This editorial refers to ‘Emerging mechanisms of T-tubule remodelling in heart failure’ by A. Guo et al., pp. 204–215, this issue and ‘Ultrastructural uncoupling between T-tubules and sarcoplasmic reticulum in human heart failure’ by H.-B. Zhang, et al., pp. 269–276, this issue.
Extensive intracellular contacts of the endo/sarcoplasmic reticulum (ER/SR) with transverse tubules (TTs) are fundamental for intracellular Ca2+ cycling in cardiac muscle. For this, TTs extend from the cytoplasmic surface deep into the cell, setting up a complex three-dimensional (3D) membrane network, referred to as ‘transverse axial tubular system’ (TATS) (Figure 1A). The TATS extends over several orders of magnitude in size, e.g. from local TT diameters <100 nm to cell-wide continuous networks expanding over 100 μm, in order to provide thousands of subcellular TT contacts with the ER organelle via junctional SR (jSR) contacts in a typical ventricular myocyte (VM). Not surprisingly, approaches to study the TT membrane structures and properties of the TATS network encompass different imaging and physiological methods. However, until recently ultrastructural TT resolution vs. continuous representation of TATS regions had to be traded against each other. Just imagine representative EM studies (smallest field of view, possible resolution ≤1 nm) when compared with confocal fluorescence microscopy of histological samples (field of view of ∼150 × 150 µm at ∼250 nm resolution using ×63 objectives). Meanwhile this methodological gap is addressed by recent super-resolution fluorescence microscopy approaches (see below).1 Despite apparent gaps between imaging resolution and the nanometric nature of subcellular membrane compartments, conventional fluorescence microscopy is essential to characterize TATS membranes in living samples. In particular, functional imaging studies of isolated cells and two-photon imaging of intact heart tissue have related typical TATS architectures to subcellular functions like Ca2+ release events (sparks) in resting cells (for diastole) or Ca2+ transients in electrically stimulated cells (for systole).
Figure 1.
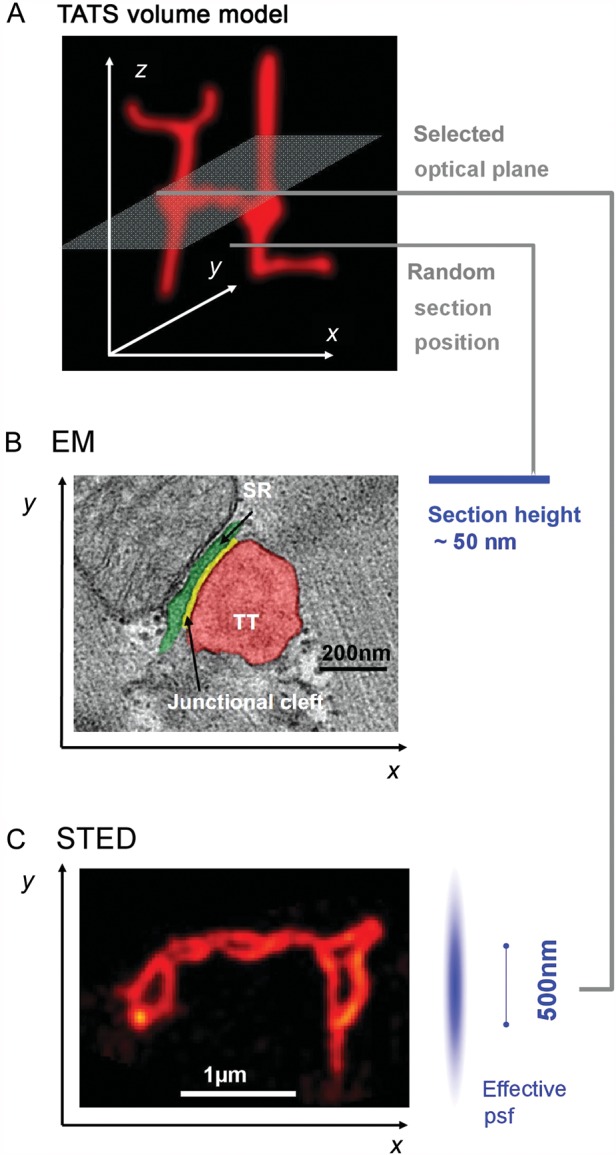
The cardiac cell membrane exerts deep intracellular functions through TT invaginations, forming a continuous three-dimensional TATS network composed of transversal and longitudinal components. (A) Minimal three-dimensional TATS volume model composed of 2 transversal TTs continuously connected with longitudinal TT elements; example imaging/sectioning planes in (B) and C) are indicated. (B) Individual TT–jSR contacts were investigated in control and HF samples in end-stage DCM/ICM by Zhang et al.8 Increased spatial separation between Z-lines and jSR junctions (green) was shown among other observations in human HF samples. While ultrathin sectioning allows for precise EM measurements of TT–jSR membrane architectures, sectioning occurs randomly relative to the complex three-dimensional membrane structures. (C) STED image of deep intracellular TT membrane structures specifically labelled with di-8-ANEPPS showing abnormally enlarged TT cross-sections and TATS network remodelling 4 weeks post-MI in mouse hearts.4 Optical planes can be directed to specific objects (e.g. transverse-axial TATS intersections) in the focal plane based on image contrast. Image analysis based on identified objects can be semi-automated for quantification. The effective psf indicates a lateral resolution of ∼55 nm and a z resolution of ∼500 nm, the latter limiting 3D resolution similar to confocal imaging here; imaging by z stacks allows for targeted three-dimensional sampling. The Field of view can be adapted to the TATS network dimensions or individual TT objects as needed. B and C reproduced with permission.
