Abstract
Since the discovery of triadin >20 years ago as one of the major proteins located in the junctional sarcoplasmic reticulum, the field has come a long way in understanding the pivotal role of triadin in orchestrating sarcoplasmic reticulum Ca2+-release and hence excitation–contraction (EC) coupling. Building on the information gathered from earlier lipid bilayer and myocyte overexpression studies, the gene-targeted ablation of Trdn demonstrated triadin's indispensable role for maintaining the structural integrity of the couplon. More recently, the discovery of inherited and acquired diseases displaying altered expression and function of triadin has further emphasized the role of triadin in health and disease. Novel therapeutic approaches could be aimed at correcting the loss of triadin in diseased hearts, and thereby correcting the sub-cellular EC coupling defect. This review summarizes current concepts of the impact of triadin on cardiac EC coupling with a focus towards triadin's role for ventricular arrhythmia.
Keywords: Cardiac calcium handling, Ventricular arrhythmia, Cardiac couplon ultrastructure, Triadin
1. Introduction
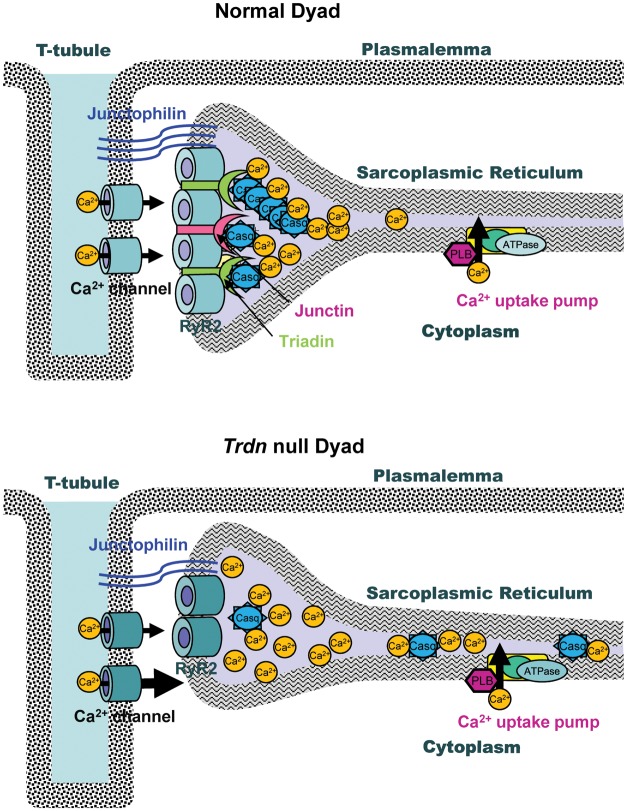
In the heart, triadin and junctin are two specialized proteins that link the Ca2+-buffering protein calsequestrin (Casq2) to the sarcoplasmic reticulum (SR) ryanodine receptor Ca2+-release channels (RyR2) in the junctional SR (jSR).1 In the jSR, RyR2s are in turn juxtaposed to L-type Ca2+ channels located on the sarcolemma-forming couplons which are the Ca2+-release units (CRUs) pivotal to cardiac excitation–contraction (EC) coupling (Figure 1).
Figure 1.
Cartoon illustrating the structure and protein composition of the dyads in normal and Trdn null cardiomyocytes.
Triadin was discovered in the rabbit skeletal muscle more than 20 years ago.2 Subsequently, its presence was also demonstrated in the heart3 where it exists in three isoforms.1 The predominant cardiac isoform is the 35-kDa protein triadin-1.4 Additionally, two other isoforms with larger molecular weight are detected in minor quantities4,5 which may be due to non-specific binding of the antibody since they are also detected in murine hearts with gene-targeted ablation of Trdn.6 This review summarizes the role of triadin for maintaining couplon ultrastructure and healthy EC coupling and triadin's impact on microdomain Ca2+ signalling in the jSR with a focus towards ventricular arrhythmia. For the purpose of this review, the cardiac isoform of triadin CT-1 is simply referred to as triadin.
