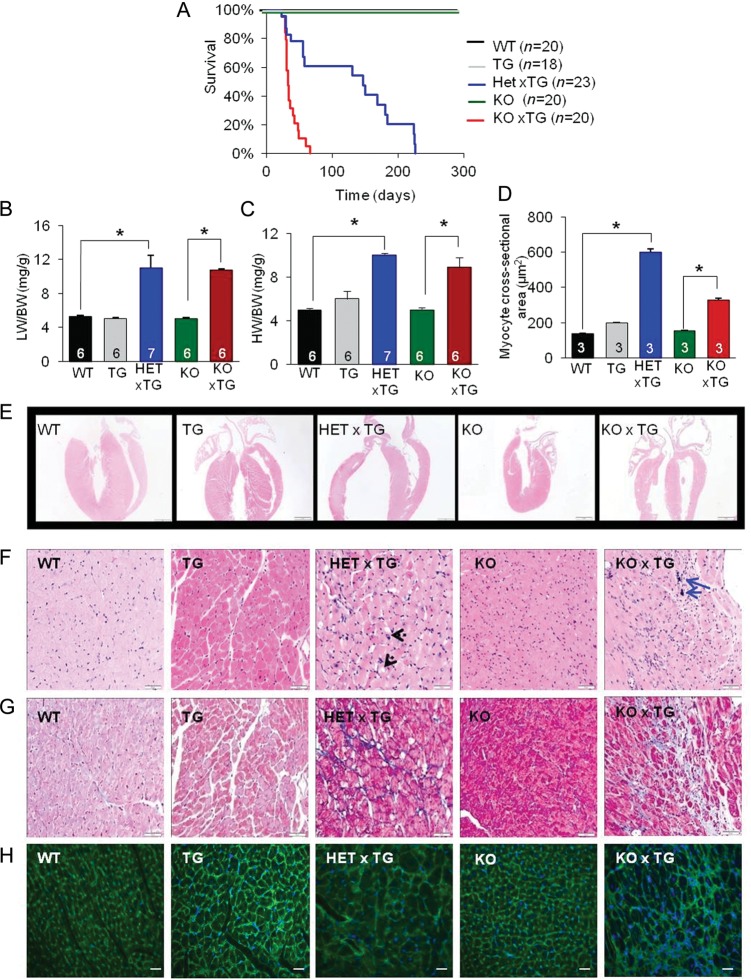
Double-mutant mice for SERCA1a expression with either partial (HETxTG) or complete ablation of CASQ2 (KOxTG) died early. 100% mortality rates were 66 days (37 ± 2.6) and 226 days (115 ± 3.4) in the KOxTG and HETxTG mice, respectively (Figure 1A). No animals died in the WT, KO, and TG groups during the study period (>15 months). Post-mortem examinations of the KOxTG and HETxTG animals revealed cardiac enlargement and wall thinning (Figure 1E) with evidence of lung oedema and significantly increased lung weight-to-body weight ratios [WT: 5.27 ± 0.16; HETxTG: 11.04 ± 1.50, (P < 0.05); KO: 5 ± 0.02; and KOxTG: 10.75 ± 0.15, (P < 0.05) mg/g] compared with the KO and the WT groups, respectively (Figure 1B). The heart-to-body weight ratios were increased approximately two-fold in the HETxTG and the KOxTG [WT: 5.00 ± 0.12, HETxTG: 10.00 ± 0.20 (P < 0.05), KO: 5.00 ± 0.19, KOxTG: 8.91 ± 0.90 (P < 0.05) (mg/g)] mice compared with the age-matched WT and KO mice, respectively (Figure 1C).
Figure 1.
Premature mortality and pathological structural remodelling of the myocardium in the HETxTG and the KOxTG mice. (A) Kaplan–Meier survival plot. (B) Lung weight/body weight ratio. (C) Heart weight/body weight ratio. (D) Average cardiomyocyte cross-sectional area. (E) Representative Haematoxylin and Eosin stained paraffin sections show wall thinning and dilation in the HETxTG (3.5 months old) and the KOxTG (30 days old) hearts compared with the age-matched WT and KO hearts (×10 magnification) (n = 5). (F) Vacuolation and megakaryosis in the HETxTG mice hearts (indicated by black arrows) and mineralization in the KOxTG hearts (indicated by blue arrows) (n = 5). (G) Fibrosis demonstrated by Masson Trichrome staining in the HETxTG and the KOxTG hearts (n = 5). (H) WGA staining of cell membranes in the HETxTG and the KOxTG hearts (n = 3). All values reported as mean ± SEM (*P < 0.05); sample sizes are indicated within the respective column (HET, heterozygous and KO, knockout for CASQ2; TG, expresses SERCA1a transgene).
3.2. CASQ2-deficient mice overexpressing SERCA1a display dilated cardiac hypertrophy and contractile dysfunction
We further examined the structural and functional alterations in the double-mutant hearts using histological and haemodynamic assays. Haematoxylin and Eosin staining revealed widespread signs of degenerative changes in HETxTG and KOxTG cardiomyocytes including loss of striations, vacuolation, megakaryosis, and mineralization (Figure 1F). Additionally, Masson trichrome staining exposed focal areas of moderate interstitial fibrosis in sections from double-mutant hearts (Figure 1G) compared with the normal histology from the age-matched WT and KO cardiac sections. No significant differences in the cardiomyocyte size were observed in the TG (199 ± 1 µm2) and the KO (153 ± 4 µm2) hearts compared with the WT (138 ± 3 µm2) hearts. A significant increase in the myocyte size was detected in both the HETxTG (599 ± 20 µm2; P < 0.05) and the KOxTG (328 ± 10 µm2; P < 0.05) hearts compared with the age-matched WT and KO hearts, respectively (Figure 1D and H). In both genotypes, myocyte hypertrophy was not regionally restricted but observed consistently both transmurally and from the apex to the base of the ventricles.
