Abstract
The β-thalassemias are characterized by a quantitative deficiency of β-globin chains underlaid by a striking heterogeneity of molecular defects. Although most of the molecular lesions involve the structural β gene directly, some down-regulate the gene through distal cis effects, and rare trans-acting mutations have also been identified. Most β-thalassemias are inherited in a Mendelian recessive fashion but there is a subgroup of β-thalassemia alleles that behave as dominant negatives. Unraveling the molecular basis of β-thalassemia has provided a paradigm for understanding of much of human genetics.
Reduced β-globin chain production, which underlies β-thalassemias, is caused by a spectrum of genetic lesions. Almost 300 β-thalassemia alleles have been characterized.
This article outlines the molecular mechanisms underlying the quantitative reduction in β-globin production. Mutations that completely inactivate the β gene resulting in no β-globin production cause β0-thalassemia. Other mutations allow the production of some β globin, and depending on the degree of quantitative reduction in the output of the β chains, are classified as β+- or β++- (“silent”) thalassemia. A quantitative reduction in β globin results in the accumulation of excess α-globin chains that are responsible for the pathophysiology of the disorder (Thein 2005). Thus, the severity of the phenotype is usually related to the degree of imbalance between α- and non-α-globin chain synthesis, and the size of the free α-chain pool. The primary determinant of β-thalassemia severity is the type of β allele (β0, β+, β++), ameliorated by coinheritance of interacting α-thalassemia (which reduces the pool of free α chains) and coinheritance of an innate ability to increase production of γ chains. The additional γ-globin chains will partner the excess α globin to form fetal hemoglobin (α2γ2).
Some structurally abnormal β-chain variants are also quantitatively reduced, with a phenotype of β-thalassemia, in which case they are referred to as “thalassemic hemoglobinopathies” (e.g., HbE [β26 Glu → Lys]) (Weatherall and Clegg 2001). Other β-globin chain variants, although synthesized in normal amounts, are extremely unstable and are not capable of forming stable hemoglobin tetramers, causing a functional deficiency of β globin, and a thalassemia phenotype (Thein 1999). These β alleles are rare and result in moderately severe anemia even when present in a single copy. They are thus inherited as dominant negatives unlike the common forms of β-thalassemias that are inherited as haploinsufficient Mendelian recessives where inheritance of two copies of β-thalassemia alleles are required to produce disease.
Almost all the mutations down-regulating the β-globin gene are physically linked to the gene and behave as alleles of the β-globin locus (i.e., they are cis-acting), but mutations that alter β-globin gene expression and segregate independently of the β-globin cluster (i.e., trans-acting) have also been identified in recent years (Viprakasit et al. 2001; Yu et al. 2002; Cantor 2005).
Almost 300 β-thalassemia alleles have now been characterized (http://globin.cse.psu.edu). Unlike α-thalassemia, in which deletions in the α-globin gene cluster account for most of the mutations, the vast majority of β-thalassemias are caused by mutations involving one (or a limited number of nucleotides) within the β gene or its immediate flanking regions (Giardine et al. 2011). Figure 1 summarizes the mechanisms underlying β-thalassemia.
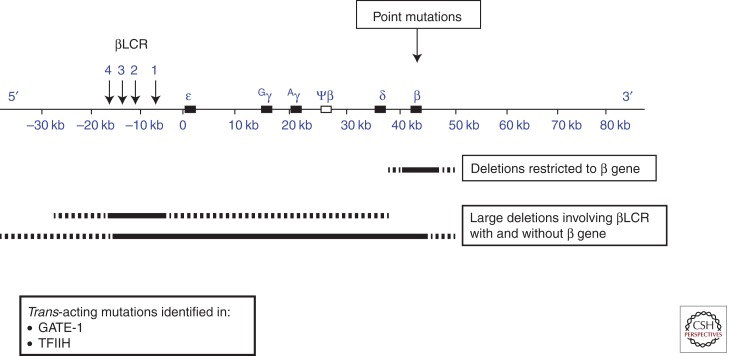
Figure 1.
Mutations causing β-thalassemia. The upper panel depicts the β-globin gene cluster with the upstream locus control region (βLCR). The mutations can be cis-acting and include point mutations affecting the structural β gene, deletions restricted to the β gene, and large deletions involving the βLCR with or without the β gene. The dashed lines represent variations in the amount of flanking DNA removed by the deletions—detailed in Figures 3 and 4, respectively.
NONDELETION FORMS OF β-THALASSEMIA
These defects account for the vast majority of the β-thalassemia alleles (Thein and Wood 2009). They include single base substitutions, small insertions, or deletions within the gene or its immediate flanking sequences and affect almost every known stage of gene expression (Fig. 2). They are listed in Table 1 according to the mechanism by which they affect gene function: transcription, RNA processing or translation of β-globin mRNA.
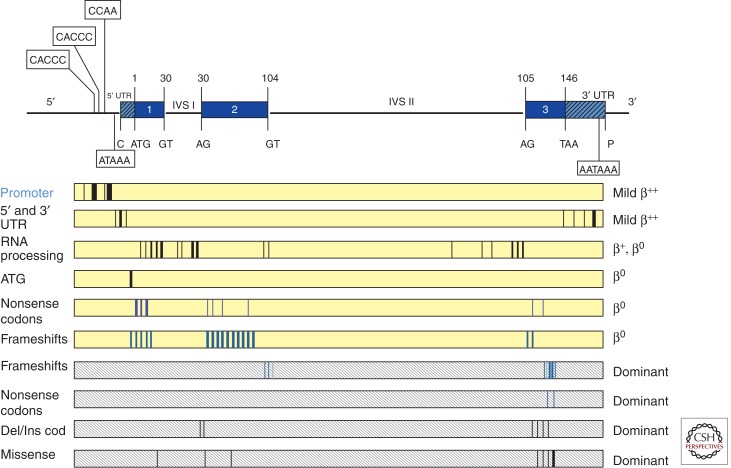
Figure 2.
Point mutations causing β-thalassemia. The β-globin gene is depicted in the upper panel with conserved sequences in the 5′ and 3′ UTRs, and the invariant dinucleotides in exon–intron junctions of the gene, important in the control of gene expression. The boxes represent the different categories of mutants. The vertical lines within the boxes represent the sites of the different mutations. Dominantly inherited mutants are found within shaded boxes.
Table 1.
Point mutations that cause β-thalassemia
| Mutation | Type | Distribution |
|---|---|---|
| I. Transcriptional mutations | ||
| Promoter regulatory elements | ||
| 1) −101 (C → T) | β++ (silent) | Mediterranean |
| 2) −101 (C → G) | β++ (silent) | Ashkenazi Jew |
| 3) −92 (C → T) | β++ (silent) | Mediterranean |
| 4) −90 (C → T) | β+ | Portuguese |
| 5) −88 (C → T) | β++ | U.S. Blacks, Asian Indians |
| 6) −88 (C → A) | β+ | Kurds |
| 7) −87 (C → G) | β++ | Mediterranean |
| 8) −87 (C → T) | β++ | German, Italian |
| 9) −87 (C → A) | β++ | U.S. Blacks |
| 10) −86 (C → G) | β+ | Thai, Lebanese |
| 11) −86 (C → A) | β++ | Italian |
| 12) −73 (A → T) | β++ | Chinese |
| 13) −32 (C → A) | β+ | Taiwanese |
| 14) −32 (C → T) | β+ | Hispanic |
| 15) −31 (A → G) | β+ | Japanese |
| 16) −31 (A → C) | β+ | Italian |
| 17) −30 (T → A) | β+ | Mediterranean, Bulgarian |
| 18) −30 (T → C) | β+ | Chinese |
| 19) −29 (A → G) | β+ | U.S. Blacks, Chinese |
| 20) −29 (A → C) | β+ | Jordanian |
| 21) −29 (G → A) | β+ | Turkish |
| 22) −28 (A → C) | β+ | Kurds |
| 23) −28 (A → G) | β+ | Blacks, SE Asians |
| 24) −27 (A → T) | β+ | Corsican |
| 25) −27 to –26 (−AA) | β+ | African American |
| 26) −25 (G → C) | β+ | African American |
| 5′ UTR | ||
| 27) CAP +1 (A → C) | β++ (silent) | Asian Indian |
| 28) CAP +8 (C → T) | β++ (silent) | Chinese |
| 29) CAP +10 (−T) | β++ (silent) | Greeks |
| 30) CAP +20 (C → T)a | ? | Bulgarian |
| 31) CAP +22 (G → A) | β++ | Mediterranean, Bulgarian |
| 32) CAP +33 (C → G) | β++ (silent) | Greek Cypriot |
| 33) CAP +40 to +43 (−AAAC) | β+ | Chinese |
| 34) CAP +45 (G → C) | β+ | Italian |
| II. RNA processing | ||
| Splice junction | ||
| 1) IVS1-(−2) CD30 (AGG → GGG) | β0 | Sephardic Jews |
| 2) IVS1-(−2) CD30 (AGG → CGG) | β0 | Italian Canadian |
| 3) IVS1-(−1) CD30 (AGG → ACG) (Arg → Thr) | β0 | Mediterranean, U.S. Blacks, N. African, Kurds, UAE |
| 4) IVS1-(−1) CD30 (AGG → AAG) | β0 | Bulgaria, UAE |
| 5) IVS1-1 (G → A) | β0 | Mediterranean |
| 6) IVS1-1 (G → T) | β0 | Asian Indian, SE Asian, Chinese |
