Abstract
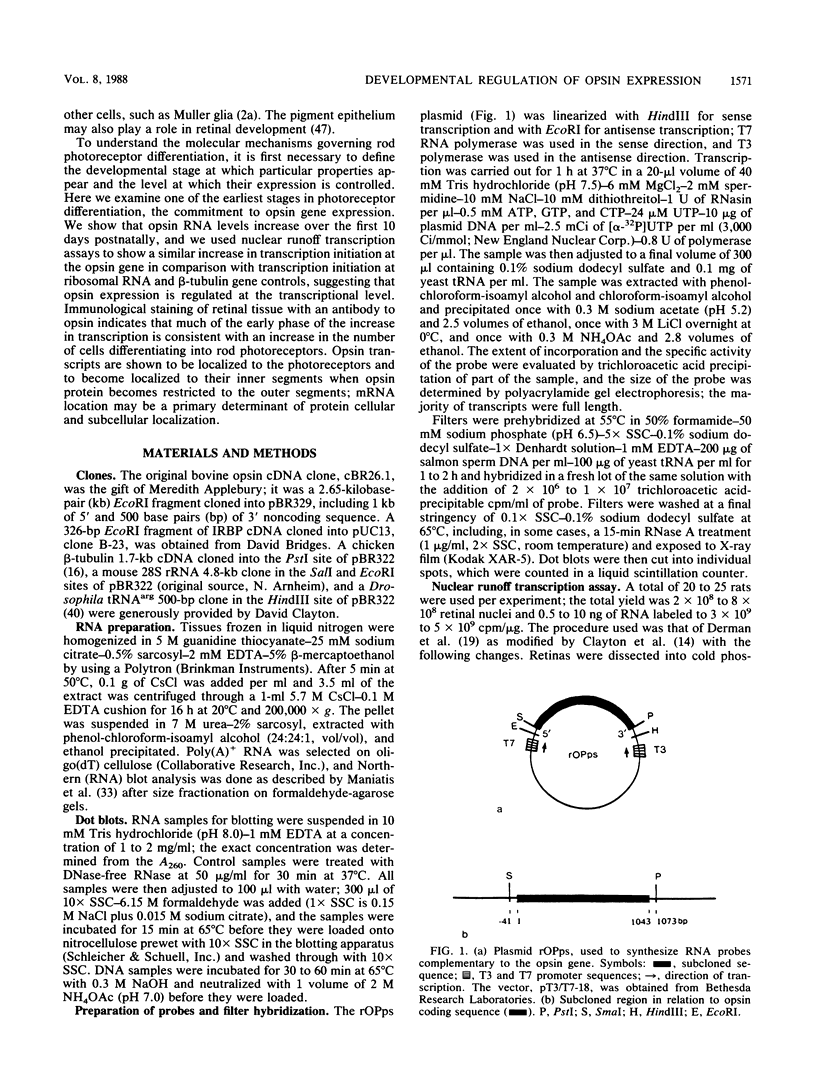
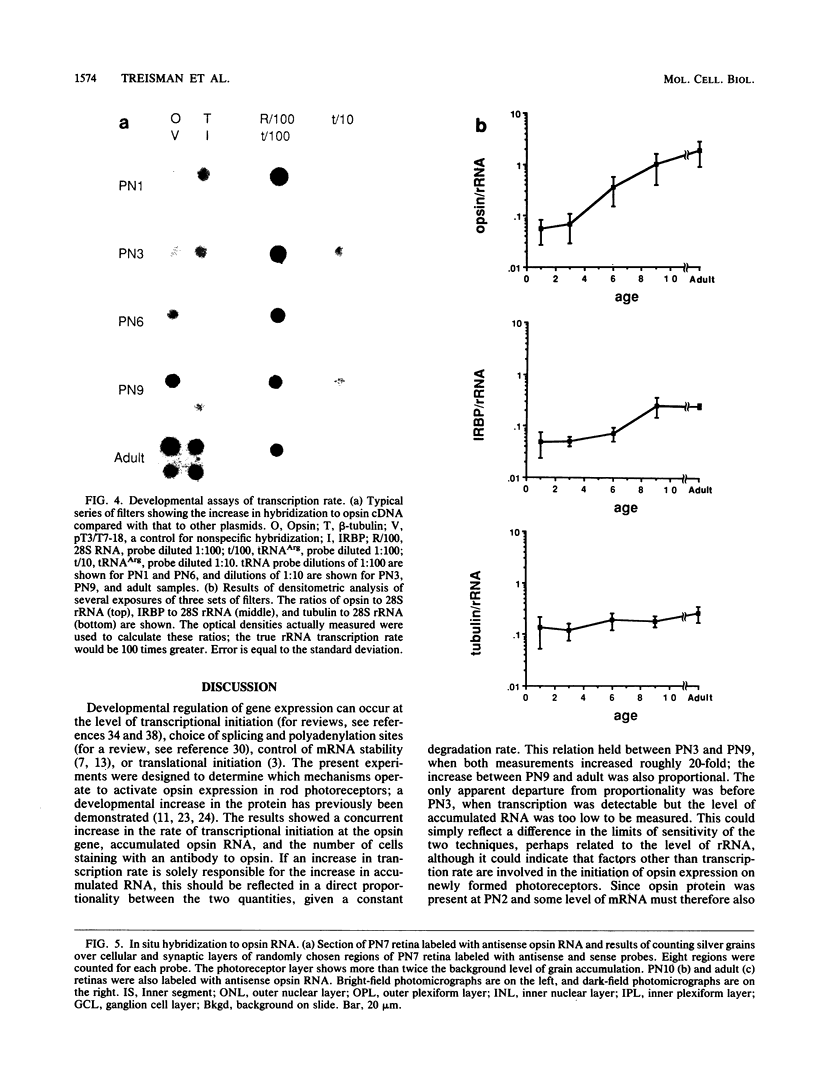
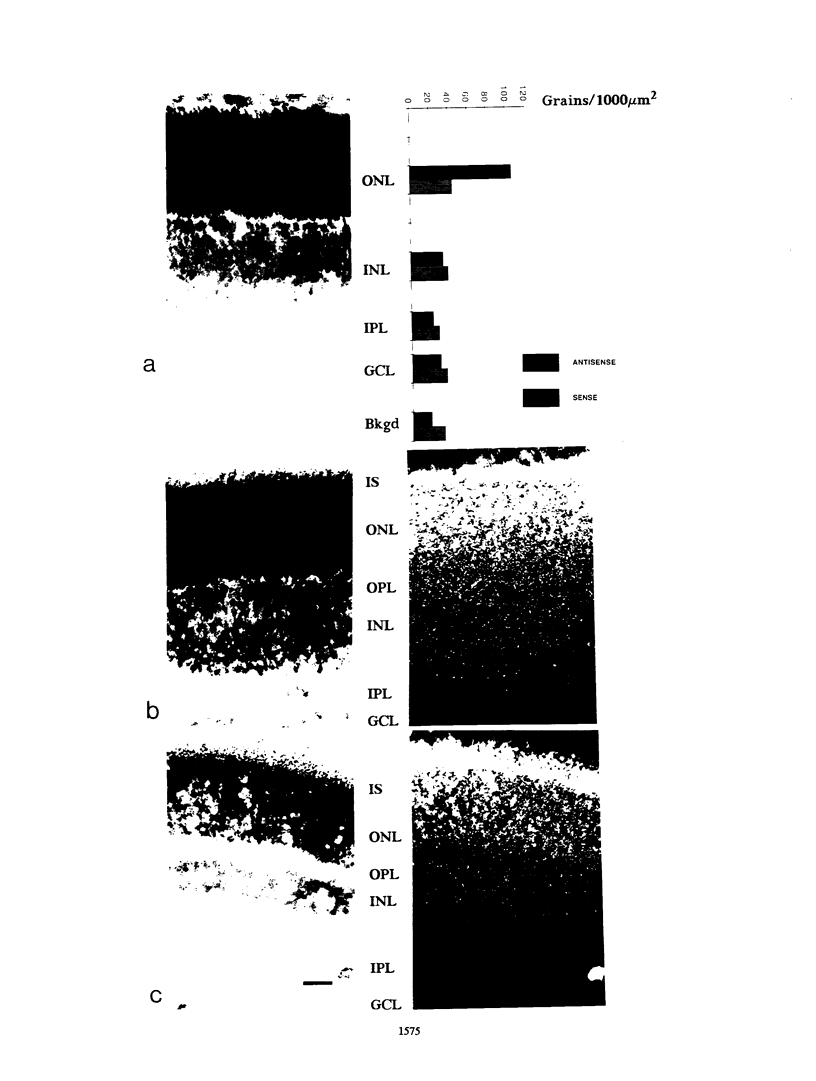
The gene for rhodopsin, the primary light sensor of the visual system, is specifically expressed in the rod photoreceptor cells of the retina. We show here that in the rat, opsin RNA first accumulates to detectable levels at postnatal day 2 (PN2) and that nascent transcripts can be detected at PN1; this is the time when peak numbers of photoreceptor cells are generated by the final division of their neuroepithelial precursors. Accumulated opsin RNA then increases to reach the adult level, 0.06% of total retinal RNA, at about PN10. The transcription rate of the opsin gene increases to a similar extent over the same time course between PN3 and adulthood, suggesting that transcriptional activation is responsible for the increase in opsin expression. We used the antibody RET-P1 to show that rhodopsin protein is also detectable at PN2 and that the number of cells expressing the protein increases with time in a central-to-peripheral gradient in the retina. This increase in the number of differentiating photoreceptors in the tissue appears to account for much of the increase in opsin gene transcription and RNA accumulation. In situ hybridization to opsin RNA shows that it is restricted to the photoreceptor layer from the time it can first be detected, at PN7. Later in development, when RET-P1 staining shifts to the photoreceptor outer segments, opsin RNA becomes localized to the inner segments, suggesting that the distributions of opsin protein and RNA are related.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R. Developmental predetermination of the structural and molecular polarization of photoreceptor cells. Dev Biol. 1986 Oct;117(2):520–527. doi: 10.1016/0012-1606(86)90319-2. [DOI] [PubMed] [Google Scholar]
- Akagawa K., Barnstable C. J. Identification and characterization of cell types in monolayer cultures of rat retina using monoclonal antibodies. Brain Res. 1986 Sep 24;383(1-2):110–120. doi: 10.1016/0006-8993(86)90012-0. [DOI] [PubMed] [Google Scholar]
- Akagawa K., Hicks D., Barnstable C. J. Histiotypic organization and cell differentiation in rat retinal reaggregate cultures. Brain Res. 1987 Dec 29;437(2):298–308. doi: 10.1016/0006-8993(87)91644-1. [DOI] [PubMed] [Google Scholar]