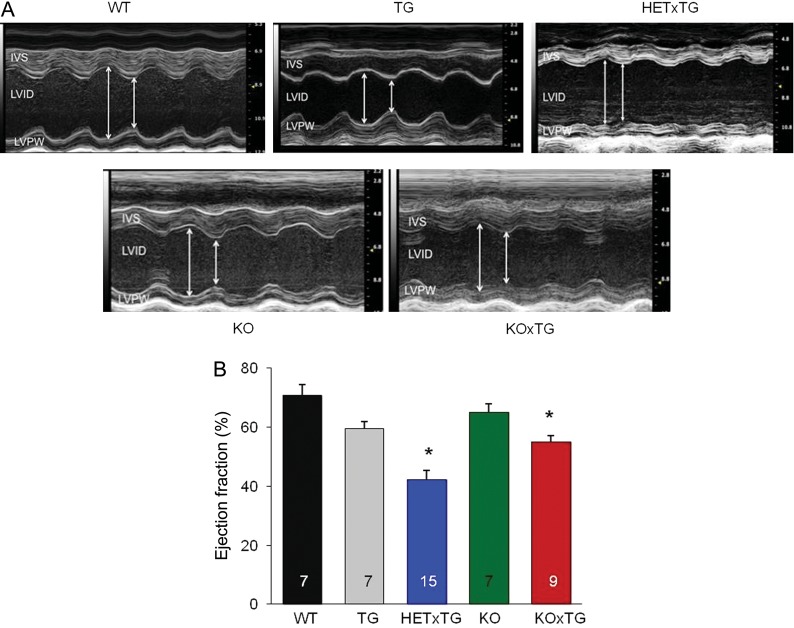
Left ventricular (LV) enlargement and impaired cardiac function in mice combining CASQ2 ablation and SERCA1a expression were indicated by echocardiography (Figure 2A). The end-diastolic diameter of the left ventricle was significantly enlarged (3.54 ± 0.13 vs. 4.48 ± 0.2 mm, P < 0.05) and LV posterior wall dimension diastole (LVPWD) was decreased (0.95 ± 0.06 vs. 0.73 ± 0.05 mm, P < 0.05) in the WT vs. the HETxTG mice (see Supplementary material online, Table S1). Ejection fraction was reduced in double-mutant mice (Figure 2B). Taken together, these results suggest that double-mutant mice developed dilated cardiomyopathy.
Figure 2.
LV contractile function is decreased in the HETxTG and the KOxTG mice. (A) Representative images of M-mode echocardiograms. IVS, interventricular septum; LVPW, left ventricular posterior wall; RV, right ventricle; LVID, left ventricular internal diameter. (B) Mean ± SEM of LV ejection fraction (EF%); *P < 0.05. (HET, heterozygous and KO, knockout for CASQ2; TG, expresses SERCA1a transgene).
3.3. Catecholamine-induced arrhythmias are exacerbated in CASQ2-deficient mice overexpressing SERCA1a
ECG traces were recorded from lightly anaesthetized mice before and after catecholaminergic challenge with ISO. Continuous ECG monitoring showed a nearly normal sinus rhythm at the baseline, except for a relatively few cases of premature ventricular complexes (PVCs) in the HETxTG group (see Supplementary material online, Figure S1A). Following the ISO challenge, both the HETxTG and the KOxTG groups showed a marked increase in the incidence of arrhythmias (see Supplementary material online, Figure S1B). Importantly, while the HETxTG mice primarily displayed simple ventricular arrhythmias (consisting of PVCs and fusion beats), the KOxTG mice also developed complex ventricular arrhythmias, including ventricular tachycardia (see Supplementary material online, Figure S1B–D).