| 7) IVS1-1 (G → C) | β0 | Italian Canadian, Japanese |
| 8) IVS1-2 (T → G) | β0 | Tunisian |
| 9) IVS1-2 (T → C) | β0 | U.S. Blacks |
| 10) IVS1-2 (T → A) | β0 | Algerian, Italian |
| 11) IVS2-1 (G → A) | β0 | Mediterranean, U.S. Blacks |
| 12) IVS2-1 (G → C) | β0 | Iranian |
| 13) IVS2-2 (T → A) | ? β0 | Turkish |
| 14) IVS2-2 (−T) | β0 | Chinese |
| 15) IVS1-3′ del 17 bp | β0 | Kuwaiti |
| 16) IVS1-3′ end del 25 bp | β0 | Asian Indian, UAE |
| 17) IVS1-3′ end del 44 bp | β0 | Mediterranean |
| 18) IVS1-3′ end duplication 22 bp | β0 | Thai |
| 19) IVS1-130 (G → C) | β0 | Italian, Japanese, UAE |
| 20) IVS1-130 G → A | β0 | Egyptian |
| 21) IVS1-130 (+1) CD30 (AGG → AGC) (Arg → Ser) | β0 | Middle East |
| 22) IVS2-849 (A → G) | β0 | U.S. Blacks |
| 23) IVS2-849 (A → C) | β0 | U.S. Blacks |
| 24) IVS2-850 (G → C) | β0 | Yugoslavian |
| 25) IVS2-850 (G → A) | β0 | N. European |
| 26) IVS2-850 (G → T) | β0 | Japanese |
| 27) IVS2-850 (−G) | β0 | Italian |
| Consensus splice sites | ||
| 28) IVS1-5 (G → C) | β0 | Asian Indian, SE Asian, Melanesian |
| 29) IVS1-5 (G → T) | β+ | Mediterranean, N. European |
| 30) IVS1-5 (G → A)b | β+ | Mediterranean, Algerian |
| 31) IVS1-6 (T → C) | β++ | Mediterranean |
| 32) IVS1- (−3) CD29 (GGC → GGT) | β+ | Lebanese |
| 33) IVS1-128 (T → G) | β+ | Saudi Arabian |
| 34) IVS1-129 (A → G) | German | |
| 35) IVS2-5 (G → C) | β+ | Chinese |
| 36) IVS2-843 (T → G) | β+ | Algerian |
| 37) IVS2-844 (C → G) | β++ (silent) | Italian |
| 38) IVS2-844 (C → A) | β++ (silent) | Ghanaian |
| 39) IVS2-848 (C → A) | β+ | UB Blacks, Egyptian, Iranian |
| 40) IVS2-848 (C → G) | β+ | Japanese |
| Cryptic splice sites | ||
| 41) IVS1-110 (G → A) | β+ | Mediterranean |
| 42) IVS1-116 (T → G) | β0 | Mediterranean |
| 43) IVS2-654 (C → T) | β0/β+ | Chinese, SE Asians, Japanese |
| 44) IVS2-705 (T → G) | β+ | Mediterranean |
| 45) IVS2-745 (C → G) | β+ | Mediterranean |
| 46) IVS2-837 (T → G) | ? | Asian Indian |
| 47) CD10 (GCC → GCA) | Asian Indian | |
| 48) CD19 (AAC → AGC) Hb Malay (Asn → Ser) | β++ | SE Asian |
| 49) CD24 (GGT → GGA) | β++ | U.S. Black, Japanese |
| 50) CD26 (GAG → AAG) (Glu → Lys, Hb E) |
β+ | SE Asian, European |
| 51) CD26 (GAG → GCG) (Glu → Ala, Hb Tripoli) |
β+ | Libyan |
| 52) CD27 (GCC → TCC) (Ala → Ser, Knossos)c |
β+ | Mediterranean |
| RNA cleavage—Poly A signal | ||
| 53) AATAAA → AACAAA | β++ | U.S. Blacks |
| 54) AATAAA → AATGAA | β++ | Mediterranean |
| 55) AATAAA → AATAGA | β++ | Malay |
| 56) AATAAA → AATAAG | β++ | Kurd |
| 57) AATAAA → AA–AA | β+ | French, U.S. Blacks |
| 58) AATAAA → A —— | β+ | Kurd, UAE |
| 59) AATAAA → AAAAAA | β+ | Tunisian |
| 60) AATAAA → CATAAA | β++ (silent) | Chinese |
| 61) AATAAA → GATAAA | β+ | Czechoslovakian, Mediterranean Yugoslavian, Canadian |
| 62) AATAAA → —— | β+ | Nigerian |
| Others in 3′ UTR | ||
| 63) Term CD +6, C → G | β++ (silent) | Greek |
| 64) Term CD +90, del 13 bp | β++ (silent) | Turkish, Persian (Hamid and Akbari 2011) |
| 65) Term CD +47 (C → G | β++ | Armenian |
| III. RNA translation | ||
| Initiation codon | ||
| 1) ATG → GTG | β0 | Japanese |
| 2) ATG → CTG | β0 | Northern Irish |
| 3) ATG → ACG | β0 | Yugoslavian |
| 4) ATG → AGG | β0 | Chinese |
| 5) ATG → AAG | β0 | N. European |
| 6) ATG → ATC | β0 | Japanese |
| 7) ATG → ATA | β0 | Italian, Swedish |
| 8) ATG → ATT | β0 | Iranian |
| 9) 45 bp insertion (−22 to +23) | ? | Maori, Polynesian |
| Nonsense codons | ||
| 1) CD6 GAG → TAG | β0 | Brazilian |
| 2) CD7 GAG → TAG | β0 | English |
| 3) CD15 TGG → TAG | β0 | Asian Indian, Japanese |
| 4) CD15 TGG → TGA | β0 | Portuguese, Japanese |
| 5) CD17 AAG → TAG | β0 | Chinese, Japanese |
| 6) CD22 GAA → TAA | β0 | Reunion Island |
| 7) CD26 GAG → TAG | β0 | Thai |
| 8) CD35 TAC → TAA | β0 | Thai |
| 9) CD37 TGG → TGA | β0 | Saudi Arabian |
| 10) CD39 CAG → TAG | β0 | Mediterranean |
| 11) CD43 GAG → TAG | β0 | Chinese, Thai |
| 12) CD59 AAG → TAG | β0 | Italian-American |
| 13) CD61 AAG → TAG | β0 | Black |
| 14) CD90 GAG → TAG | β0 | Japanese |
| 15) CD112 TGT → TGA | β0 | Slovenian |
| 16) CD121 GAA → TAA | β0 | Czechoslovakian |
| Frameshift | ||
| 1) CD1 −G | β0 | Mediterranean |
| 2) CD2/3/4 (−9 bp, +31 bp) | β0 | Algerian |
| 3) CD2−4, 5−9, 7, 10 | β0 | Algerian |
| 4) CD5-CT | β0 | Mediterranean |
| 5) CD6 −A | β0 | Mediterranean, U.S. Blacks |
| 6) CD8 −AA | β0 | Mediterranean |
| 7) CD8/9 +G | β0 | Asian Indian, Japanese |
| 8) CD9 +TA | ? β0 | Tunisian |
| 9) CD9/10 +T | β0 | Greek, Arab |
| 10) CD11 −T | β0 | Mexican |
| 11) CD14/15 +G | β0 | Chinese |
| 12) CD15 −T | β0 | Malay |
| 13) CD15/16 −G | β0 | German |
| 14) CD15/16 +G | β0 | Chinese |
| 15) CD16 −C | β0 | Asian Indian |
| 16) CD22/23/24 −7 bp (−AAGTTGG) | β0 | Turkish |
| 17) CD24 −G; +CAC | β0 | Egyptian |
| 18) CD24/25 −GGT | ? | No additional information |
| 19) CD25/26 +T | β0 | Tunisian |
| 20) CD26 +T | β0 | Japanese |
| 21) CD27/28 +C | β0 | Chinese, Thai |
| 22) CD28 −C | β0 | Egyptian |
| 23) CD28/29 −G | β0 | Japanese, Egyptian |
| 24) CD31 −C | β0 | Chinese |
| 25) CD35 −C | β0 | Malay |
| 26) CD36/37 −T | β0 | Kurd, Iranian |
| 27) CD37/38/39 del 7 bp (−GACCCAG) | β0 | Turkish |
| 28) CD38/39 −C | β0 | Czechoslovkian |
| 29) CD38/39 −CC | β0 | Belgian |
| 30) CD40 −G | β0 | Japanese |
| 31) CD40/41 +T | β0 | Chinese |
| 32) CD41 −C | β0 | Thai |
| 33) CD41/42 −TTCT | β0 | Chinese, SE Asian, Indian |
| 34) CD42/43 +T | β0 | Japanese |
| 35) CD42/43 +G | β0 | Japanese |
| 36) CD44 −C | β0 | Kurdish |
| 37) CD45 −T | β0 | Pakistani |
| 38) CD45 +T | β0 | Turkish |
| 39) CD47 +A | β0 | Surinamese |
| 40) CD47/48 +ATCT | β0 | Asian Indian |
| 41) CD49 −C | β0 | Jordanian |
| 42) CD51 −C | β0 | Hungarian |
| 43) CD53/54 +G | β0 | Japanese |
| 44) CD54 −T | β0 | Swedish |
| 45) CD54/58 (−T ATG GGC AAC CCT) | β0 | Chinese (Li et al. 2009) |
| 46) CD55 −A | β0 | Asian Indian |
| 47) CD54/55 +A | β0 | Asian Indian |
| 48) CD56-60 +14 bp | β0 | Iranian |
| 49) CD57/58 +C | β0 | Asian Indian |
| 50) CD59 −A | β0 | Italian |
| 51) CD62/63/64 del 7 bp (−TCATGGC) | β0 | Asian Indian |
| 52) CD64 −G | β0 | Swiss |
| 53) CD67 −TG | β0 | Filipino |
| 54) CD71/72 +T | β0 | Chinese |
| 55) CD71/72 +A | β0 | Chinese |
| 56) CD72/73 −AGTGA, +T | β0 | British |
| 57) CD74/75 −C | β0 | Turkish |
| 58) CD76 GCT → –T | β0 | North African |
| 59) CD76 −C | β0 | Italian |
| 60) CD82/83 −G | β0 | Czech, Azerbaijani |
| 61) CD81-87 (−22 bp) | β0 | Asian Indian |
| 62) CD83-86 del 8 bp (−CACCTTTG) |
β0 | Japanese |
| 63) CD84/85 +C | β0 | Japanese |
| 64) CD84/85/86 +T | β0 | Japanese |
| 65) CD88 +T | β0 | Asian Indian |
| 66) CD88 −TG | β0 | Japanese |
| 67) CD89/90 −GT | β0 | Japanese |
| 68) CD95 +A | β0 | SE Asian |
| 69) CD106/107 +G | β0 | U.S. Black, Egyptian |
| 70) CD109 (GTG → GT−) | ? | Irish |
| 71) CD120/121 +Ad | β0 | Philippino |
| 72) CD130/131 +GCCT | ? β0 | German |
| 73) CD142/143 (−CC) | ? | French Caucasian |
References to these mutations can be found in Forget (2001), Weatherall and Clegg (2001), and Thein and Wood (2009).