- Ballantine J. E., Woodland H. R., Sturgess E. A. Changes in protein synthesis during the development of Xenopus laevis. J Embryol Exp Morphol. 1979 Jun;51:137–153. [PubMed] [Google Scholar]
- Barnstable C. J. A molecular view of vertebrate retinal development. Mol Neurobiol. 1987 Spring-Summer;1(1-2):9–46. doi: 10.1007/BF02935263. [DOI] [PubMed] [Google Scholar]
- Barrett D. J., Redmond T. M., Wiggert B., Oprian D. D., Chader G. J., Nickerson J. M. cDNA clones encoding bovine interphotoreceptor retinoid binding protein. Biochem Biophys Res Commun. 1985 Sep 30;131(3):1086–1093. doi: 10.1016/0006-291x(85)90202-5. [DOI] [PubMed] [Google Scholar]
- Bastos R. N., Volloch Z., Aviv H. Messenger RNA population analysis during erythroid differentiation: a kinetical approach. J Mol Biol. 1977 Feb 25;110(2):191–203. doi: 10.1016/s0022-2836(77)80068-5. [DOI] [PubMed] [Google Scholar]
- Brann M. R., Cohen L. V. Diurnal expression of transducin mRNA and translocation of transducin in rods of rat retina. Science. 1987 Jan 30;235(4788):585–587. doi: 10.1126/science.3101175. [DOI] [PubMed] [Google Scholar]
- Brann M. R., Young W. S., 3rd Localization and quantitation of opsin and transducin mRNAs in bovine retina by in situ hybridization histochemistry. FEBS Lett. 1986 May 12;200(2):275–278. doi: 10.1016/0014-5793(86)81151-6. [DOI] [PubMed] [Google Scholar]
- Caron J. M., Jones A. L., Rall L. B., Kirschner M. W. Autoregulation of tubulin synthesis in enucleated cells. Nature. 1985 Oct 17;317(6038):648–651. doi: 10.1038/317648a0. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson L. D., LaVail M. M. Rods and cones in the mouse retina. II. Autoradiographic analysis of cell generation using tritiated thymidine. J Comp Neurol. 1979 Nov 15;188(2):263–272. doi: 10.1002/cne.901880205. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson L., Alvarez R. A., Fong S. L., Liou G. I., Sperling H. G., Bridges C. D. Rhodopsin, 11-cis vitamin A, and interstitial retinol-binding protein (IRBP) during retinal development in normal and rd mutant mice. Dev Biol. 1986 Aug;116(2):431–438. doi: 10.1016/0012-1606(86)90144-2. [DOI] [PubMed] [Google Scholar]