While fluorescence microscopy studies traditionally focused on the transverse TT elements and subcellular changes at striations, it is important to note that both transversal and longitudinal (axial) TT elements underlie the continuous TATS membrane network, as documented by EM studies and luminal TATS tracing, and more recently by live cell STED microscopy.2–4 Furthermore, both transversal and longitudinal TT elements exhibit the crucial TT–jSR contacts (dyads; here reviewed5) with highly variable shapes and sizes, which ultimately function as intracellular Ca2+ release units (CRUs).6 Accordingly, current myocyte models predict that among thousands of CRUs per typical ventricular myocyte, each may individually represent a junctional membrane compartment with quasi-autonomous elementary Ca2+ release functions (e.g. sparks), synchronized at the cellular level during excitation–contraction (EC) coupling by tight association between L-type Ca2+ channels (CaV1.2; here reviewed7) and cardiac ryanodine receptors (RyR2).2,5
Therefore, from the perspective of individual membrane compartments, EM studies can address important aspects of local TT–jSR architectures. Traditionally individual TT–jSR contact domains were analysed by EM near sarcomeric Z-discs, where they happen to be frequently visualized as membrane cross-sections of a TT contacting a jSR domain through a dyadic cleft of ∼15 nm width (Figure 1B). Within this framework, Zhang et al.8 in this issue compare EM data from human end-stage dilative (DCM) and ischaemic (ICM) heart failure (HF) vs. control samples. With a low number of human tissue samples they show quantitatively that the alignment of TT–jSR junctions with Z-discs is significantly altered (Figure 1B) or junction size is decreased or junctions are entirely lost (∼60% in the given DCM/ICM sample population). This confirms the original hypothesis by Lederer, Cheng, and colleagues9,10 that local mechanisms of EC un-coupling are central to HF, and further extends into the context of common forms of human heart disease. Zhang et al. attribute the TT–jSR membrane un-coupling to down-regulation of junctophilin-2, a cardiac membrane-associated disease mechanism proposed by Wehrens and colleagues,11 and recently confirmed by our group.4 While subcellular changes of the junctional Ca2+ release channels (RyR2 clusters) during cardiac remodelling have been discussed but not directly visualized, previous EM studies have characterized the size of individual RyR2 channel clusters by counting the number of electron dense RyR2 ‘feet’ in cross-sections and extrapolating cluster sizes based on presumed circular geometries of jSR domains.12 Furthermore, 3D EM has been applied to study local TT diameters, membrane branching and to estimate the size of junctions.13 As with all imaging applications, there are relevant limitations inherent to EM for investigations concerning subcellular CRU variability and extrapolation to consequences of altered dyadic coupling in HF (see below).