aOccurs in cis to the IVS2-745 C → G mutation.
bAlso occurs in cis to 7201 bp deletion involving δ gene.
cOccurs in cis to δ59 –A.
dThis frameshift leads to predicted truncated β variant of 138aa with an abnormal carboxyl terminal. Heterozygotes do not appear to have an unusually severe phenotype.
Transcriptional Mutants
Point mutations involving the conserved DNA sequences that form the β-globin promoter (from 100 bp upstream to the site of the initiation of transcription, including the functionally important CACCC, CCAAT, and ATAA boxes) and the stretch of 50 nucleotides in the 5′ UTR have been identified in patients of different ethnic groups. In general, mutations of the β-globin gene promoter within the CCAAT box cause mild β-thalassemia phenotypes, consistent with the relatively minor deficit in β-globin production and minor globin-chain imbalance. This finding is also consistent with transient expression studies of the mutant genes in tissue culture cells, which show that the defects allow an output of β-globin mRNA ranging from 10% to 25% of normal (Treisman et al. 1983).
Some of these β-thalassemia alleles are so mild that heterozygotes (carriers) are “silent” with near normal red cell indices and HbA2 levels, the only abnormality being an imbalanced globin-chain synthesis (Gonzalez-Redondo et al. 1989). Overall, the “silent” β-thalassemia alleles are uncommon except for the –101 C → T mutation, which has been observed fairly frequently in the Mediterranean region in which it interacts with a variety of more severe β-thalassemia mutations to produce milder forms of β-thalassemia (Maragoudaki et al. 1999).
Other “silent” mutations include those in the 5′ UTR. Because the description of the CAP +1 A-C allele (Wong et al. 1987), other molecular defects including single base substitutions and minor deletions distributed along the stretch of 50 nucleotides have been identified. As in the −101 C → T mutation, heterozygotes for this class of mutations are “silent”; the extremely mild phenotype is exemplified in a homozygote for the +1 A → C mutation who has the hematologic values of a thalassemia carrier (Wong et al. 1987). It is not known whether the CAP mutation causes β-thalassemia by decreasing β-globin gene transcription or by decreasing the efficiency of capping (posttranscriptional addition of m7G) and mRNA translation. In vivo and in vitro studies show that the +33 C → G mutation leads to a reduction of β mRNA that is 33% of the output from a normal β gene, milder than the mutations involving the promoter elements (Ho et al. 1996).
Within this group of transcriptional mutants, ethnic variation in phenotype has been observed. Black individuals homozygous for the −29 A → G mutation have an extremely mild disease (Safaya et al. 1989), whereas a Chinese individual homozygous for the same mutation had thalassemia major (Huang et al. 1986). The cause of this striking difference in phenotype is not known but may be related to the different chromosomal backgrounds on which the apparently identical mutations have arisen or to the C-T polymorphism at position –158 upstream of the Gγ-globin gene (Xmn1-Gγ site), which is associated with increased fetal hemoglobin production under conditions of erythropoietic stress. The Xmn1-Gγ site is present in the β chromosome carrying the –29 A → G mutation in Blacks but absent in that of the Chinese.
Mutants Affecting RNA Processing
A wide variety of mutations interfere with processing of the primary mRNA transcript. Those that affect the invariant dinucleotides GT or AG at the exon–intron splice junction completely abolish normal splicing and produce the phenotype of β0-thalassemia. These mutations can be base substitutions that change one or the other of invariant dinucleotides or short deletions that remove them. Flanking the invariant dinucleotides are sequences that are fairly well conserved and a consensus sequence can be recognized at the exon–intron boundaries. They encompass the last three nucleotides of the exon and the first six nucleotides of the intron for the 5′ donor site; and the last 10 nucleotides of the intron and the first nucleotide of the exon for the 3′ acceptor site.
Mutations within the consensus sequences at the splice junctions reduce the efficiency of normal splicing to varying degrees and produce a β-thalassemia phenotype that ranges from mild to severe. For example, mutations at position 5 IVS1 G → C, T or A, considerably reduce splicing at the mutated donor site compared with normal. The mutations appear to activate the use of three “cryptic” donor sites, two in exon 1 and one in IVS1, which are used preferentially to the mutated donor site (Treisman et al. 1983). On the other hand, the substitution of C for T in the adjacent nucleotide, intron 1 position 6, only mildly affects normal RNA splicing even though it activates the same three cryptic donor sites as seen in the IVS1-5 mutants. Although the IVS1-6 T → C mutation is generally associated with milder β-thalassemia, studies have shown that in some cases, apparently identical mutations can be severe; and again this is presumably related to the chromosomal background on which the mutations have arisen (Rund et al. 1997).
Both exons and introns also contain “cryptic” splice sites, which are sequences very similar to the consensus sequence for a splice site but are not normally used. Mutations can occur in these sites creating a sequence that resembles more closely the normal splice site. During RNA processing the newly created site is used preferentially, leading to aberrant splicing. Such mutations have been identified in both introns 1 and 2, and exon 1 (Tables 1 and 2). The associated phenotype may be either β+- or β0- thalassemia, depending on the site and nature of the mutation.
Table 2.
Dominantly inherited β-thalassemia alleles
| Mutations | Exon | Hb variant | Ethnic group |
|---|---|---|---|
| I. Missense mutations | |||
| 1) CD 28 (CTG → CGG) Leu to Arg | 1 | Hb Chesterfielda | English |
| 2) CD 32 (CTG → CAG) Leu to Glu in cis with CD 98 (GTG → ATG) Val to Met, Hb Köln | 2 | Hb Medicine Lakea | U.S. Caucasian |
| 3) CD 60 (GTG → GAG) Val to Glu | 2 | Hb Cagliaria | Italian |
| 4) CD 106 (CTG → CGG) Leu to Arg | 3 | Hb Terre Haute | N. European, French |
| 5) CD 110 (CTG → CCG) Leu to Pro | 3 | Hb Showa-Yakushiji | Japanese |
| 6) CD 114 (CTG → CCG) Leu to Pro | 3 | Hb Durham NC/Hb Bresciaa | U.S. Irish, Italian |
| 7) CD 115 (GCC → GAC) Ala to Asp | 3 | Hb Hradec Kralove | Czech |
| 8) CD 127 (CAG → CCG) Gln to Pro | 3 | Hb Houston | U.S. English |
| 9) CD 127 (CAG → CGG) Gln to Arg | 3 | Hb Dieppe | French |
| 10) CD 128 Ala to Pro | 3 | Hb Mont Saint Aignan | French Caucasian |
| II. Deletion or insertion of intact codons → destabilization | |||
| 1) CD 3 (+T), CD5 (−C) Leu-Thr-Pro to Ser-Asp-Ser | 1 | Hb Antalya | Turkish |
| 2) CD 30-31 (+CGG) +Arg | 1/2 | Spanish | |
| 3) CD 32-34 (−GGT) Val-Val to Val | 2 | Hb Koreaa | Korean |
| 4) CD 33-35 (−TGGTCT) Val-Val-Tyr to 0-0-Asp | 2 | Hb Dresden | German |
| 5) CD 108-112 (−12 bp) Asn-Val-Leu-Val-Cys to Ser | 3 | Swedish | |
| 6) CD 124-126 (+CCA) +Pro | 3 | Armenian | |
| 7) CD 127-128 (−AGG) Gln-Ala to Pro | 3 | Hb Gunma | Japanese |
| 8) CD 134-137 (−12, +6) Val-Ala-Gly-Val to Gly-Arg | 3 | Portuguese | |
| 9) CD 137-139 (−TGGCTA) Val-Ala-Asn to Asp | 3 | Hb Stara Zagnoraa | Bulgarian |
| III. Premature termination | |||
| 1) CD 121 (GAA → TAA) Glu to Term (120aa)b,c | 3 | N. European Japanese |
|
| 2) CD 127 (CAG → TAG) Gln to Term (127aa) | 3 | English, French | |
| IV. Frameshift mutations | |||
| 1) IVSII: 2,3 (+11, −2) | IVSII | Iranian | |
| 2) IVSII: 4, 5 (−AG) → aberrant splicing | IVSII | Portuguese | |
| 3) IVSII: 535 to CD108 (+23, −310, +28) to CD105-108 Leu-Leu-Glu-Asn to Val-Pro-Ser-Val-Thr-Leu-Phe-Phe-Asp | IVSII (Exon 3) | Hb Jambola | Bulgarian |
| 4) CD 91 (CTG → CG) → 156aa | 2 | Hb Morgantown | Irish |
| 5) CD 94 (+TG) → 156aa | 2 | Hb Agnanaa | S. Italian |
| 6) CD 100 (−CTT, +TCTGAGAACTT) → 158aa | S. African | ||
| 7) CD 104 (AGG → AG ) → 156aac | 2 | German Caucasian | |
| 8) CD 109 (GTG → TG ) → 156aa | 3 | Hb Manhatten | Lithuanian |
| 9) CD 113 (GTG → TG) → 156aa | 3 | Canadian, N. European | |
| 10) CD 114 (−CT, +G) → 156aa | 3 | Hb Geneva | Swiss-French |
| 11) CD 118 (−T) | 3 | Hb Sainte Seve | French Caucasian |
| 12) CD 120-121 (+a) → 138aa | 3 | Philippino | |
| 13) CD 123 (−A) → 156aa | 3 | Hb Makabe | Japanese |
| 14) CD 123-125 (−ACCCCACC) → 135aa | 3 | Hb Khon Kaend | Thai |
| 15) CD 124 (−A) → 156aa | 3 | Russian | |
| 16) CD 124-126 (+CCA) → Pro-Pro-Val to Pro-Pro-Val | 3 | Armenian | |
| 17) CD 125 (−A) → 156aa | 3 | Japanese | |
| 18) CD 126 (−T) → 156aa | 3 | Hb Vercellia | N. Italian |
| 19) CD 126-131 (−17 bp) → 132aa | 3 | Hb Westdaled | Asian Indian, Pakistani |
| 20) CD 128-129 (−4, +5, −11) → 153aa | 3 | Irish | |
| 21) CD 131-132 (−GA) → 138aa | 3 | Swiss | |
| 22) CD 131-134 (−11 bp) → 134aa | 3 | Spanish | |
| 23) CD 140-141 (−C) → 156aa | 3 | Hb Florida | Argentinian Spanish |
Some of these variants are not associated with elevated A2 in heterozygous state (e.g., Hb Dresden, Hb Jambol, Hb Morgantown, Hb Stara Zagnora). References to these mutations can be found in Thein (2001) and Thein and Wood (2009).