3.4. Intrinsic cardiac muscle contractility is preserved in CASQ2-deficient mice expressing SERCA1a
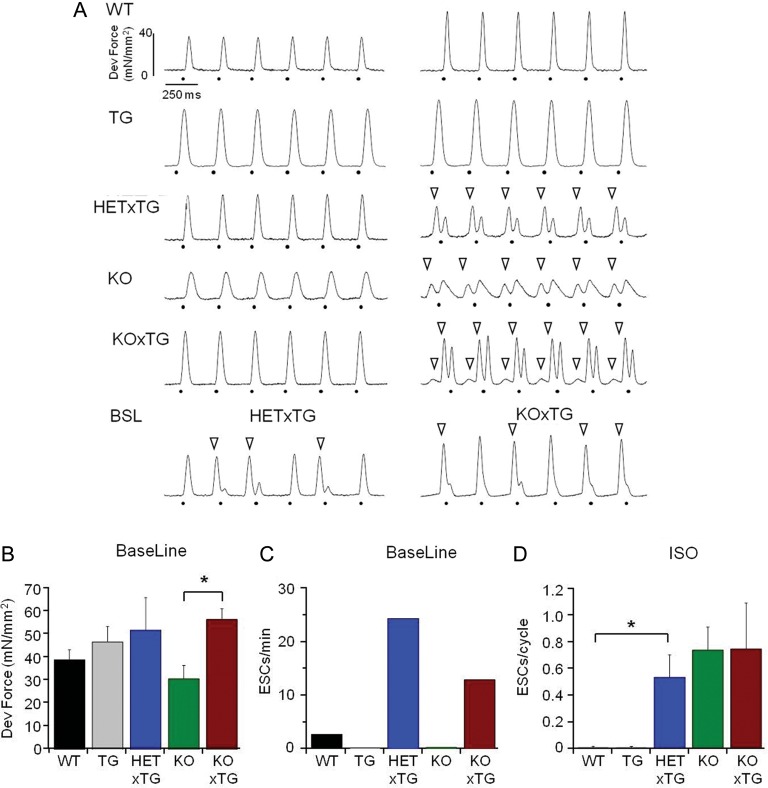
To determine whether the impaired LV haemodynamic performance in double-mutant mice is associated with weakening of intrinsic myocardial contractility, we performed contractile force measurements in ventricular papillary muscles paced at 4 Hz at 37°C. As shown in Figure 3, the systolic contractile force in the HETxTG muscles, rather than being weaker, tended to be stronger than in the age-matched WT muscles. Similarly, the KOxTG muscles contracted more strongly than the KO muscles. Of note, both HETxTG and KOxTG muscles showed occasional spontaneous diastolic contractions, which were rarely observed in the WT, TG, and the KO muscles (Figure 3A, bottom panels; Figure 3C). Exposure to ISO (0.3–1 µM) led to regular diastolic contractions in all the three types of CASQ2-deficient muscles (HETxTG, KOxTG, and KO) but not in the WT or the TG muscles. Thus, in vivo cardiac contractile dysfunction in mice combining CASQ2 deficiency with up-regulated SR Ca2+ uptake develops in the absence of decreased intrinsic cardiac muscle contractility. Consistent with the in vivo ECG studies, muscles from the double-mutant mice showed an enhanced propensity towards diastolic, spontaneous contractile activity that was further exacerbated by ISO.
Figure 3.
Intrinsic cardiac muscle contractility is preserved in CASQ2-deficient mice overexpressing SERCA1a. (A) Representative contraction traces at baseline (37°C, 4 Hz, left traces) and in the presence of β-adrenergic stimulation (0.3–1 µM ISO, right traces) in the different groups. Dots show electrical pacing; empty arrowheads show spontaneous extra-systolic contractions (ESCs). Occasionally, the HETxTG and the KOxTG muscles showed ESCs at baseline (bottom row). (B) Average developed force at baseline. (C) Frequency of ESCs at baseline. (D) Frequency of ESCs in the presence of ISO. *P < 0.05. (HET, heterozygous and KO, knockout for CASQ2; TG, expresses SERCA1a transgene).
3.5. Cardiomyocytes from CASQ2-deficient mice expressing SERCA1a display enhanced diastolic SR Ca2+ release
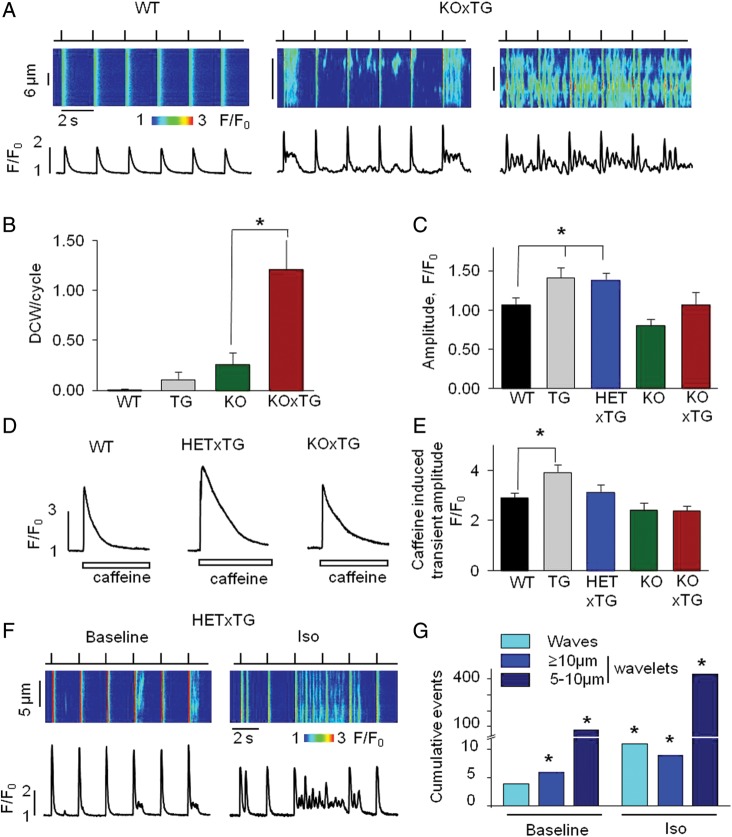
To directly examine the effects of combining CASQ2 ablation with expression of SERCA1a on myocyte Ca2+ handling, we performed intracellular confocal Ca2+ imaging and Ca2+ current measurements in isolated cardiomyocytes from double-mutant mice and the three control groups (i.e. WT, KO, and TG). As expected, expressing SERCA1a in the background of deficient CASQ2 expression resulted in a marked increase in diastolic Ca2+ release that manifested in repetitive multiple propagating wave- or wavelet-like signals in both KOxTG and HETxTG myocytes (Figure 4A and F). All the other groups, including TG and KO myocytes, exhibited such spontaneous releases at much lower rates under the same experimental conditions (Figure 4B). In addition, average diastolic Ca2+, as a total measure of diastolic release, was significantly increased in the double-mutant myocytes compared with the other groups (see Supplementary material online, Figure S2). Of note, the amplitude of systolic Ca2+ transients and SR Ca2+ content in the double-mutant myocytes were either higher or similar to those in the WT but lower than those in the TG myocytes. The reduced SR Ca2+ content of the double-mutant myocytes relative to the SERCA1a myocytes is evidently due to the increased diastolic release in the double-mutant myocytes which would be expected to deplete the SR Ca2+ store (Figure 4C–E). Ca2+ currents measured in separate experiments in the double-mutant myocytes were not significantly different in amplitude compared with the WT and the other groups (see Supplementary material online, Figure S3). Additionally, key Ca2+-handling proteins, including SERCA1a, SERCA2a, CASQ2, RyR2, and PLB, were assessed by western blotting in the hearts from different groups (see Supplementary material online, Figure S4). SERCA1a levels were similar in all the groups overexpressing this protein. Consistent with previous results, endogenous SERCA2a13 and RyR217 were decreased in the hearts overexpressing SERCA1a.