- Chung S., Landfear S. M., Blumberg D. D., Cohen N. S., Lodish H. F. Synthesis and stability of developmentally regulated dictyostelium mRNAs are affected by cell--cell contact and cAMP. Cell. 1981 Jun;24(3):785–797. doi: 10.1016/0092-8674(81)90104-5. [DOI] [PubMed] [Google Scholar]
- Clayton D. F., Harrelson A. L., Darnell J. E., Jr Dependence of liver-specific transcription on tissue organization. Mol Cell Biol. 1985 Oct;5(10):2623–2632. doi: 10.1128/mcb.5.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Havercroft J. C. Is apparent autoregulatory control of tubulin synthesis nontranscriptionally regulated? J Cell Biol. 1983 Sep;97(3):919–924. doi: 10.1083/jcb.97.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Crowder C. M., Merlie J. P. DNase I-hypersensitive sites surround the mouse acetylcholine receptor delta-subunit gene. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8405–8409. doi: 10.1073/pnas.83.21.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Fekete D. M., Barnstable C. J. The subcellular localization of rat photoreceptor-specific antigens. J Neurocytol. 1983 Oct;12(5):785–803. doi: 10.1007/BF01258151. [DOI] [PubMed] [Google Scholar]
- Fong H. K., Hurley J. B., Hopkins R. S., Miake-Lye R., Johnson M. S., Doolittle R. F., Simon M. I. Repetitive segmental structure of the transducin beta subunit: homology with the CDC4 gene and identification of related mRNAs. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2162–2166. doi: 10.1073/pnas.83.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks D., Barnstable C. J. Different rhodopsin monoclonal antibodies reveal different binding patterns on developing and adult rat retina. J Histochem Cytochem. 1987 Nov;35(11):1317–1328. doi: 10.1177/35.11.3655327. [DOI] [PubMed] [Google Scholar]
- Hicks D., Barnstable C. J. Lectin and antibody labelling of developing rat photoreceptor cells: an electron microscope immunocytochemical study. J Neurocytol. 1986 Apr;15(2):219–230. doi: 10.1007/BF01611658. [DOI] [PubMed] [Google Scholar]
- Hicks D., Molday R. S. Differential immunogold-dextran labeling of bovine and frog rod and cone cells using monoclonal antibodies against bovine rhodopsin. Exp Eye Res. 1986 Jan;42(1):55–71. doi: 10.1016/0014-4835(86)90017-5. [DOI] [PubMed] [Google Scholar]