aSpontaneous mutations.
bSeveral families reported including one spontaneous mutation.
cCoinheritance of extra α-globin gene (genotype ααα/αα) contributed to unusually severe thal intermedia in proband.
dDifficult to evaluate phenotypes of heterozygotes as only homozygote and compound heterozygotes reported.
The IVS1-110 G to A mutation was the first base substitution identified in a β-thalassemia gene (Spritz et al. 1981; Westaway and Williamson 1981). It is one of the most common forms of β-thalassemia in the Mediterranean population. The G to A substitution creates an alternative acceptor AG, 19 bp 5′ to the normal acceptor AG of IVS1. In vitro expression studies have shown that this newly created alternative splice site is preferentially used in 80% to 90% of the transcripts (Busslinger et al. 1981). The mutant mRNA is hardly detected in affected erythroid cells presumably because the 19 bp segment of retained intronic sequence contains an in-phase premature termination codon. Only 10%–20% of the transcripts are normally spliced, hence the severe β+-thalassemia. In the case of the T → G substitution in position 116 of IVS1, however, all transcripts are aberrantly spliced utilizing the newly created 3′ acceptor site, resulting in little or no normal β mRNA and a β0-thalassemia phenotype (Metherall et al. 1986).
Other β-thalassemia genes have substitutions within intron 2 that generate new donor sites. They include the IVS2 position 654 C → T, 705 T → G, and 745 C → G (Orkin et al. 1982a; Dobkin et al. 1983; Cheng et al. 1984). In each case, an identical upstream acceptor site at position 579 is activated such that the normal 5′ donor site at exon 2/IVS2 is spliced to the activated site at position 579 while the newly created donor site is spliced to the normal 3′ acceptor site at IVS1/exon 3. This two-stage splicing results in the retention of 73 bp, 121 bp and 151 bp of IVS2 in the misspliced β mRNA for the IVS2-654, IVS2-705, and IVS2-745 mutations, respectively. Variable amounts of splicing from the normal donor to the normal acceptor also occurs, resulting in phenotypes that range from β+- to β0- thalassemia. The β0 versus β+ phenotype associated with these different mutations must be related to different affinities of the enzymatic splicing complex for a given mutant splice site versus the normal splice sites.
Four mutations have been identified in exon 1 that are associated with activation of alternative splice sites. Three of these mutations modify the cryptic splice site spanning codons 24 to 27 in exon 1 so that it more closely resembles the consensus splice sequence AAGGTGAGT. The codon 24 GGT-GGA mutation is translationally silent (Goldsmith et al. 1983), whereas codon 26 GAG-AAG and codon 27 GCC-TCC result in the βE and βKnossos variants, respectively (Orkin et al. 1982b, 1984). The mutation in codons 26 and 27 lead to a minor use of the alternative pathway so that there is a reasonable level of normally spliced products that result in the mild β+-thalassemic phenotype of the βE and βKnossos alleles, respectively.
Similarly, the A → G mutation in codon 19 activates another cryptic donor site spanning codons 17 to 19 resulting in a reduced level of normally spliced β mRNA with the codon 19 mutation encoding Hb Malay (Yang et al. 1989).
Mutations Causing Abnormal Posttranscriptional Modification
The nascent precursor globin mRNA molecule has to be modified at both of its ends to be functional; a methylated (m7G) cap structure is added at the 5′ end, and a string of adenylic acid residues (poly-A) added at the 3′ end. Proper cleavage of the primary RNA transcript and polyadenylation of the 3′ end of the mRNA is guided by a consensus hexanucleotide sequence (AATAAA) about 20 nucleotides upstream of the poly-A tail. Mutations affecting the AATAAA sequence include seven base substitutions at different locations; two short deletions of 2 and 5 bp each, and one deletion of the total AATAAA sequence (Table 1). These mutations markedly decrease the efficiency of the cleavage-polyadenylation process, only about 10% of the mRNA is properly modified (Orkin et al. 1985). Therefore, the associated phenotype is that of β+-thalassemia of moderate severity. The remainder of the transcripts extend far beyond the normal polyadenylation site and are probably cleaved and polyadenylated after the next AATAAA consensus sequences, which occur about 0.9–3 kb downstream. Mutations affecting other sites in the 3′ UTR, a C → G substitution at nucleotide 6, and a 13 bp deletion at nucleotides 90 downstream from the termination codon, also result in β+-thalassemia (Rund et al. 1992; Hamid and Akbari 2011).
Mutants Affecting Translation of β-Globin mRNA
Mutations Affecting the Initiation Codon
Mutations affecting the initiation codon (ATG) all produce β0-thalassemia (Table 1). One mutation involves an insertion of 45 bp between positions –22 to +23, thus affecting the initiation codon. The rest are single base substitutions, two affecting the first (A), three the second (T) and, three the third (G) nucleotide of ATG (Jankovic et al. 1990; Lam et al. 1990; Hattori et al. 1991; Saba et al. 1992; Ohba et al. 1997; Forget 2001; Blacklock et al. 2005; Thein and Wood 2009). It is theoretically possible for mutant β mRNAs to be initiated at the next downstream initiation codons, which are located at codons 21 and 22, or codon 55. However, it is predicted that these alternative initiation codons would result in premature termination, and that mutant mRNAs would be nonfunctional and subjected to nonsense mediated decay surveillance.
Premature Termination Codons
Approximately half the β-thalassemia alleles result from the introduction of premature termination codons, either because of direct mutations creating a stop codon or a change in the reading frame by insertion or deletion of a single to a few nucleotides. These frameshifts lead to premature termination further downstream when the next nonsense codon is reached.
One of the first nonsense mutations to be characterized and extensively studied was the mutation at codon 39 (CAG to TAG) (Humphries et al. 1984; Takeshita et al. 1984; Huang and Benz 2001). This mutation is the second most common cause of β-thalassemia in the Mediterranean population and accounts for most of the cases of β-thalassemia in Sardinia. An interesting feature of this and other nonsense mutations is the finding of very low levels of the mutant β-globin mRNA in affected erythroid cells. Initial studies of this phenomenon revealed that the gene was transcribed normally, but there appeared to be defective β-mRNA stability in the nucleus or defective processing and/or transport of the mRNA from nucleus to cytoplasm; mRNA stability in the cytoplasm appeared to be normal. It is now clear that reduced levels of the mutant β mRNA is a result of the nonsense-mediated decay quality-control mechanism (Isken and Maquat 2007; Schoenberg and Maquat 2012).
The frameshift and nonsense mutations that cause recessively inherited β0-thalassemia typically result in premature termination within exon 1 and 2 with minimal steady-state levels of abnormal β mRNA (Fig. 2). In heterozygotes for such cases, no β chain is produced from the mutant allele, resulting in a typical asymptomatic phenotype (Hall and Thein 1994). In contrast, the mutant mRNA associated with mutations that produce in-phase termination later in the β sequence, in exon 3, does not undergo nonsense-mediated decay and presumably, gets translated into variant β chains. The abnormal β chains together with the concomitant excess α chains overcome the proteolytic machinery of the cell, increasing the ineffective erythropoiesis resulting in a severe phenotype. These mutants are usually dominantly inherited, in contrast to the typical recessive inheritance of β-thalassemia (see below).