2. Co-expression of jSR proteins is highly regulated and well co-ordinated
The human triadin gene TRDN is fragmented and located on chromosome 6.7 It covers 420 kb and comprises at least 41 exons, with an average of 58 bp per exon.7 The isoforms of triadin are derived from a single gene by alternative splicing and are tissue specific.7 It is now known that splicing factor SRp38 directly regulates alternative splicing of triadin and that its loss leads to changes in triadin pre-mRNA splicing, and altered intracellular Ca2+ handling.8 Interestingly, SRp38 ‘knock-out’ also down-regulates Casq2 mRNA and protein expression but does not affect other SR proteins indicating tightly co-ordinated co-expression of SR Ca2+-handling proteins during cardiac development.8 Furthermore, there is growing evidence that additional regulator proteins such as the tail-anchored membrane proteins from the myocardium known as the sarcolemmal membrane-associated proteins9 or the transcription factor early growth response-110 are involved in orchestrating SR Ca2+-handling proteins co-expression and hence Ca2+ dynamics.
The interdependence of expression of jSR Ca2+-handling proteins is evident from mouse models with gene-targeted ablation of either Casq2 or Trdn. In both models, knockout of Casq2 or triadin causes a near-complete loss of the associated proteins Casq2, junctin, and triadin, suggesting that in vivo the three proteins form a protein complex.6,11 Notably, junctin requires Casq2 and triadin for its expression but its gene-targeted ablation does not down-regulate the expression of the latter two proteins, suggesting that junctin's role for stability of the jSR protein complex is redundant.12 However, it is now known that despite sharing a close homology with triadin, junctin serves a different function than triadin. Junctin directly regulates SR Ca2+ release without affecting the expression of related jSR Ca2+-handling proteins or the architecture of the couplon.13 Junctin seems to have a dual effect on the response of RyR2s to luminal Ca2+.13 At low luminal Ca2+ (<1 mmol/L), junctin's loss attenuates SR Ca2+ release; conversely, at high luminal Ca2+ (i.e. with β-adrenergic stimulation) junctin's absence sensitizes the RyR2s and increases SR Ca2+ leak.13 However, this dual effect may be related to regulation of RyR2 by Casq2 and triadin in the absence of junctin which cannot be entirely ruled out.14
3. Why study triadin in cardiac muscle?
Although the structure of triadin has extensively been studied,1,15 triadin's function in the heart still remains to be fully elucidated.12 In humans, both missense and nonsense mutations in TRDN have recently been linked to a genetic form of ventricular arrhythmia and sudden cardiac death—catecholaminergic polymorphic ventricular tachycardia (CPVT, discussed in detail below).16 Overexpression of junctin in murine hearts was shown to increase susceptibility to atrial fibrillation.17 Down-regulation of triadin protein has been demonstrated in human heart failure, the significance of which is largely unknown.18 Furthermore, there are preliminary in vitro data showing impaired contractile recovery and increased injury following myocardial ischaemia in the absence of the jSR proteins, junctin, or triadin.19 The aforementioned discoveries highlight the need for a better understanding of the role of triadin in healthy and diseased cardiac muscle.
4. Triadin sensitizes RyR2 in lipid bilayer and overexpression studies
The earliest functional studies showed that triadin protein sensitizes RyR2 channels and enhances SR Ca2+ release when applied to purified RyR2 channels incorporated in lipid bilayers.20 However, when applied together with Casq2 and junctin, it may regulate RyR2 open probability in response to increasing luminal Ca2+. This finding has prompted investigators to postulate that triadin together with Casq2 and junctin functions as a hetero-trimeric ‘luminal Ca2+ sensor’,20 a notion that has remained controversial.12,21 Triadin also sensitizes the RyR2 when overexpressed using adenovirus-mediated gene transfer in isolated rat myocytes.14 Interestingly, gene-targeted α-myosin heavy-chain promoter-driven overexpression of triadin in murine hearts causes depressed contractility, elevated diastolic Ca2+ with slower Ca2+ transient decay without affecting SR Ca2+ release.22 These conflicting data suggest the role of triadin in EC coupling to be multifaceted, and therefore the function of native triadin in EC coupling in intact myocytes still remains largely unknown.