- Hollyfield J. G., Fliesler S. J., Rayborn M. E., Fong S. L., Landers R. A., Bridges C. D. Synthesis and secretion of interstitial retinol-binding protein by the human retina. Invest Ophthalmol Vis Sci. 1985 Jan;26(1):58–67. [PubMed] [Google Scholar]
- Kaneko A. Physiology of the retina. Annu Rev Neurosci. 1979;2:169–191. doi: 10.1146/annurev.ne.02.030179.001125. [DOI] [PubMed] [Google Scholar]
- LaVail M. M. Kinetics of rod outer segment renewal in the developing mouse retina. J Cell Biol. 1973 Sep;58(3):650–661. doi: 10.1083/jcb.58.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Intracellular localization of messenger RNAs for cytoskeletal proteins. Cell. 1986 May 9;45(3):407–415. doi: 10.1016/0092-8674(86)90326-0. [DOI] [PubMed] [Google Scholar]
- Leff S. E., Rosenfeld M. G., Evans R. M. Complex transcriptional units: diversity in gene expression by alternative RNA processing. Annu Rev Biochem. 1986;55:1091–1117. doi: 10.1146/annurev.bi.55.070186.005303. [DOI] [PubMed] [Google Scholar]
- Liou G. I., Bridges C. D., Fong S. L., Alvarez R. A., Gonzalez-Fernandez F. Vitamin A transport between retina and pigment epithelium--an interstitial protein carrying endogenous retinol (interstitial retinol-binding protein). Vision Res. 1982;22(12):1457–1467. doi: 10.1016/0042-6989(82)90210-3. [DOI] [PubMed] [Google Scholar]
- Lochrie M. A., Hurley J. B., Simon M. I. Sequence of the alpha subunit of photoreceptor G protein: homologies between transducin, ras, and elongation factors. Science. 1985 Apr 5;228(4695):96–99. doi: 10.1126/science.3856323. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983 Oct;34(3):807–814. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Pittenger M. F., Cleveland D. W. Retention of autoregulatory control of tubulin synthesis in cytoplasts: demonstration of a cytoplasmic mechanism that regulates the level of tubulin expression. J Cell Biol. 1985 Nov;101(5 Pt 1):1941–1952. doi: 10.1083/jcb.101.5.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman S., Schmidt O., Söll D., Hovemann B. The nucleotide sequence of a cloned Drosophila arginine tRNA gene and its in vitro transcription in Xenopus germinal vesicle extracts. J Biol Chem. 1979 Oct 25;254(20):10290–10294. [PubMed] [Google Scholar]