GENE DELETIONS
In contrast to α-thalassemia, the β-thalassemias are rarely caused by major gene deletions with two exceptions: a group of deletions that are restricted to the β-globin gene (Fig. 3) and a second group of larger deletions affecting the upstream βLCR with or without the β-globin gene (Fig. 4).
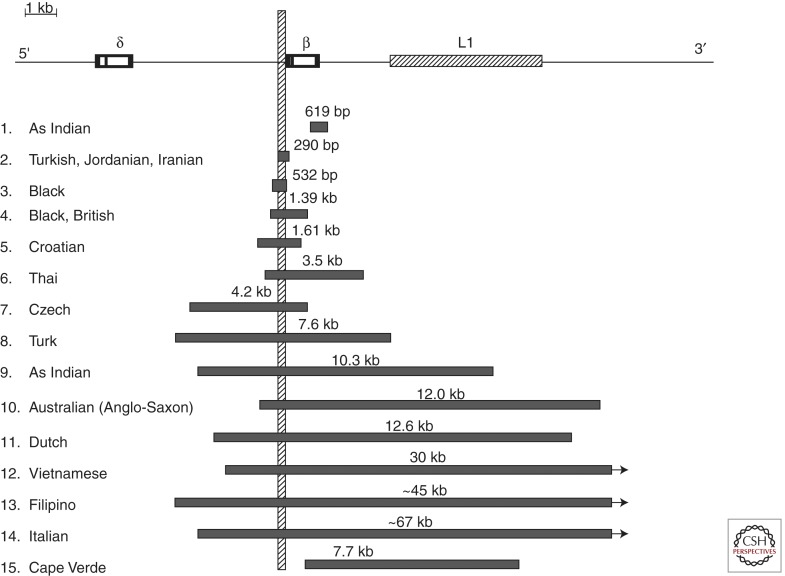
Figure 3.
β-thalassemia deletions that are restricted to the gene. The vertical bar indicates the promoter region that is removed in common by these deletions except for the 619 bp Asian Indian and the 7.7 kb Cape Verde deletions.
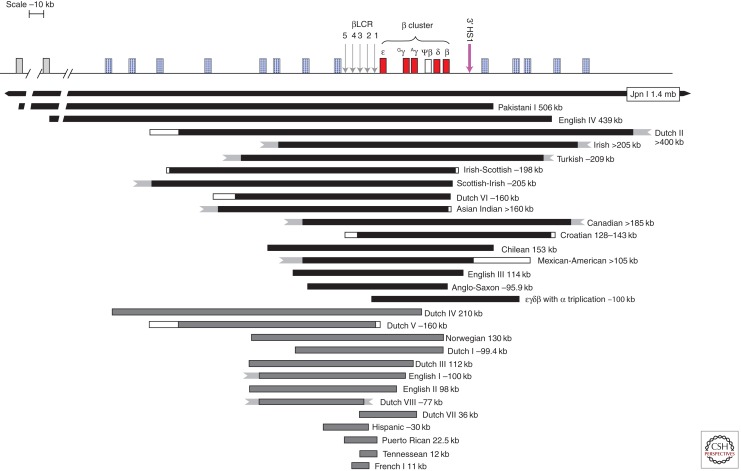
Figure 4.
Deletions causing β-thalassemia as part of (εγδβ)0-thalassemia. The deletions can be classified into two groups: group I deletions (in black) remove all or most of the cluster including the βLCR and the β-globin gene, whereas group II deletions remove all or part of βLCR leaving the β gene intact. Only about one-third of the breakpoints of these deletions have been defined; the white boxes and jagged ends indicate undefined breakpoints.
Deletions Restricted to the β-Globin Gene
Deletions affecting only the β-globin gene ranged from 105 bp to ∼67 kb in size (Fig. 3). The phenotype associated with these deletions is that of β0-thalassemia (Thein and Wood 2009).
Two deletions remove the 3′ end but preserve the integrity of the 5′ end of the β-globin gene. The 0.6 kb deletion involving the 3′ end of the β-globin gene is a relatively common cause of β0-thalassemia in Asian Indians and accounts for about one-third of the cases of β-thalassemia in this population (Thein et al. 1984; Varawalla et al. 1991). The second deletion was recently described in compound heterozygosity with HbS in a woman from Cape Verde Islands who presented with sickle cell disease (Andersson et al. 2007). The deletion removes 7.7 kb, starting in IVS2 of the β-globin gene and extending 7.1 kb downstream, in the midst of the Kpn I family of L1 repeat elements. The other deletions differ widely in size, but remove in common a region in the β promoter (from positions –125 to +78 relative to the mRNA cap site), which includes the CACCC, CCAAT, and TATA elements. They are associated with unusually high levels of HbA2 and variable increases of HbF in heterozygotes. It has been proposed that deletion of the β promoter removes competition for the upstream βLCR and limiting transcription factors, allowing greater interaction of the LCR with the cis δ and γ genes, thus enhancing their expression. Indeed, studies of an individual heterozygous for the 1.39 kb β-thalassemia deletion and a δ-chain variant showed that there is a disproportionate increase of HbA2 derived from the δ gene in cis to the β-gene deletion (Codrington et al. 1990). The β promoter can also be inactivated by point mutations and again, carriers have unusually high HbA2 and HbF levels (Huisman 1997). Although the increases in Hb F are variable and modest in heterozygotes for such mutations, they can be sufficiently increased to partially compensate for the complete absence of β globin in homozygotes; two individuals homozygous for different deletions in this group are not transfusion dependent with a mild disease (Schokker et al. 1966; Gilman 1987; Craig et al. 1992).
Upstream Deletions and (εγδβ)0-Thalassemia
This group of deletions affects the upstream regulatory locus control region (βLCR) and expression of the β-globin gene is down-regulated together with all the linked globin genes in the cluster on chromosome 11p, as part of (εγδβ)0-thalassemia (Fig. 4). The deletions are classified molecularly into two categories: a larger group (I) that removes all or most of the complex, including the HBB gene and the βLCR, and group II that removes the upstream LCR but leaves the HBB gene itself intact (Thein and Wood 2009; Rooks et al. 2012). It was the characterization of the upstream deletions that indicated the importance of long-range regulatory elements in the control of the β-globin locus (Kioussis et al. 1983; Grosveld et al. 1987). Adult heterozygotes for these deletions have a hematological phenotype similar to that of β-thalassemia trait, but the HbA2 and HbF levels are within normal limits and the red blood cells tend to be relatively more hypochromic microcytic. Newborns have anemia and hemolysis; some requiring intrauterine and perinatal blood transfusions to tide them over the perinatal period. The severity of anemia and hemolysis is variable (even within a family with identical mutations) (Verhovsek et al. 2012), and appears to show no correlation with type (group I or II) or size of deletions. Only heterozygotes have been identified; homozygotes, presumably would not survive early gestation. These deletions are rare and unique to the families in which they have been described.
DOMINANTLY INHERITED β-THALASSEMIA
The syndrome comprises a distinct set of mutations affecting the HBB gene that are associated with typical hematological features of β-thalassemia (i.e., increased HbA2 levels and imbalanced α-/β-globin-chain biosynthesis in heterozygotes), yet cause a disease phenotype when present in single copy (Thein 1999, 2001). Unlike the common recessive forms of β-thalassemia, which are prevalent in malaria-endemic regions, “dominantly inherited” β-thalassemia has been described in dispersed geographical regions, a large number of the mutations are spontaneous. Severe dyserythropoiesis associated with inclusion bodies in bone marrow erythroblasts and peripheral red blood cells after splenectomy were frequently observed in affected individuals prompting the term “inclusion body β-thalassemia” (Weatherall et al. 1973; Fei et al. 1989).
Globin-chain biosynthesis studies of both bone marrow erythroblasts and peripheral blood reticulocytes show imbalanced synthesis of α- and β-globin chains typical of those found in heterozygous β-thalassemia (Weatherall et al. 1973; Ho et al. 1997). As the molecular lesions of an increasing number of such cases were characterized, it became apparent that the defects were extremely heterogeneous; some mutations were highly complex involving deletions interrupted by insertions similar to the complex arrangement originally described in the Irish family (Weatherall et al. 1973). All mutations were associated with the synthesis or predicted synthesis of extremely unstable β-globin-chain variants (Fig. 2). In the majority of cases, the abnormal β variant is not detectable but there is a significant pool of free α chains, which presumably form the characteristic cytoplasmic inclusions (Weatherall et al. 1973).
The predicted synthesis is supported by the presence of substantial amounts of mutant β-globin mRNA in the peripheral blood reticulocytes, comparable in amounts to that of the other normal β-globin allele (Hall and Thein 1994). In dominantly inherited β-thalassemia, not only is there a functional deficiency of β globin, but precipitation of the β-chain variants with the concomitant excess α chains overload the proteolytic intracellular mechanisms increasing ineffective erythropoiesis. Indeed, the large intraerythroblastic inclusions, that are so characteristic of this form of β-thalassemia, have subsequently been shown to be composed of both α- and β-globin chains (Ho et al. 1997). In contrast, the inclusion bodies in homozygous β-thalassemia consisted only of precipitated α globin. The molecular mechanisms underlying the instability include: substitution of the critical amino acids in the hydrophobic heme pocket displacing heme leading to aggregation of the globin variant; disruption of secondary structure because of replacement of critical amino acids; substitution or deletion of amino acids involved in αβ dimer formation; and elongation of subunits by a hydrophobic tail.