5. Triadin maintains the structural and hence functional integrity of the couplon and is required for normal EC coupling
To address the physiological role of triadin in cardiac muscle, our group used a gene-targeted ablation approach and studied the phenotype of Trdn null mice.6 Interestingly, as one would not have predicted from in vitro or overexpression studies, triadin appears to be required for maintaining the structural and hence functional integrity of the couplon, both being tightly interdependent to each other (Figure 1).
Ablation of Trdn is not lethal and the mice survive to old age with survival rates similar to that of wild-type littermates.6 In Trdn null hearts, there is reduced expression of the jSR proteins RyR2 (50%), Casq2 (∼60%), junctin (∼92%), and junctophilin 1 and 2 (∼30–40%) without any significant change in the non-jSR proteins: SERCA2, phospholamban (Figure 1).6 Expression of L-type Ca2+ channel protein was unchanged.6 Since this change occurred at the level of the jSR proteins but not the mRNA, these data suggest that triadin somehow regulates either stability, targeting, or retention of binding partner proteins in the jSR. This is also supported by the finding that trdn null myocytes retain only a small amount of Casq2 in jSR cisternae with a significant fraction of the protein escaping and localizing in the free SR (Figure 1). Hence, a major role of triadin in cardiac muscle is that of a critical anchoring protein that links Casq2 to RyR2 and thereby retains Casq2 in the jSR.6
The deletion of triadin also causes a significant remodelling of the structure of the couplon such that there is overall 50% reduction in the close association between jSR and T-tubules, without any change in SR volume (Figure 1).6 This is evident from the electron microscopy of the dyads and co-localization studies using immunolabelling with anti-RyR2 antibody and anti-L-type Ca2+ channel antibody in isolated myocytes lacking triadin. An important consequence of the couplon remodelling is that ∼50% of L-type Ca2+ channels are not juxtaposed to RyR2 SR Ca2+ release channels. The alteration of couplon architecture is not unique to Trdn deletion since Casq2 deletion increases SR volume by ∼50%11 suggesting their pivotal role in maintaining the architecture of the couplon (unlike junctin13) besides being instrumental in executing SR Ca2+ release. Interestingly, α-myosin heavy-chain promoter-induced expression of missense Casq2 mutation D307H in the Casq2 null background partially restores the ultrastructural changes reported with gene-targeted ablation of Casq2,11 reiterating that the presence of Casq2 (even if mutated) is critical for maintaining the normal jSR architecture.23 It is unclear how either triadin or Casq2, both of which are entirely confined to the membrane and/or lumen of jSR, would be able to stabilize the formation of T-tubule jSR interface. A possible explanation comes from the observation that junctophilin 1 and 2, proteins that have two membrane-spanning domains and likely serve as anchors for the T-tubule jSR couplon,24 are both down-regulated in the triadin null hearts (Figure 1). Thus, it is intriguing to speculate that triadin may be required for either targeting or stabilizing junctophilin in the jSR.
The paucity of RyR2 and L-type Ca2+ channel juxtapositions in Trdn null myocytes has important functional consequences. There is an increase in SR Ca2+ load resulting from uninhibited Ca2+ influx via L-type Ca2+ channels due to the loss of negative feedback of SR Ca2+ release on L-type Ca2+ currents (Figure 1).6 On the other hand, Ca2+-induced SR Ca2+ release is significantly reduced despite larger SR loads.6 Loss of junctin probably contributes to the attenuation of SR Ca2+ release.13 However, the mismatch of RyR2s and L-type Ca2+ channels appears to be the major contributor, since blocking the L-type Ca2+ channels with nifedipine prevents excessive SR Ca2+ loading.6
Furthermore, gating of L-type Ca2+ channels is altered in Trdn null myocytes.6 At baseline, ICa inactivation is significantly slower in Trdn null mycocytes compared with wild-type myocytes. With β-adrenergic stimulation there is a greater increase in ICa amplitude in Trdn null myocytes, which is again accompanied by very slow ICa inactivation. Since preventing SR Ca2+-release by blocking RyR2 channels with ryanodine or depleting the SR by incubating with thapsigargin abolishes the differences in Ica amplitude and most of the differences in inactivation, this finding was interpreted as being related to the impaired negative feedback on Ica from SR Ca2+-release resulting from the structural disruption of the couplon and consequent impaired microdomain signalling. Furthermore, activation and inactivation of ICa remain significantly slower in Trdn null myocytes using Ba2+ as a charge carrier, which does not trigger SR Ca2+ release and does not cause Ca2+-dependent inactivation. Hence, in addition to impaired SR Ca2+ release-induced Ica inactivation, there appears to be altered intrinsic ICa gating in Trdn null myocytes, the mechanism of which remains to be determined.6 On the other hand, NaCa exchanger function was not significantly changed in Trdn null myocytes.6