Until recently and complementary to EM, confocal or two-photon fluorescence microscopy were the only strategies to gain insight about TT membranes through larger field of views, higher sample throughput for representative cellular analysis, and through live cell data acquisition. Guo et al.14 highlight this particular research area with their timely review about mechanisms of TT remodelling in heart failure. Here, the unique cell biology and imaging strategies of leading EC coupling investigators are discussed in-depth and aligned with historical EM data. In particular, confocal and two-photon imaging strategies which may translate between cell and intact tissue are comprehensively discussed.14 As Guo et al. point out, live fluorescence microscopy is essential to study native membranes in the intact cellular environment, and can be combined with patch-clamp or tissue-based strategies. While imaging of individual cells in intact tissue can be beneficial, both perfusion of tissue or isolation of myocytes carries inherent advantages and limitations for fluorescence microscopy, which require more study and side-to-side comparison.
In addition, super-resolution fluorescence microscopy has emerged as a powerful field allowing for ultrastructural insight with up to 10 times higher lateral resolution when compared with confocal microscopy. We have recently reviewed existing super-resolution approaches, including different applications and limitations to cellular membrane and protein questions, particularly for cardiac CRUs and disease changes.1 To date super-resolution microscopy of entire, intact TT structures have been demonstrated exclusively for STED microscopy by our group.4 At the same time, this represents the only super-resolution application for living cardiac myocytes so far. Using a customized imaging setup with a documented lateral resolution of ∼55 nm, we could reproduce and extend individual TT size measurements from EM and directly measure ultrastructural changes of TATS membrane networks 4 weeks post-MI (Figure 1C). In parallel, super-resolution imaging of fixed cardiac myocytes showed caveolin-3 (Cav3) clusters directly adjacent to RyR2 clusters.15 Since Cav3 is an essential membrane protein of caveolae, it is possible that CRUs and caveolae represent functionally associated compartments, yet caveolae may have autonomous transcriptional Ca2+ signalling functions as discussed in this issue.7
To sum up, the discussion of different TT imaging strategies and biological scenarios helps to outline future developments. EM provides molecular resolution and ultrastructural insight, e.g. to identify large protein complexes like the cytosolic domains of RyR2 channel clusters, dyadic subspace properties, or caveolae. Yet, the extremely small subcellular volumes represented by EM images (Figure 1B) and potential artefacts from physicochemical processing of membranes clearly limit stand-alone insight from EM analysis and require balancing of approaches through independent methods. Furthermore, EM sections will frequently capture TT–jSR couplings perpendicular to the jSR membrane plane. But such EM sections may represent either 2D or 3D couplings, that inherently vary in size, shape, and orientation.5 While cautious interpretation is mandatory, some limitations were overcome by 3D EM or, if available, recent combination with super-resolution microscopy. In fact, the same rationale and limitation applies to all imaging strategies with limited spatial resolution in the third dimension, including confocal imaging.
To date confocal and two-photon microscopy are widely accessible techniques through commercially available microscopes and labelling agents. Notably, recent cardiac super-resolution microscopy studies—not surprisingly—used customized setups, dye testing, and data analysis software. In parallel, new protocols with regard to fluorophores and sample preparation are continuously added for super-resolution microscopy, because there is a clear need for more flexible choices, especially for live labelling strategies and alternatives to fluorescent protein tags. Thus, in the context of TTs and other cell membrane studies, specialized imaging facilities and new biotechnical developments for live labelling appear to be promising steps.