The molecular defects include missense mutations, deletions or insertion of intact codons, nonsense mutations causing premature termination codons in exon 3, which leads to a failure of the nonsense mediated decay of the RNA (Fig. 2; Table 2). Frameshifts may also result in aberrant splicing producing elongated or truncated β-globin-chain variants with abnormal carboxy-terminal ends similar to the variant predicted in the Irish family (Weatherall et al. 1973).
Missense Mutations
An example of a missense mutation causing β-thalassemia intermedia is Hb Terre Haute (β106 Leu → Arg) (Coleman et al. 1991). This mutant was initially described as Hb Indianapolis (β112 Cys → Arg) (Adams et al. 1979), which was subsequently reported in two families (Spanish and Italian) (Baiget et al. 1986; De Biasi et al. 1988) in whom affected members had evidence of mild hemolytic anemia with 2%–4% reticulocytosis. In two patients heterozygous for Hb Terre Haute, globin-chain biosynthesis studies showed an α:non-α ratio of ∼1.0 in bone marrow erythroblasts compared with a ratio of ∼2.0 in peripheral blood reticulocytes. Although the variant β-globin chain was synthesized at a level almost equal to that of the normal β-globin chain, most of it was rapidly precipitated on the red cell membrane. The half-life of this globin variant was less than 10 min, and the abnormal hemoglobin was not detectable by standard techniques.
Other examples of missense variants include Hb Chesterfield (Thein et al. 1991), Hb Cagliari (Podda et al. 1991), Hb Showa-Yakushiji (Kobayashi et al. 1987), Hb Durham NC/Brescia (de Castro et al. 1994), Hb Houston (Kazazian et al. 1992), and more recently, Hb Mont Saint Aignan (Wajcman et al. 2001). In the example of Hb Chesterfield, an abnormal peak in the position expected for the β-globin-chain variant but without detectable corresponding protein was demonstrated by globin-chain biosynthesis studies. Hb Mont Saint Aignan (β128 [Hb] Ala → Pro), in comparison, appeared mildly unstable; the abnormal β chain could be isolated by selective isopropanol precipitation and the structure determined by protein chemistry methods. Mass spectrometry electrospray analysis estimated Hb Mont Saint Aignan to be ∼20% of total hemoglobin.
Most of the other abnormal hemoglobins were not detected by routine hemoglobin electrophoresis.
Deletion or Insertion of Intact Codons
Deletions or insertions of entire codons allow the reading frame to remain in phase, and the remaining amino acids are normal. Both Hb Korea (Park et al. 1991) and Hb Gunma (Fucharoen et al. 1990a) have 145 amino acid residues each; in Hb Gunma, the β127–128 Gln-Ala dipeptide is replaced by a proline residue because of the deletion of three bases (AGG), whereas in Hb Korea, the deletion of three bases (GGT) removes one of the Val residues from codons 32–34. Other β-globin-chain variants have extra residues, and include the insertion of Arg in codons 30–31 in a Spanish family (Arjona et al. 1996) and insertion of a single proline in codons 124–126 in an Armenian patient (Çürük et al. 1994).
In all cases, no trace of abnormal hemoglobin could be detected by the standard techniques of IEF, HPLC, or heat stability tests. One mechanism that could explain the lack of detection of these structural β-globin-chain variants is that the affected amino acids are involved in α1β1 contacts. In the normal β-globin chain, β30 Arg (B 12), β33 Val (B 15), β34 Val (B 16), β108 Asn (G 10), β112 Cys (G 14), β124 Pro (H2), β125 Pro (H3), β127 Gln (H5), and β128 Ala (H6) are essential for α1β1 dimer formation (Bunn and Forget 1986). Deletion or substitution of these critical amino acids would be likely to prevent the formation of αβ subunits and, effectively, lead to a functional loss of half of the β-globin chains.
Another example in this category is a deletion of 12 in combination with an insertion of six nucleotides, leading to the substitution of the normal Val-Ala-Gly-Val by Gly-Arg in codons 134–137 and a β-globin subunit that was two amino acids shorter than normal. Affected individuals of this Portuguese family had moderately severe anemia, splenomegaly, and leg ulcers (Öner et al. 1991).
Hb Stara Zagnora, found in a 2-year-old Bulgarian boy, results from the deletion of 6 bp spanning β codons 137–139 such that Val-Ala-Asn is replaced by Asp (Efremov 2007). Hb Stara Zagnora was hyperunstable; the affected 2-year-old had hemolysis and dyserythropoietic anemia with mild globin-chain imbalance (α/β synthesis ratio 1.40) although HbA2 levels were within normal limits.
Premature Termination (Nonsense Mutation)
All the dominantly inherited β alleles are rare and unique to the families in which they have been described with one exception. The GAA → TAA termination codon at codon 121, which leads to the synthesis of a truncated β-globin chain, has been described in several families of different ethnic backgrounds (Stamatoyannopoulos et al. 1974; Kazazian et al. 1986; Fei et al. 1989; Thein et al. 1990; Ohba et al. 1997), and in some families, heterozygotes do not have an unusually severe phenotype (i.e., the mutation is not dominantly inherited). Substantial amounts of mutant β-globin mRNA could be demonstrated in individuals in whom the mutation is dominantly inherited, but demonstration of the presence of the truncated β-globin variant has been difficult. Presence of the predicted truncated variant, however, was implicated from a large difference between the total radioactivity incorporated into newly synthesized chains and the total amount of protein in globin biosynthesis studies. In one study, the truncated β-globin chain was estimated to comprise only 0.05% to 0.1% of the total non-α globin (Ho et al. 1997).
Heterozygosity for a premature stop codon in β127 (CAG TO TAG) has also been to cause thalassemia intermedia in an English woman (Hall et al. 1991), and a 29-year-old French Caucasian woman (Prehu et al. 2005).
Elongated or Truncated Variants with Abnormal Carboxy-Terminal Ends
In general, the elongated or truncated β-globin gene variants in this group have arisen from frameshift mutations that generated distal premature termination codons. Again, the abnormal β-globin variants were not detected in any of the cases by hemoglobin electrophoresis or globin biosynthesis studies, but in all cases there was imbalanced synthesis of α- and β-globin chains.
Elongated β-globin subunits could also arise from aberrant splicing of precursor mRNA, as described in a deletion of two nucleotides affecting the IVS2 consensus donor splice site in a Portuguese family (Faustino et al. 1998). The mutation was associated with unusually severe anemia and intraerythroblastic inclusions, transmitted as a single allele in five generations of the family.
In Hb Jambol, an insertion of 23 nucleotides and a deletion of 310 nucleotides in βIVS2 extending to exon 3 of the HBB gene results in the replacement of Leu-Leu-Glu-Asn at codon 105 to 108 with nine residues and was associated with severe hemolytic anemia and mildly imbalanced globin synthesis ratio (Efremov 2007).
Recessive versus Dominant Inheritance
Frameshift mutations and premature termination codons (PTCs) that are recessively inherited terminate in exon 1 or 2, whereas those that are dominantly inherited, terminate much later in the sequence of the β-globin gene, in the 3′ part of exon 2 and exon 3 (Fig. 2). These in-phase termination mutants appear to have differential effects on triggering the surveillance mechanism of nonsense mediated decay (NMD). NMD is an in-house mRNA quality-control mechanism that degrades abnormal mRNAs that arise from mistakes in gene expression such as those caused by premature termination codons. In mammalian cells, termination codons are recognized as “premature” if it is located upstream of a boundary of 50–55 nucleotides 5′ to the final exon-exon junction (Nagy and Maquat 1998; Schoenberg and Maquat 2012). In the β-globin gene this boundary corresponds to a position 54 bp upstream of exon 2–exon 3 junction in intron 2; premature termination mutants that reside upstream are associated with minimal mutant β mRNA. However, exceptions to the “50–55 nt boundary rule” have been reported. β mRNA with nonsense mutations in codons 5, 15, and 17, all within exon 1, were detected at high levels, similar to those of wild type β-globin mRNA. It is possible that early PTCs within β-globin mRNA and proximity to the translation initiation codon (ATG) can override the “50–55 nt boundary rule” (Romao et al. 2000).
Similarly, frameshift mutations that occur later in the sequences, terminate later and tend to lead to accumulation of the mutant message and the synthesis of elongated β-globin variants. These elongated variants have abnormal carboxy-terminal ends made up of hydrophobic sequences causing their instability. Furthermore, these β-globin variants would not be able to form αβ dimers as most of the α1β1 contact residues would have been removed. Because the heme contact site codons—mostly located in exon 2—are retained, these elongated variants should have some tertiary structure, be less susceptible to proteolytic degradation, and presumably, form the characteristic inclusion bodies. Indeed, prominent inclusions were noted in individuals heterozygous for Hb Geneva (Beris et al. 1988), Hb Makabe (Fucharoen et al. 1990b), Hb Agnana (Ristaldi et al. 1990), Hb Vercelli (Murru et al. 1991), and the frameshift mutation at codon 128 in the Irish family (Weatherall et al. 1973; Thein et al. 1990).
VARIANTS OF β-THALASSEMIA
Normal HbA2 β-Thalassemias
Normal HbA2 β-thalassemias (previously referred to as type 2) refers to the form in which the blood picture is typical of heterozygous β-thalassemia (i.e., hypochromic microcytic red blood cells) except for the normal levels of Hb A2. Most cases of normal HbA2 β-thalassemia result from coinheritance of δ-thalassemia (δ0 or δ+) in cis or trans to a β0- or β+-thalassemia gene (Thein 1998).