As described in the previous section, Trdn ablation impairs ICa-induced SR Ca2+ release despite the increased SR Ca2+ content by virtue of reduced RyR2s and L-type Ca2+ channels juxtaposition.6 In addition, the severe (<90%) reduction of junctin, which can desensitize RyR2 channels,13 may contribute to the reduction in SR Ca2+ release. Somewhat paradoxically, there is an increase in spontaneous SR Ca2+ releases in Trdn null myocytes upon β-adrenergic stimulation, and catecholamine-induced ventricular arrhythmias in Trdn null mice.6 It is tempting to speculate that the disruption of the couplon architecture due to Trdn deletion primes the heart for ventricular arrhythmias by a number of mechanisms: First, by reduced co-expression and non-retention of Casq2 in the jSR, with reduced Casq2 protein being an established primary cause of Ca2+ released triggered arrhythmias even in structurally normal cardiac muscle.6,25 Secondly, by the drastic reduction in junctin expression.13 Thirdly, by causing increased Ca2+ influx and SR Ca2+ overload due to impaired ICa inactivation, leading to spontaneous Ca2+ release and delayed after-depolarizations with catecholaminergic surge.6,11,25 Fourthly, although hypothetical and not yet evaluated in the triadin model, the impaired ICa inactivation in theory may cause action potential prolongation, which is a well-established mechanism for early after-depolarizations (EADs) and EAD-mediated arrhythmias.26
Interestingly, at the level of the SR, it is likely the loss of Casq225 and junctin13 coupled with SR Ca2+ overload during β-adrenergic stimulation which triggers the CPVT phenotype in the Trdn null mice. This is supported by observation in multiple murine models of CPVT where the common theme is reduction (100%, 60%, or modest 25%) in Casq2 with or without reduction in triadin and/or Junctin.11,25,27 Additionally, the loss of junctin in isolation is also shown to increase SR Ca2+ leak during catecholaminergic surge.13 It is important to note that the direct effect of loss of native triadin on the RyR2 complex and SR Ca2+ release still remains largely unknown and that experiments in Trdn null myocytes have not ruled out the previously held notion that loss of triadin desensitizes the RyR2 complex.6 It is possible that the functional consequences of loss of Casq2 and junctin dominate over the loss of triadin on SR Ca2+ release upon Trdn ablation. Future experiments that measure gating in native RyR2s incorporated in lipid bilayer or Ca2+ waves in permeablized myocytes from Trdn null or heterogygous mice may clarify this further, although reduction of other SR Ca2+-handling proteins notably Casq2 and junctin will make it difficult to gauge the effect of triadin loss in isolation.
6. Loss of function mutations in TRDN as a cause of CPVT in humans
CPVT is a genetic disorder characterized by stress-induced polymorphic VT and sudden death in the absence of structural heart disease.28 It is primarily a paediatric disease affecting juveniles and young adults. In up to 70% of cases, mutations in the RYR2 or CASQ2 are the underlying culprit,28 the mechanism being delayed after-depolarization-induced triggered VT during catecholamine excess.28
Although TRDN has been an attractive candidate gene that could harbour CPVT mutations given that the Trdn null mouse reported in 2009 displayed a CPVT phenotype,6 none had been found until 2012.16 Using a candidate gene approach, mutations in the triadin (TRDN) and in the junctin (ASPH) genes were sought in a cohort of 97 CPVT patients who did not harbour RYR2 or CASQ2 mutations.16 No ASPH mutations were found; however, three mutations in TRDN co-segregated with the disease and displayed a recessive mode of transmission in two families.16
The proband in the first family was a 2-year-old boy who experienced syncope followed by cardiac arrest while playing with his 7-year-old brother and later died due to anoxic brain injury. His ECG after cardiac arrest showed runs of polymorphic and bidirectional VT typical of CPVT which was also displayed by the Trdn null mouse.6,16 Molecular analysis revealed a c.del53_56ACAG homozygous deletion in exon 2 of TRDN which led to the deletion of four nucleotides resulting in a frame-shift of the amino acid sequence from position 18 leading to a premature stop codon at position 31. Putatively, this would lead to the deletion of the major part of the triadin protein making it either non-functional or absent.16 Three unaffected relatives who had a normal phenotype tested heterozygous for this mutation.