Figure 4.
Cardiomyocytes from CASQ2-deficient mice expressing SERCA1a display enhanced diastolic SR Ca2+ release. (A) Representative line-scan images and temporal profiles of Fluo-3 fluorescence recorded in the WT and the KOxTG myocytes stimulated at 0.5 Hz. Top: field-stimulation protocol. Timing of electrical stimulations is indicated by upward deflections. (B) Distributions of diastolic Ca2+ wave (DCW)/cycle (KOxTG vs. KO *P < 0.05). (C) Average values of Ca2+ transient amplitude (*P < 0.05 TG and HETxTG vs. WT). (D) Representative traces of Ca2+ transients evoked with 10 mM caffeine in the WT, HETxTG, and the KOxTG myocytes. (E) Average values of caffeine-induced Ca2+ transients (*P < 0.05 TG vs. WT). (F) Representative line-scan images and temporal profiles of Fluo-3 fluorescence at baseline and in the presence of ISO in the HETxTG myocytes stimulated at 0.5 Hz. (G) Average data of diastolic Ca2+ release events at baseline and in the presence of ISO in the HETxTG myocytes (*P < 0.05). Experiments were performed at room temperature (RT) with 1 mM Ca2+ in the external solution. (HET, heterozygous and KO, knockout for CASQ2; TG, expresses SERCA1a transgene).
The effects of ISO on Ca2+ handling were investigated in the HETxTG myocytes (Figure 4F and G). At baseline, the myocytes exhibited occasional spontaneous releases of different sizes from small wavelets to full waves. All spontaneous release events were grouped into three categories according to their size (i.e. full waves and wavelets with width of either ≥ 10 μm or 5–10 μm) (Figure 4G). ISO caused a marked increase in the rate of occurrence of all types of events including full Ca2+ waves.
3.6. Increased cardiomyocyte apoptosis in CASQ2-deficient mice overexpressing SERCA1a
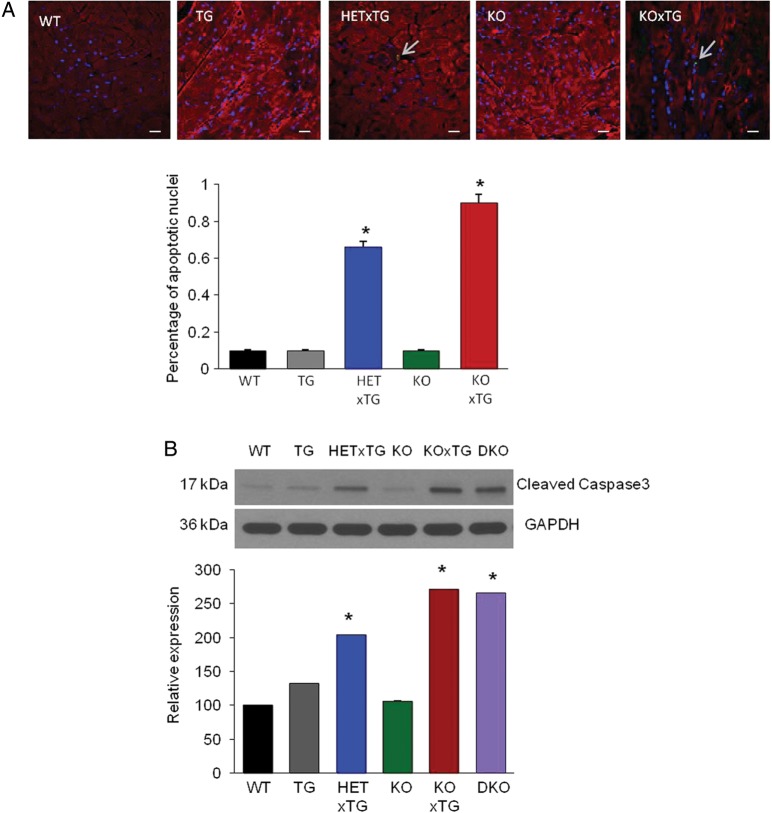
Collectively, the data presented above indicate that weakening of in vivo mechanical performance of double-mutant hearts occurs without a corresponding weakening of systolic Ca2+ release and contractile force in cardiac myocytes. This apparent discrepancy could be explained by increased myocyte loss leading to LV chamber dilation, a possibility also consistent with the significant LV dilation revealed by our histological analyses (Figure 1E). TUNEL staining of tissue sections from both the HETxTG and the KOxTG hearts displayed significant increases in the fraction of apoptotic nuclei [0.6 and 0.9%, respectively (P < 0.05)] compared with the WT and the KO groups (0.1%; P < 0.05) (Figure 5A). Additionally, expression of the pro-apoptotic form of Caspase 3 (17 kDa fragment) was significantly increased in the HETxTG (one fold; P < 0.05) and KOxTG (1.7 fold; P < 0.05) mice compared with the WT and the KO hearts, respectively. (Figure 5B). Thus, myocyte loss could indeed contribute to the in vivo cardiac contractile dysfunction in double-mutant mice by reduced myocyte number and increased LV diameter, thereby reducing LV pressure (see ‘Section 4’).