One relatively common form of normal Hb A2-thalassemia in the Middle East and Mediterranean is that associated with Hb Knossos (β27 Ala → Ser). Like Hb E, the mutation β27(GCC → TCC) activates an alternative splice site reducing the amount of normal transcript that contains the variant. Unlike Hb E, the Hb A2 level is not elevated in heterozygotes as there is a δ0-thalassemia (Cd59-A) mutation in cis to the β27 Ala → Ser mutation (Olds et al. 1991).
Another relatively common cause of normal Hb A2 β-thalassemia phenotype in the Greek population is the Corfu form of δβ-thalassemia, a 7.2 kb deletion that includes the 5′ part of the δ gene (Wainscoat et al. 1985; Traeger-Synodinos et al. 1991). The β-globin gene in cis is down-regulated by a G → A mutation in position 5 of the IVS1 (Kulozik et al. 1988). Heterozygotes for the Corfu β-thalassemia allele have a slight increase in Hb F (1.1%–2.8%) and low to normal Hb A2 levels but homozygotes have a milder than expected phenotype of thalassemia intermedia. They have almost 100% Hb F with no Hb A2 and trace levels of Hb A, suggesting that the effect of the deletion is to allow increased γ-chain production under the stress of anemia. Studies in primary erythroid cell cultures from heterozygotes, homozygotes and compound heterozygotes for the Corfu deletion suggest that γ-mRNA accumulation and HbF expression is indirectly dependent on the total amount of viable β mRNA (Chakalova et al. 2005). Reduction of β mRNA below a critical threshold, as in compound heterozygotes and homozygotes, allows full expression of HbF, and hence the unusually high HbF in such individuals. The Corfu mutation has been described as separate lesions in two different populations. The β gene in cis to the 7.2 kb deletion in an Italian individual is normal and expressed at normal levels (Galanello et al. 1990) whereas Algerian homozygotes for the βIVS1-5 G → A mutation have a severe transfusion-dependent anemia.
The phenotype of normal Hb A2 β-thalassemia is also seen in heterozygotes for εγδβ- thalassemia (see above).
“Silent” β-Thalassemia (See also Transcriptional Mutants)
Heterozygotes for “silent” β-thalassemias do not have any evident hematological phenotype; the only abnormality being a mild imbalance of globin-chain synthesis. “Silent” β-thalassemia alleles are not common except for the C → T mutation at position –101 of the β-globin gene, which accounts for most of the milder forms of β-thalassemia in the Mediterranean (Maragoudaki et al. 1999). It has been noted that carriers for this mutation have highly variable HbA2 levels despite similar hematological parameters and globin-chain synthesis ratios. Coinheritance of δ-thalassemia mutations were implicated but sequence analysis of the δ-globin genes in one family excluded this possibility (Ristaldi et al. 2001). A C → G transversion has also been reported in the same −101 position and heterozygotes have a “silent” phenotype (Moi et al. 2004). Several other mutations in the 5′ and 3′ UTRs are also “silent” (Table 1).
It has been suggested that the [TA]x[T]y sequence variation at position –530 of the β-globin gene may be responsible for some “silent” β-thalassemia carriers and that the reduced β- globin expression may be related to increased binding of the BP1 repressor protein (Berg et al. 1991). However, population surveys and clinical studies do not show a consistent correlation between the [TA]x[T]y variants and a β- thalassemia phenotype, suggesting that it is a neutral polymorphism (Wong et al. 1989).
β-Thalassemia Trait with Unusually High HbA2
Despite the vast heterogeneity of mutations, the increased levels of HbA2 observed in heterozygotes for the different β-thalassemia alleles in different ethnic groups are remarkably uniform, usually 3.5%–5.5% and rarely exceeding 6%. Unusually high levels of HbA2 (over 6.5%) seem to characterize the subgroup of β-thalassemias caused by lesions (point mutations or deletions) that inactivate the regulatory elements in the β promoter (Huisman 1997; Thein and Wood 2009). The unusually high HbA2, often accompanied by modest increases in HbF, may be related to the removal of competition for the upstream LCR and transcription factors, allowing an increased interaction with the cis δ and γ genes.
UNUSUAL CAUSES OF β-THALASSEMIA
Insertion of a Transposable Element Causing β-Thalassemia
Transposable elements may occasionally disrupt human genes and result in their inactivation. The insertion of such an element, a retrotransposon of the L1 family has been reported with the phenotype of β+-thalassemia (Kimberland et al. 1999). Despite the insertion of 6–7 kb DNA into its IVS2, the affected gene expresses full length β-globin transcripts at a level corresponding to about 15% of normal β-globin mRNA.
Trans-Acting Mutations
Population studies have shown that ∼1% of β-thalassemias remain uncharacterized despite extensive sequence analysis, including the flanking regions of the β-globin genes. In the last 10 years several rare trans-acting mutations that affect HBB and its linked HBD gene have been identified. Mutations in XPD that cause trichothiodystrophy (TTD) are frequently associated with a phenotype of β-thalassemia trait, supported by reduced levels of β-globin synthesis and reduced β-globin mRNA (Viprakasit et al. 2001). The XPD protein is a subunit of the general transcription factor TF11H, which is involved in basal transcription and DNA repair. Some mutations in the erythroid-specific transcription factor GATA-1 on the X-chromosome have also been reported to cause β-thalassemia in association with thrombocytopenia (Yu et al. 2002). In other cases of β-thalassemia, no mutations have been detected in the HBB gene, and intrafamilial segregation implicates trans-acting regulatory factors (Murru et al. 1992; Thein et al. 1993; Pacheco et al. 1995).
More recently, rare variants in KLF1, an erythroid-specific transcription factor, have been described in association with isolated borderline increases in HbA2 (α2δ2) levels in the absence of mutations in HBB gene (Perseu et al. 2011). It has been suggested that the increased HBD expression is indirect, via impaired interaction of the KLF1 variant with HBB gene in favor of the competing HBD gene.
Somatic Deletion of β-Globin Gene/Uniparental Isodidomy
Somatic deletions involving HBB gene have been described in three unrelated families of French (Badens et al. 2002) and Italian (Galanello et al. 2004) origins. Affected individuals had moderately severe anemia with hepatosplenomegaly despite being constitutionally heterozygous for a typical β-thalassemia mutation (β0 39 C → T) with a normal α genotype. Subsequent investigations revealed a somatic deletion of chromosome 11p15, including the β-globin complex, in trans to the mutation, in a subpopulation of erythroid cells. This results in a somatic mosaic—10% to 20% of erythroid cells were heterozygous with one normal copy of β-globin gene, and the rest hemizygous (i.e., without any normal β-globin gene). The implication for gene therapy is that expression in ∼20% of erythroid cells may be sufficient to convert a transfusion-dependent state to transfusion independence.
Uniparental isodisomy of a segment of chromosome 11p containing the HBB cluster has also been described in two cases. In one family of Chinese origin (Chang et al. 2008), uniparental isodisomy in a carrier for β-thalassemia has resulted in homozygosity for the β-thalassemia allele and thalassemia major. In another, paternal isodisomy of a segment of chromosome 11p in a carrier for βS has resulted in a subpopulation of erythroid cells homozygous for HbS and sickle cell disease (Swensen et al. 2010).
CONCLUDING REMARKS
A complete spectrum of genetic lesions affecting the β-globin gene giving rise to a spectrum of phenotypic severity has been described. Characterization of the β-thalassemia alleles has involved a multitude of methodologies, mirroring the changing DNA technology.
ACKNOWLEDGMENTS
I thank Claire Steward for help with preparation of the manuscript and Helen Rooks for help in preparation of some of the figures.