The proband in the second family was a 26-year-old male with recurrent episodes of syncope during exercise since infancy. Interestingly, he also displayed proximal muscle weakness suggesting the absence of triadin protein in both the cardiac and skeletal muscle which would be predicted since the same gene (TRDN) encodes for the skeletal and cardiac isoforms by alternative splicing.7 Nonetheless, the phenotype in humans consequent to a non-functional TRDN mutation appears to be primarily cardiac, which is consistent with the relatively preserved skeletal muscle contractile function found in trdn null mice.29 CPVT was also diagnosed in his dizygotic twin-brother. Molecular analysis in this family revealed that the two affected patients were compound heterozygous for a c.176C>G missense mutation in exon 2 that led to the substitution of a threonine by an arginyl residue at position 59 (p.T59R) and a c.613C>T nonsense mutation in exon 8 that introduced a premature stop codon at position 205 in the amino acid sequence (p.Q205*). The latter mutation is clearly pathogenic since it would lead to either the absence of protein or the presence of a non-functional protein. Although cardiac biopsy was not performed from the probands in the two families, their mutations would predict complete absence of the triadin protein in the hearts and the skeletal muscle akin to the Trdn null mouse.6,16
The p.T59R missense mutation in the second family proband resulted in the introduction of a positively charged arginine instead of threonine at position 59 in the transmembrane domain of the protein. Since the consequence of this mutation was unknown, it was further studied by expressing the p.T59R mutant in COS-7 cells which resulted in intracellular retention and degradation of the mutant protein.16 This was confirmed with in vivo expression of the mutant triadin in Trdn null mouse by viral transduction.16
No echocardiographic data were reported from the patients harbouring TRDN mutations. But if one were to extrapolate from the Trdn null mouse phenotype, the LV wall thickness and the LV ejection fraction may be increased.6 The latter was a surprising finding, since at the individual myocyte level, loss of triadin causes reduced EC coupling gain and decreased contractility,6 a cellular phenotype that has previously been associated with impaired contractile function of myocytes isolated from experimental models of hypertrophy and heart failure.30 Clearly, more detailed studies, in particular, of the patients harbouring TRDN mutations, are needed to solve this puzzle.
7. Conclusions
In summary, triadin serves a crucial role in maintaining structural integrity of the cardiac CRUs and in doing so ensures functional cardiac EC coupling and tightly controlled SR Ca2+ release. In mice, the loss of function of Trdn causes a phenotype of catecholamine-induced SR Ca2+ overload, premature spontaneous Ca2+ release and Ca2+-triggered polymorphic ventricular tachycardia. In humans, the loss of function mutations in the TRDN gene has been associated with CPVT, which is consistent with the findings from the Trdn null mouse model. However, the significance of the dramatic down-regulation of triadin and junctin in failing hearts is largely unknown and needs further elucidation. Several questions remain and need more investigation, and it is crucial that they are answered for progress to occur in this field. ‘Necessity is the mother of invention’ and understanding the role of SR Ca2+-handling proteins in health and disease is crucial for pioneering new therapeutic options for treating heart failure and ventricular arrhythmias, possibly by targeting the sub-cellular defect in SR Ca2+ release.
Conflict of interest: none declared.
Funding
This work was supported in part by the US National Institutes of Health (grants HL88635 and HL71670 to B.C.K.) and by the American Heart Association Established Investigator Award (0840071N to B.C.K.).