Figure 5.
Increased incidence of cardiomyocyte apoptosis in the HETxTG and the KOxTG hearts. (A) Cardiac sections from the WT, TG, HETxTG, KO, and the KOxTG hearts stained for TUNEL-positive nuclei (green), α-actin marking sarcomeres (red) and DAPI (blue) (n = 3). (B) Representative western blot images of cleaved Caspase 3 expression in the WT, TG, HETxTG, KO, KOxTG, and the DKO hearts; summary data of cleaved Caspase 3 expression (n = 6). All values reported as mean ± SEM (*P < 0.05). (HET, heterozygous and KO, knockout for CASQ2; TG, expresses SERCA1a transgene).
3.7. Combining CASQ2 ablation with either ablation of PLB or expression of SERCA1a produces similar phenotypes
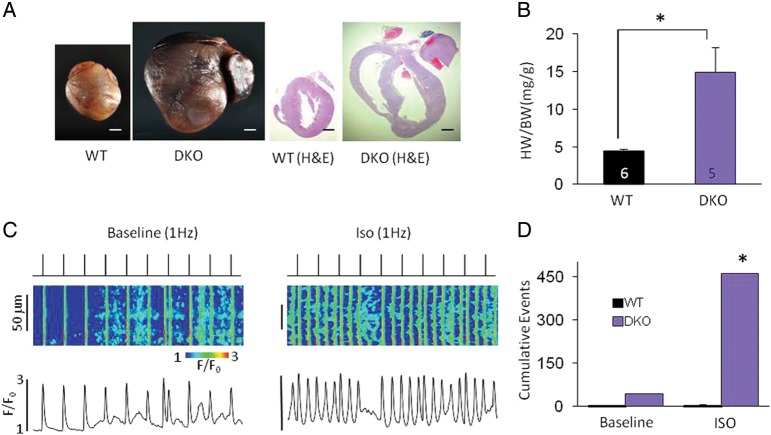
Despite lack of detrimental effects of SERCA1a expression on cardiac function, by itself13 or even in combination with ablation of PLB,18 this exogenous SERCA isoform might not be considered fully compatible with cardiac myocytes. Therefore, confirmatory experiments were performed in a new mouse model of increased diastolic Ca2+ release by crossing CASQ2.KO mice with mice deficient in PLB14, the endogenous inhibitor of SERCA2a (DKO). We found that this DKO model reproduces the main findings obtained with KOxTG and HETxTG mice including early mortality [average dying age 12 ± 0.4 months, (n = 19)], cardiac contractile dysfunction, hypertrophy, severe chamber dilation [HW/BW ratio: WT (5.00 ± 0.22) DKO (15.00 ± 3.27, P < 0.05), pulmonary congestion (LW/BW ratio: WT (5 ± 0) DKO (12 ± 2, P < 0.05], abnormal myocyte Ca2+ cycling (Figure 6), and increased Caspase 3 fragments (Figure 5B). Although the results were qualitatively similar, the DKO mice lived relatively longer than the HETxTG and the KOxTG mice, indicating that the number of active pumps could play a significant role in modulating the magnitude of RyR2 leak and hence its deleterious effects.
Figure 6.
Up-regulation of SERCA2a Ca2+ uptake by PLB ablation in the CASQ2 KO mouse reproduces the dysregulated SR Ca2+ release found in the KOxTG mice, causing HF. (A) Representative hearts from the WT and the DKO mice. (B) Heart weight/body weight ratio. (C) Representative line-scan images and temporal profiles of Fluo-3 fluorescence recorded in the WT and the DKO myocytes stimulated at 1 Hz. Top: field-stimulation protocol. Timing of electrical stimulations is indicated by upward deflections. (D) Average values of diastolic Ca2+ release events at baseline and in the presence of ISO in the WT (n = 15) and the DKO (n = 12) myocytes, normalized to total cell number. All values reported as mean ± SEM. Sample size for each group is indicated within the respective bar (*P < 0.05) (DKO, double knockout for both CASQ2 and PLB).