We note that different microscopy techniques combined with image processing have led to the TT data discussed in this issue. Among available image processing tools, deconvolution, 3D reconstruction, and power analysis are frequently applied for TT analysis. Power analysis of periodic fluorescence signals as a regional readout of the TATS network has successfully identified disease changes, e.g. a loss of transversal TT elements in a hypertension model of chronic HF.9 While analysis of spatial frequencies can efficiently compare different physiological scenarios and sample groups, it cannot provide any ultrastructural insight, nor identify specific image objects like individual TTs. In contrast, recent super-resolution data allowed for direct, object-based quantitative approaches that identified a significantly increased length and complexity of the TATS network 4 weeks post-MI in living cells.4
Complementary to structural analysis new analytical strategies have been established, which may translate subcellular data at different orders of magnitude into predictions about EC coupling. While physiological and computational models (here reviewed16) of cardiac EC coupling traditionally reflected uniform subcellular architectures, the highly variable and dynamic nature of the TT contacts at jSR junctions is an important emergent area of membrane physiology and disease mechanisms. Ventricular myocytes (VMs) may critically depend on the variability and integrity of thousands of TT-associated CRUs in order to sustain ∼3 000 000 000 contraction–relaxation cycles during a typical human lifespan. However, significant TT changes were documented in HF, particularly in human VMs.4,17,18 Using computational modelling of 20 000 CRUs to test heterogeneous mechanisms of TT remodelling in HF, we have recently shown that spatial un-coupling at only 25% of CRUs may cause significant diastolic Ca2+ leak with preserved SR Ca2+ load leading to action potential prolongation.4 To further increase the physiological realism of such simulations, an important area for future research will be for VM models to incorporate recent methods that consider CRU populations with heterogeneous properties.19
Several decades after their discovery, the cardiac membrane–protein complexes which underlie TT–jSR contacts are again at the centre of intriguing studies and testimony to this special issue of frontier research. A growing number of different fluorescence microscopy techniques and strategies address the apparent complexity of subcellular TT changes. At the same time further examination of cellular TT–jSR domain variability and analysis of different cardiac regions will contribute to a more realistic understanding of heart function. In parallel, novel integrative efforts like mathematical modelling will extend insight into leading contractile and arrhythmogenic pathologies in HF. Profound imaging, molecular biology, disease models, and computational strategies framed by interdisciplinary exchange including cardiologists and electrophysiologists are likely to thrust this exciting membrane field forward. While there is much to be learned about cardiac cell biology and disease mechanisms, the time has never been better to test therapeutic opportunities and translate these back to the patient.
Acknowledgements
We would like to thank Eric A. Sobie and Xander H.T. Wehrens for critical comments
Conflict of interest: none declared.
Funding
This work was supported by Deutsche Forschungsgemeinschaft through KFO 155 (LE 1313/2-2) and SFB 1002 (subprojects A05 and B05). A DAAD exchange program supported T.K. at the University of Maryland. The research leading to these results has received funding from the European Community's Seventh Framework Program FP7/2007–2013 under grant agreement n°. HEALTH-F2-2009-241526, EUTrigTreat (to S.E.L.).