References
- 1.Guo W, Jorgensen AO, Jones LR, Campbell KP. Biochemical characterization and molecular cloning of cardiac triadin. J Biol Chem. 1996;271:458–465. doi: 10.1074/jbc.271.1.458. doi:10.1074/jbc.271.1.458. [DOI] [PubMed] [Google Scholar]
- 2.Caswell AH, Brandt NR, Brunschwig JP, Purkerson S. Localization and partial characterization of the oligomeric disulfide-linked molecular weight 95000 protein (triadin) which binds the ryanodine and dihydropyridine receptors in skeletal muscle triadic vesicles. Biochemistry. 1991;30:7507–7513. doi: 10.1021/bi00244a020. doi:10.1021/bi00244a020. [DOI] [PubMed] [Google Scholar]
- 3.Peng M, Fan H, Kirley TL, Caswell AH, Schwartz A. Structural diversity of triadin in skeletal muscle and evidence of its existence in heart. FEBS Lett. 1994;348:17–20. doi: 10.1016/0014-5793(94)00556-7. doi:10.1016/0014-5793(94)00556-7. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi YM, Jones LR. Identification of triadin 1 as the predominant triadin isoform expressed in mammalian myocardium. J Biol Chem. 1999;274:28660–28668. doi: 10.1074/jbc.274.40.28660. doi:10.1074/jbc.274.40.28660. [DOI] [PubMed] [Google Scholar]
- 5.Hong CS, Ji JH, Kim JP, Jung DH, Kim DH. Molecular cloning and characterization of mouse cardiac triadin isoforms. Gene. 2001;278:193–199. doi: 10.1016/s0378-1119(01)00718-1. doi:10.1016/S0378-1119(01)00718-1. [DOI] [PubMed] [Google Scholar]
- 6.Chopra N, Yang T, Asghari P, Moore ED, Huke S, Akin B, et al. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation–contraction coupling, and cardiac arrhythmias. Proc Natl Acad Sci USA. 2009;106:7636–7641. doi: 10.1073/pnas.0902919106. doi:10.1073/pnas.0902919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thevenon D, Smida-Rezgui S, Chevessier F, Groh S, Henry-Berger J, Beatriz Romero N, et al. Human skeletal muscle triadin: gene organization and cloning of the major isoform, Trisk 51. Biochem Biophys Res Commun. 2003;303:669–675. doi: 10.1016/s0006-291x(03)00406-6. doi:10.1016/S0006-291X(03)00406-6. [DOI] [PubMed] [Google Scholar]
- 8.Feng Y, Valley MT, Lazar J, Yang AL, Bronson RT, Firestein S, et al. SRp38 regulates alternative splicing and is required for Ca(2+) handling in the embryonic heart. Dev Cell. 2009;16:528–538. doi: 10.1016/j.devcel.2009.02.009. doi:10.1016/j.devcel.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nader M, Westendorp B, Hawari O, Salih M, Stewart AF, Leenen FH, et al. Tail-anchored membrane protein SLMAP is a novel regulator of cardiac function at the sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol. 2012;302:H1138–1145. doi: 10.1152/ajpheart.00872.2011. doi:10.1152/ajpheart.00872.2011. [DOI] [PubMed] [Google Scholar]
- 10.Kasneci A, Kemeny-Suss NM, Komarova SV, Chalifour LE. Egr-1 negatively regulates calsequestrin expression and calcium dynamics in ventricular cells. Cardiovasc Res. 2009;81:695–702. doi: 10.1093/cvr/cvn357. doi:10.1093/cvr/cvn357. [DOI] [PubMed] [Google Scholar]
- 11.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knollmann BC. New roles of calsequestrin and triadin in cardiac muscle. J Physiol. 2009;587:3081–3087. doi: 10.1113/jphysiol.2009.172098. doi:10.1113/jphysiol.2009.172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altschafl BA, Arvanitis DA, Fuentes O, Yuan Q, Kranias EG, Valdivia HH. Dual role of junctin in the regulation of ryanodine receptors and calcium release in cardiac ventricular myocytes. J Physiol. 2011;589:6063–6080. doi: 10.1113/jphysiol.2011.215988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terentyev D, Cala SE, Houle TD, Viatchenko-Karpinski S, Gyorke I, Terentyeva R, et al. Triadin overexpression stimulates excitation–contraction coupling and increases predisposition to cellular arrhythmia in cardiac myocytes. Circ Res. 2005;96:651–658. doi: 10.1161/01.RES.0000160609.98948.25. doi:10.1161/01.RES.0000160609.98948.25. [DOI] [PubMed] [Google Scholar]