4. Discussion
In the present study, we investigated the consequences of combining dysregulated RyR2 function with facilitated SR Ca2+ uptake by crossing genetically altered mice deficient in expression of CASQ2 with mice overexpressing SERCA1a (or ablation of PLB, a negative regulator of SERCA2a). Myocytes from the crossbred mice displayed increased diastolic Ca2+ release with preserved myocyte systolic Ca2+ release and contractility. Strikingly, the hybrid mice died prematurely and developed cardiac failure characterized by enhanced myocyte apoptotic death, hypertrophic chamber dilation, and haemodynamic deficiency. These findings present important implications for the pathophysiology and treatment of HF.
4.1. Diastolic SR Ca 2+ release as a cause of HF
Although dysfunctional RyR2s have been associated with HF6–8 and exacerbation of the disease phenotype in pressure-overload induced HF,9 it is unknown whether chronic SR Ca2+ leak by itself can trigger pathological downstream changes leading to HF. Notably, gain of function RyR2 mutations associated with ‘leaky’ RyR2 channels have not been shown to cause HF. In the present study, we demonstrate that a chronic increase in diastolic RyR2-mediated SR Ca2+ release can lead to a severe cardiac mechanical dysfunction and hypertrophy, thus providing strong support for the notion that excessive diastolic SR Ca2+ release through RyR2s can cause HF. At the same time, our results demonstrate clearly that, because of the self-limiting nature of diastolic release via RyR2s,19,20 chronically elevated Ca2+ release that is large enough to cause HF can be attained only under conditions of facilitated SR Ca2+ uptake. According to previous longitudinal studies, in a tachypacing model of HF,21 RyR2-mediated leak that results from hyperphosphorylation and oxidation of the RyR2 presents one of the earliest alterations in myocyte Ca2+ handling. Similarly, increased β-adrenergic stimulation of the heart expected to increase SR Ca2+ uptake (via PLB phosphorylation) typically occurs early, during the compensatory phase of HF development and has been shown to persist through up-regulation of the β2 adrenoreceptors at more advanced stages.22,23 Therefore, on the basis of our findings, altered RyR2 function would be expected to causally contribute to HF but only when coinciding with adrenergically mediated increase in SR Ca2+ uptake, thus potentially contributing to the heterogeneity of HF aetiology in various pathological settings.24
4.2. LV remodelling due to cell loss and hypertrophy but with preserved myocyte contractility causes haemodynamic impairment in Ca2+-dependent HF
Decreased cardiomyocyte SR Ca2+ content and weakened systolic SR Ca2+ release and contractility are considered to be important characteristics of the failing heart, and indeed, contractile dysfunction in HF is commonly attributed to depressed myocyte contractility, i.e. myocyte failure.25–27 Our results show that decreased systolic SR Ca2+ release and weakened myocyte contractility are not essential for HF development. Notably, because of the facilitated SERCA-mediated SR Ca2+ re-uptake, SR Ca2+ load, systolic Ca2+ release, and contractile force were preserved, or even improved, in intact muscles and isolated ventricular myocytes from the double-mutant mice compared with the WT and the KO hearts despite the substantial diastolic loss of SR Ca2+ in the double mutants (Figures 3 and 4). Furthermore, our data demonstrate that under conditions of sustained RyR2-mediated diastolic release, HF can develop without signs of myocyte contractile failure as a result of myocyte loss and dilation of the LV chamber. Indeed according to the Laplace's law, ventricular pressure will fall with increasing chamber diameter and wall thickness even if wall tension due to myocyte contraction is preserved. Notably, the ∼50% increase in end-diastolic LV diameter and the ∼20% decrease in LV wall thickness observed in the HETxTG mice at a constant wall tension translate into nearly two-fold decrease in systolic LV pressure consistent with the ∼2.5-fold decrease in ejection fraction in these mice (Figure 2).
It is well established that mitochondrial Ca2+ overload results in mitochondrial injury associated with myocyte apoptosis or necrosis.28–30 In addition, several major signalling pathways have been implicated in the activation of the cardiac hypertrophic response following elevations of intracellular Ca2+, including the calcineurin/NFAT and the CaMKII/HDAC pathways.31–33 Despite their important role in the development of cardiac pathological remodelling, the exact sub-cellular sources of Ca2+ involved in mitochondria-mediated cell death and Ca2+-dependent hypertrophy remain poorly defined. Our results clearly point to a role for SR-derived Ca2+ in these pathological processes.
4.3. Increased SR Ca2+ uptake and arrhythmogenesis in CPVT
In CPVT, episodes of arrhythmia are provoked by exercise or emotional stress associated with increased levels of circulating catecholamines in both patients and genetic mouse models of this syndrome.3 Although the catecholamine-dependency of CPVT is likely to have complex causes, it has been suggested that stimulation of SERCA2a (via phosphorylation of PLB) is a potential mechanism for the pro-arrhythmic effects of catecholamines.4,34 Facilitated SERCA-mediated SR Ca2+ uptake is expected to increase myocyte arrhythmic potential by maintaining the SR Ca2+ content at levels conducive to spontaneous Ca2+ release generation. Consistent with this expectation, CASQ2-deficient myocytes overexpressing SERCA1a exhibited a higher propensity for spontaneous SR Ca2+ release than either the WT or the KO myocytes in the absence of β-adrenergic stimulation (Figure 4). Surprisingly, increased spontaneous SR Ca2+ release propensity did not translate into higher arrhythmia vulnerability in double-mutant mice under baseline conditions (no ISO). However, the severity of arrhythmias provoked by ISO was markedly exacerbated in the double-mutant mice compared with the KO mice (see Supplementary material online, Figure S1). Other factors such as functional heterogeneities in the heart during catecholamine stimulation35 (as opposed to homogeneous functional changes resulting from cardiac expression of SERCA1a) seem to be required to provide a necessary substrate for arrhythmias. Further studies will have to look into this important issue.