- Sorge L. K., Levy B. T., Maness P. F. pp60c-src is developmentally regulated in the neural retina. Cell. 1984 Feb;36(2):249–257. doi: 10.1016/0092-8674(84)90218-6. [DOI] [PubMed] [Google Scholar]
- Stryer L. Transducin and the cyclic GMP phosphodiesterase: amplifier proteins in vision. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):841–852. doi: 10.1101/sqb.1983.048.01.087. [DOI] [PubMed] [Google Scholar]
- Turner D. L., Cepko C. L. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987 Jul 9;328(6126):131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Fox L. E., Moscona A. A. Accumulation of c-src mRNA is developmentally regulated in embryonic neural retina. Mol Cell Biol. 1986 Nov;6(11):4109–4111. doi: 10.1128/mcb.6.11.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardimon L., Fox L. E., Moscona A. A. Developmental regulation of glutamine synthetase and carbonic anhydrase II in neural retina. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9060–9064. doi: 10.1073/pnas.83.23.9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer G., Layer P. G. An in vitro model of proliferation and differentiation of the chick retina: coaggregates of retinal and pigment epithelial cells. J Neurosci. 1986 Jul;6(7):1885–1896. doi: 10.1523/JNEUROSCI.06-07-01885.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidman T. A., Kuwabara T. Postnatal development of the rat retina. An electron microscopic study. Arch Ophthalmol. 1968 Apr;79(4):470–484. doi: 10.1001/archopht.1968.03850040472015. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985 Oct;42(3):705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Young R. W. Cell differentiation in the retina of the mouse. Anat Rec. 1985 Jun;212(2):199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- Young R. W. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967 Apr;33(1):61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen T., Katial A., Shinohara T., Barrett D. J., Wiggert B., Chader G. J., Nickerson J. M. Retinal photoreceptor neurons and pinealocytes accumulate mRNA for interphotoreceptor retinoid-binding protein (IRBP). FEBS Lett. 1986 Nov 10;208(1):133–137. doi: 10.1016/0014-5793(86)81547-2. [DOI] [PubMed] [Google Scholar]