- 15.Knudson CM, Stang KK, Moomaw CR, Slaughter CA, Campbell KP. Primary structure and topological analysis of a skeletal muscle-specific junctional sarcoplasmic reticulum glycoprotein (triadin) J Biol Chem. 1993;268:12646–12654. [PubMed] [Google Scholar]
- 16.Roux-Buisson N, Cacheux M, Fourest-Lieuvin A, Fauconnier J, Brocard J, Denjoy I, et al. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Hum Mol Genet. 2012;21:2759–2767. doi: 10.1093/hmg/dds104. doi:10.1093/hmg/dds104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong CS, Cho MC, Kwak YG, Song CH, Lee YH, Lim JS, et al. Cardiac remodeling and atrial fibrillation in transgenic mice overexpressing junctin. FASEB J. 2002;16:1310–1312. doi: 10.1096/fj.01-0908fje. [DOI] [PubMed] [Google Scholar]
- 18.Gergs U, Berndt T, Buskase J, Jones LR, Kirchhefer U, Muller FU, et al. On the role of junctin in cardiac Ca2+ handling, contractility, and heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H728–734. doi: 10.1152/ajpheart.01187.2006. doi:10.1152/ajpheart.01187.2006. [DOI] [PubMed] [Google Scholar]
- 19.Cai WF, Pritchard T, Florea S, Lam CK, Han P, Zhou X, et al. Ablation of junctin or triadin is associated with increased cardiac injury following ischaemia/reperfusion. Cardiovasc Res. 2012;94:333–341. doi: 10.1093/cvr/cvs119. doi:10.1093/cvr/cvs119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. doi:10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. doi:10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirchhefer U, Neumann J, Baba HA, Begrow F, Kobayashi YM, Reinke U, et al. Cardiac hypertrophy and impaired relaxation in transgenic mice overexpressing triadin 1. J Biol Chem. 2001;276:4142–4149. doi: 10.1074/jbc.M006443200. doi:10.1074/jbc.M006443200. [DOI] [PubMed] [Google Scholar]
- 23.Kalyanasundaram A, Bal NC, Franzini-Armstrong C, Knollmann BC, Periasamy M. The calsequestrin mutation CASQ2D307H does not affect protein stability and targeting to the junctional sarcoplasmic reticulum but compromises its dynamic regulation of calcium buffering. J Biol Chem. 2010;285:3076–3083. doi: 10.1074/jbc.M109.053892. doi:10.1074/jbc.M109.053892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 25.Chopra N, Kannankeril PJ, Yang T, Hlaing T, Holinstat I, Ettensohn K, et al. Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ Res. 2007;101:617–626. doi: 10.1161/CIRCRESAHA.107.157552. doi:10.1161/CIRCRESAHA.107.157552. [DOI] [PubMed] [Google Scholar]
- 26.Knollmann BC, Roden DM. A genetic framework for improving arrhythmia therapy. Nature. 2008;451:929–936. doi: 10.1038/nature06799. doi:10.1038/nature06799. [DOI] [PubMed] [Google Scholar]
- 27.Rizzi N, Liu N, Napolitano C, Nori A, Turcato F, Colombi B, et al. Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock in mouse model. Circ Res. 2008;103:298–306. doi: 10.1161/CIRCRESAHA.108.171660. doi:10.1161/CIRCRESAHA.108.171660. [DOI] [PubMed] [Google Scholar]
- 28.Liu N, Ruan Y, Priori SG. Catecholaminergic polymorphic ventricular tachycardia. Prog Cardiovasc Dis. 2008;51:23–30. doi: 10.1016/j.pcad.2007.10.005. doi:10.1016/j.pcad.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Shen X, Franzini-Armstrong C, Lopez JR, Jones LR, Kobayashi YM, Wang Y, et al. Triadins modulate intracellular Ca(2+) homeostasis but are not essential for excitation-contraction coupling in skeletal muscle. J Biol Chem. 2007;282:37864–37874. doi: 10.1074/jbc.M705702200. doi:10.1074/jbc.M705702200. [DOI] [PubMed] [Google Scholar]
- 30.Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, et al. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. doi:10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]