4.4. SERCA up-regulation as therapy for HF
Up-regulation of SR Ca2+ re-uptake through expression of SERCA2a or ablation of PLB are promising gene therapy approaches based on their beneficial effects in a number of animal models of HF.36,37 Despite many successful examples, this approach has failed to reverse HF or has even exacerbated cardiac dysfunction in some studies.38–40 Our results demonstrate that up-regulation of SERCA-mediated SR Ca2+ uptake either by directly increasing the number of SERCA pumps or by disinhibition of endogenous SERCA pumps via ablation of PLB, when combined with dysregulated RyR2s, can be severely detrimental rather than therapeutic. RyR2s are known to become hyperactive or ‘leaky’ in certain HF settings as a result of post-translational modifications through phosphorylation and/or oxidation.6,21,41 Our data suggest that SERCA therapy should be advocated with caution in failing hearts given the possibility of pathological interaction of facilitated SR Ca2+ uptake with dysregulated RyR2 function.
4.5. Potential limitations
A potential limitation of this study is that SERCA1a, normally absent in cardiac myocytes, might be considered ‘non-physiological’ and therefore detrimental to cardiac function. However, as shown previously, SERCA1a can functionally substitute SERCA2a in the cardiac muscle without detrimental changes in phenotype.13 Additionally, when we used ablation of PLB to facilitate SR Ca2+ uptake against the background of CASQ2.KO, we obtained results similar to those attained in our original double-mutant strains. It is also to be noted that as in all studies involving manipulation of individual genes, changes in SERCA1a and CASQ2 are likely to be associated with alterations in expression/activity of other proteins; thus, we cannot rule out the possibility of such compensatory changes influencing our results.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the National Institutes of Health (HL074045 and HL063043 to S.G. HL080551 to M.P. and K01RR023083 to V.A.L.). A.K. was supported by a Post-doctoral fellowship from Great Rivers Affiliate, American Heart Association.
Supplementary Material
Acknowledgements
We thank Dr Evangelia G. Kranias PhD for providing the Phospholamban knockout mouse and Ms Summer Hayes for excellent technical assistance with Echocardiography and ECG studies.
Conflict of interest: none declared.
References
- 1.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 2.Gyorke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008;77:245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- 3.Napolitano C, Priori SG. Diagnosis and treatment of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2007;4:675–678. doi: 10.1016/j.hrthm.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 4.Gyorke S. Molecular basis of catecholaminergic polymorphic ventricular tachycardia. Heart rhythm. 2009;6:123–129. doi: 10.1016/j.hrthm.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe H, Knollmann BC. Mechanism underlying catecholaminergic polymorphic ventricular tachycardia and approaches to therapy. J Electrocardiol. 2011;44:650–655. doi: 10.1016/j.jelectrocard.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Wehrens XH, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annu Rev Physiol. 2005;67:69–98. doi: 10.1146/annurev.physiol.67.040403.114521. [DOI] [PubMed] [Google Scholar]
- 7.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 8.Gyorke S, Carnes C. Dysregulated sarcoplasmic reticulum calcium release: potential pharmacological target in cardiac disease. Pharm Therap. 2008;119:340–354. doi: 10.1016/j.pharmthera.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Oort RJ, Respress JL, Li N, Reynolds C, De Almeida AC, Skapura DG, et al. Accelerated development of pressure overload-induced cardiac hypertrophy and dysfunction in an RyR2-R176Q knockin mouse model. Hypertension. 2010;55:932–938. doi: 10.1161/HYPERTENSIONAHA.109.146449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi N, Takahashi N, Xu L, Smithies O, Meissner G. Early cardiac hypertrophy in mice with impaired calmodulin regulation of cardiac muscle Ca release channel. J Clin Invest. 2007;117:1344–1353. doi: 10.1172/JCI29515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Gyorke I, Terentyeva R, et al. Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci USA. 2005;102:14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George CH. Sarcoplasmic reticulum Ca2+ leak in heart failure: mere observation or functional relevance? Cardiovasc Res. 2008;77:302–314. doi: 10.1093/cvr/cvm006. [DOI] [PubMed] [Google Scholar]
- 13.Loukianov E, Ji Y, Grupp IL, Kirkpatrick DL, Baker DL, Loukianova T, et al. Enhanced myocardial contractility and increased Ca2+ transport function in transgenic hearts expressing the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. Circ Res. 1998;83:889–897. doi: 10.1161/01.res.83.9.889. [DOI] [PubMed] [Google Scholar]
- 14.Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, et al. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- 15.Chopra N, Kannankeril PJ, Yang T, Hlaing T, Holinstat I, Ettensohn K, et al. Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ Res. 2007;101:617–626. doi: 10.1161/CIRCRESAHA.107.157552. [DOI] [PubMed] [Google Scholar]