References
- 1.Kohl T, Westphal V, Hell SW, Lehnart SE. Super-resolution microscopy in heart—cardiac nanoscopy. J Mol Cell Cardiol. 2013 doi: 10.1016/j.yjmcc.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 2.Forssmann WG, Girardier L. A study of the T system in rat heart. J Cell Biol. 1970;44:1–19. doi: 10.1083/jcb.44.1.1. doi:10.1083/jcb.44.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fawcett DW, McNutt NS. The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. J Cell Biol. 1969;42:1–45. doi: 10.1083/jcb.42.1.1. doi:10.1083/jcb.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner E, Lauterbach MA, Kohl T, Westphal V, Williams GS, Steinbrecher JH, et al. Stimulated emission depletion live-cell super-resolution imaging shows proliferative remodeling of T-tubule membrane structures after myocardial infarction. Circ Res. 2012;111:402–414. doi: 10.1161/CIRCRESAHA.112.274530. doi:10.1161/CIRCRESAHA.112.274530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scriven DRL, Asghari P, Moore EDW. Microarchitecture of the dyad. Cardiovasc Res. 2013;98:169–176. doi: 10.1093/cvr/cvt025. [DOI] [PubMed] [Google Scholar]
- 6.Asghari P, Schulson M, Scriven DR, Martens G, Moore ED. Axial tubules of rat ventricular myocytes form multiple junctions with the sarcoplasmic reticulum. Biophys J. 2009;96:4651–4660. doi: 10.1016/j.bpj.2009.02.058. doi:10.1016/j.bpj.2009.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw RM, Colecraft HM. L-type calcium channel targeting and local signalling in cardiac myocytes. Cardiovasc Res. 2013;98:177–186. doi: 10.1093/cvr/cvt021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H-B, Li R-C, Xu M, Xu S-M, Lai Y-S, Wu H-D, et al. Ultrastructural uncoupling between T-tubules and sarcoplasmic reticulum in human heart failure. Cardiovasc Res. 2013;98:269–276. doi: 10.1093/cvr/cvt030. [DOI] [PubMed] [Google Scholar]
- 9.Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci USA. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. doi:10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, et al. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. doi:10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 11.van Oort RJ, Garbino A, Wang W, Dixit SS, Landstrom AP, Gaur N, et al. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation. 2011;123:979–988. doi: 10.1161/CIRCULATIONAHA.110.006437. doi:10.1161/CIRCULATIONAHA.110.006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franzini-Armstrong C, Protasi F, Ramesh V. Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophys J. 1999;77:1528–1539. doi: 10.1016/S0006-3495(99)77000-1. doi:10.1016/S0006-3495(99)77000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi T, Martone ME, Yu Z, Thor A, Doi M, Holst MJ, et al. Three-dimensional electron microscopy reveals new details of membrane systems for Ca2+ signaling in the heart. J Cell Sci. 2009;122:1005–1013. doi: 10.1242/jcs.028175. doi:10.1242/jcs.028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo A, Zhang C, Wei S, Chen B, Song L-S. Emerging mechanisms of T-tubule re-modelling in heart failure. Cardiovasc Res. 2013;98:204–215. doi: 10.1093/cvr/cvt020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baddeley D, Crossman D, Rossberger S, Cheyne JE, Montgomery JM, Jayasinghe ID, et al. 4D super-resolution microscopy with conventional fluorophores and single wavelength excitation in optically thick cells and tissues. PLoS One. 2011;6:e20645. doi: 10.1371/journal.pone.0020645. doi:10.1371/journal.pone.0020645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poláková E, Sobie EA. Alterations in T-tubule and dyad structure in heart disease: challenges and opportunities for computational analyses. Cardiovasc Res. 2013;98:233–239. doi: 10.1093/cvr/cvt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinzel FR, Bito V, Biesmans L, Wu M, Detre E, von Wegner F, et al. Remodeling of T-tubules and reduced synchrony of Ca2+ release in myocytes from chronically ischemic myocardium. Circ Res. 2008;102:338–346. doi: 10.1161/CIRCRESAHA.107.160085. doi:10.1161/CIRCRESAHA.107.160085. [DOI] [PubMed] [Google Scholar]
- 18.Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, et al. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci USA. 2009;106:6854–6859. doi: 10.1073/pnas.0809777106. doi:10.1073/pnas.0809777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YS, Liu OZ, Hwang HS, Knollmann BC, Sobie EA. Parameter sensitivity analysis of stochastic models provides insights into cardiac calcium sparks. Biophys J. 2013;104:1142–1150. doi: 10.1016/j.bpj.2012.12.055. doi:10.1016/j.bpj.2012.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]