- 16.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lalli MJ, Yong J, Prasad V, Hashimoto K, Plank D, Babu GJ, et al. Sarcoplasmic reticulum Ca2+ ATPase (SERCA) 1a structurally substitutes for SERCA2a in the cardiac sarcoplasmic reticulum and increases cardiac Ca2+ handling capacity. Circ Res. 2001;89:160–167. doi: 10.1161/hh1401.093584. [DOI] [PubMed] [Google Scholar]
- 18.Zhao W, Frank KF, Chu G, Gerst MJ, Schmidt AG, Ji Y, et al. Combined phospholamban ablation and SERCA1a overexpression result in a new hyperdynamic cardiac state. Cardiovasc Res. 2003;57:71–81. doi: 10.1016/s0008-6363(02)00609-0. [DOI] [PubMed] [Google Scholar]
- 19.Trafford AW, Diaz ME, Sibbring GC, Eisner DA. Modulation of CICR has no maintained effect on systolic Ca2+: simultaneous measurements of sarcoplasmic reticulum and sarcolemmal Ca2+ fluxes in rat ventricular myocytes. J Physiol. 2000;522(Pt 2):259–270. doi: 10.1111/j.1469-7793.2000.t01-2-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukyanenko V, Viatchenko-Karpinski S, Smirnov A, Wiesner TF, Gyorke S. Dynamic regulation of sarcoplasmic reticulum Ca2+ content and release by luminal Ca2+-sensitive leak in rat ventricular myocytes. Biophys J. 2001;81:785–798. doi: 10.1016/S0006-3495(01)75741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belevych AE, Terentyev D, Terentyeva R, Nishijima Y, Sridhar A, Hamlin RL, et al. The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc Res. 2011;90:493–502. doi: 10.1093/cvr/cvr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, et al. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 23.Altschuld RA, Starling RC, Hamlin RL, Billman GE, Hensley J, Castillo L, et al. Response of failing canine and human heart cells to beta 2-adrenergic stimulation. Circulation. 1995;92:1612–1618. doi: 10.1161/01.cir.92.6.1612. [DOI] [PubMed] [Google Scholar]
- 24.Toischer K, Rokita AG, Unsold B, Zhu W, Kararigas G, Sossalla S, et al. Differential cardiac remodeling in preload versus afterload. Circulation. 2010;122:993–1003. doi: 10.1161/CIRCULATIONAHA.110.943431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beuckelmann DJ, Erdmann E. Ca2+-currents and intracellular [Ca2+]i-transients in single ventricular myocytes isolated from terminally failing human myocardium. Basic Res Cardiol. 1992;87(Suppl. 1):235–243. doi: 10.1007/978-3-642-72474-9_19. [DOI] [PubMed] [Google Scholar]
- 26.O'Rourke B, Kass DA, Tomaselli GF, Kaab S, Tunin R, Marban E. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res. 1999;84:562–570. doi: 10.1161/01.res.84.5.562. [DOI] [PubMed] [Google Scholar]
- 27.Balke CW, Shorofsky SR. Alterations in calcium handling in cardiac hypertrophy and heart failure. Cardiovasc Res. 1998;37:290–299. doi: 10.1016/s0008-6363(97)00272-1. [DOI] [PubMed] [Google Scholar]
- 28.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, et al. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, et al. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Revs Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 32.Bueno OF, van Rooij E, Molkentin JD, Doevendans PA, De Windt LJ. Calcineurin and hypertrophic heart disease: novel insights and remaining questions. Cardiovasc Res. 2002;53:806–21. doi: 10.1016/s0008-6363(01)00493-x. [DOI] [PubMed] [Google Scholar]
- 33.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 34.Kashimura T, Briston SJ, Trafford AW, Napolitano C, Priori SG, Eisner DA, et al. In the RyR2(R4496C) mouse model of CPVT, beta-adrenergic stimulation induces Ca waves by increasing SR Ca content and not by decreasing the threshold for Ca waves. Circ Res. 2010;107:1483–1489. doi: 10.1161/CIRCRESAHA.110.227744. [DOI] [PubMed] [Google Scholar]
- 35.Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000;101:1960–1969. doi: 10.1161/01.cir.101.16.1960. [DOI] [PubMed] [Google Scholar]
- 36.Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Tilemann L, Ishikawa K, Weber T, Hajjar RJ. Gene therapy for heart failure. Circ Res. 2012;110:777–793. doi: 10.1161/CIRCRESAHA.111.252981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janczewski AM, Zahid M, Lemster BH, Frye CS, Gibson G, Higuchi Y, et al. Phospholamban gene ablation improves calcium transients but not cardiac function in a heart failure model. Cardiovasc Res. 2004;62:468–480. doi: 10.1016/j.cardiores.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 39.O'Donnell JM, Fields A, Xu X, Chowdhury SA, Geenen DL, Bi J. Limited functional and metabolic improvements in hypertrophic and healthy rat heart overexpressing the skeletal muscle isoform of SERCA1 by adenoviral gene transfer in vivo. Am J Physiol. 2008;295:H2483–H2494. doi: 10.1152/ajpheart.01023.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang T, Guo T, Mishra S, Dalton ND, Kranias EG, Peterson KL, et al. Phospholamban ablation rescues sarcoplasmic reticulum Ca2+ handling but exacerbates cardiac dysfunction in CaMKIIdelta(C) transgenic mice. Circ Res. 2010;106:354–362. doi: 10.1161/CIRCRESAHA.109.207423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell and Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.