Footnotes
Editors: David Weatherall, Alan N. Schechter, and David G. Nathan
Additional Perspectives on Hemoglobin and Its Diseases available at www.perspectivesinmedicine.org
REFERENCES
- Adams JGI, Boxer LA, Baehner RL, Forget BG, Tsistrakis GA, Steinberg MH 1979. Hemoglobin Indianapolis (β112[G14] Arginine). An unstable β-chain variant producing the phenotype of severe β-thalassemia. J Clin Invest 63: 931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson BA, Wering ME, Luo HY, Basran RK, Steinberg MH, Smith HP, Chui DH 2007. Sickle cell disease due to compound heterozygosity for Hb S and a novel 7.7-kb β-globin gene deletion. Eur J Haematol 78: 82–85 [DOI] [PubMed] [Google Scholar]
- Arjona SN, Maldonado Eloy-Garcia J, Gu L-H, Smetanina NS, Huisman THJ 1996. The dominant β-thalassaemia in a Spanish family is due to a frameshift that introduces an extra CGG codon (=arginine) at the 5′ end of the second exon. Br J Haematol 93: 841–844 [DOI] [PubMed] [Google Scholar]
- Badens C, Mattei MG, Imbert AM, Lapoumérouliee C, Martini N, Michel G, Lena-Russo D 2002. A novel mechanism for thalassaemia intermedia. Lancet 359: 132–133 [DOI] [PubMed] [Google Scholar]
- Baiget M, Gomez Pereira C, Jue DL, Johnson MH, McGuffey JE, Moo-Penn WF 1986. A case of hemoglobin Indianapolis [β112(G14) Cys → Arg] in an individual from Cordoba, Spain. Hemoglobin 10: 483–494 [DOI] [PubMed] [Google Scholar]
- Berg P, Mittleman M, Elion J, Labie D, Schechter AN 1991. Increased protein binding to a −530 mutation of the human β-globin gene associated with decreased β-globin synthesis. Am J Hematol 36: 42–47 [DOI] [PubMed] [Google Scholar]
- Beris P, Miescher PA, Diaz-Chico JC, Han IS, Kutlar A, Hu H, Wilson JB, Huisman THJ 1988. Inclusion-body β-thalassemia trait in a Swiss family is caused by an abnormal hemoglobin (Geneva) with an altered and extended β-chain carboxy-terminus due to a modification in codon β114. Blood 72: 801–805 [PubMed] [Google Scholar]
- Blacklock HA, Case J, Chan T, Raizis T, Doocey R, Fellowes A, Royle G, Jackson S, Brennan S, George P 2005. Novel sequence insertion in a Maori patient with transfusion-dependent β-thalassaemia. Br J Haematol 131: 400–402 [DOI] [PubMed] [Google Scholar]
- Bunn HF, Forget BG 1986. Hemoglobin: Molecular, genetic and clinical aspects. W.B. Saunders Company, Philadelphia, PA [Google Scholar]
- Busslinger M, Moschonas N, Flavell RA 1981. β+ thalassemia: Aberrant splicing results from a single point mutation in an intron. Cell 27: 289–298 [DOI] [PubMed] [Google Scholar]
- Cantor AB 2005. GATA transcription factors in hematologic disease. Int J Hematol 81: 378–384 [DOI] [PubMed] [Google Scholar]
- Chakalova L, Osborne CS, Dai YF, Goyenechea B, Metaxotou-Mavromati A, Kattamis A, Kattamis C, Fraser P 2005. The Corfu δβ thalassemia deletion disrupts γ-globin gene silencing and reveals post-transcriptional regulation of HbF expression. Blood 105: 2154–2160 [DOI] [PubMed] [Google Scholar]
- Chang JG, Tsai WC, Chong IW, Chang CS, Lin CC, Liu TC 2008. β-thalassemia major evolution from β-thalassemia minor is associated with paternal uniparental isodisomy of chromosome 11p15. Haematologica 93: 913–916 [DOI] [PubMed] [Google Scholar]
- Cheng TC, Orkin SH, Antonarakis SE, Potter MJ, Sexton JP, Giardina PJV, Li A, Kazazian HHJ 1984. β-thalassemia in Chinese: Use of in-vivo RNA analysis and oligonucleotide hybridization in systematic characterization of molecular defects. Proc Natl Acad Sci 81: 2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codrington JF, Li H-W, Kutlar F, Gu L-H, Ramachandran M, Huisman THJ 1990. Observations on the levels of Hb A2 in patients with different β-thalassemia mutations and a δ chain variant. Blood 76: 1246–1249 [PubMed] [Google Scholar]
- Coleman MB, Steinberg MH, Adams JG III 1991. Hemoglobin Terre Haute arginine β106. J Biol Chem 266: 5798–5800 [PubMed] [Google Scholar]
- Craig JE, Kelly SJ, Barnetson R, Thein SL 1992. Molecular characterization of a novel 10.3 kb deletion causing β-thalassaemia with unusually high Hb A2. Br J Haematol 82: 735–744 [DOI] [PubMed] [Google Scholar]
- Çürük MA, Molchanova TP, Postnikov YV, Pobedimskaya DD, Liang R, Baysal E, Kolodey S, Smetanina NS, Tokarev YN, Rumyantsev AG, et al. 1994. β-Thalassemia alleles and unstable hemoglobin types among Russian pediatric patients. Am J Hematol 46: 329–332 [DOI] [PubMed] [Google Scholar]
- De Biasi R, Spiteri D, Caldora M, Iodice R, Pucci P, Malorni A, Ferranti P, Marino G 1988. Identification by fast atom bombardment mass spectrometry on Hb Indianapolis [b112(G14)Cys → Arg] in a family from Naples, Italy. Hemoglobin 12: 323–336 [DOI] [PubMed] [Google Scholar]
- de Castro CM, Devlin B, Fleenor DE, Lee ME, Kaufman RE 1994. A novel β-globin mutation, β Durham-NC [β114 Leu → Pro], produces a dominant thalassemia-like phenotype. Blood 83: 1109–1116 [PubMed] [Google Scholar]
- Dobkin C, Pergolizzi RG, Bahre P, Bank A 1983. Abnormal splice in a mutant β-globin gene not at the site of a mutation. Proc Natl Acad Sci 80: 1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efremov GD 2007. Dominantly inherited β-thalassemia. Hemoglobin 31: 193–207 [DOI] [PubMed] [Google Scholar]
- Faustino P, Osório-Almeida L, Romão L, Barbot J, Fernandes B, Justiça B, Lavinha J 1998. Dominantly transmitted β-thalassaemia arising from the production of several aberrant mRNA species and one abnormal peptide. Blood 91: 685–690 [PubMed] [Google Scholar]
- Fei YJ, Stoming TA, Kutlar A, Huisman TH, Stamatoyannopoulos G 1989. One form of inclusion body β-thalassemia is due to a GAA → TAA mutation at codon 121 of the β chain. Blood 73: 1075–1077 [PubMed] [Google Scholar]
- Forget BG 2001. Molecular mechanisms of β thalassemia. In Disorders of hemoglobin: Genetics, pathophysiology, and clinical management (ed. Steinberg MH, Forget BG, Higgs DR, Nagel RL), pp. 252–276 Cambridge University Press, Cambridge, UK [Google Scholar]
- Fucharoen S, Fucharoen G, Fukumaki Y, Nakayama Y, Hattori Y, Yamamoto K, Ohba Y 1990a. Three-base deletion in exon 3 of the β-globin gene produced a novel variant (β Gunma) with a thalassemia-like phenotype. Blood 76: 1894–1896 [PubMed] [Google Scholar]
- Fucharoen S, Kobayashi Y, Fucharoen G, Ohba Y, Miyazono, Fukumaki Y, Takaku F 1990b. A single nucleotide deletion in codon 123 of the β-globin gene causes an inclusion body β-thalassaemia trait: A novel elongated globin chain βMakabe. Br J Haematol 75: 393–399 [DOI] [PubMed] [Google Scholar]
- Galanello R, Melis MA, Podda A, Monne M, Persey L, Loudianos G, Cao A, Pirastu M, Piga A 1990. Deletion δ-thalassemia: The 7.2 kb deletion of Corfu δβ-thalassemia in a non-β-thalassemia chromosome. Blood 75: 1747–1749 [PubMed] [Google Scholar]
- Galanello R, Perseu L, Perra C, Maccioni L, Barella S, Longinotti M, Cao A, Cazzola M 2004. Somatic deletion of the normal β-globin gene leading to thalassaemia intermedia in heterozygous β-thalassaemic patients. Br J Haematol 127: 604–606 [DOI] [PubMed] [Google Scholar]
- Giardine B, Borg J, Higgs DR, Peterson KR, Philipsen S, Maglott D, Singleton BK, Anstee DJ, Basak AN, Clark B, et al. 2011. Systematic documentation and analysis of human genetic variation in hemoglobinopathies using the microattribution approach. Nat Genet 43: 295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JG 1987. The 12.6 kilobase DNA deletion in Dutch β0-thalassaemia. Br J Haematol 67: 369–372 [DOI] [PubMed] [Google Scholar]
- Goldsmith ME, Humphries RK, Ley T, Cline A, Kantor JA, Nienhuis AW 1983. Silent substitution in β+-thalassemia gene activating a cryptic splice site in β-globin RNA coding sequence. Proc Natl Acad Sci 80: 2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Redondo JM, Stoming TA, Kutlar A, Kutlar F, Lanclos KD, Howard EF, Fei YJ, Aksoy M, Altay C, Gurgey A, et al. 1989. A C → T substitution at nt −101 in a conserved DNA sequence of the promoter region of the β-globin gene is associated with “silent” β-thalassemia. Blood 73: 1705–1711 [PubMed] [Google Scholar]
- Grosveld F, van Assendelft GB, Breaves DR, Kollias G 1987. Position-independent, high-level expression of the human γ-globin gene in transgenic mice. Cell 51: 975–985 [DOI] [PubMed] [Google Scholar]
- Hall GW, Thein SL 1994. Nonsense codon mutations in the terminal exon of the β-globin gene are not associated with a reduction in β-mRNA accumulation: A mechanism for the phenotype of dominant β-thalassemia. Blood 83: 2031–2037 [PubMed] [Google Scholar]
- Hall GW, Franklin IM, Sura T, Thein SL 1991. A novel mutation (nonsense β127) in exon 3 of the β-globin gene produces a variable thalassaemia phenotype. Br J Haematol 79: 342–344 [DOI] [PubMed] [Google Scholar]
- Hamid M, Akbari MT 2011. A 13-bp deletion in the 3′ untranslated region of the β-globin gene causes β-thalassemia major in compound heterozygosity with IVSII-1 mutation. Med Princ Pract 20: 488–490 [DOI] [PubMed] [Google Scholar]
- Hattori Y, Yamashiro Y, Ohba Y, Miyaji T, Morishita M, Yamamoto K, Yamamoto K, Narai S, Kimura A 1991. A new β-thalassemia mutation (initiation codon ATG → GTG) found in the Japanese population. Hemoglobin 15: 317. [DOI] [PubMed] [Google Scholar]
- Ho PJ, Rochette J, Fisher CA, Wonke B, Jarvis MK, Yardumian A, Thein SL 1996. Moderate reduction of β globin gene transcript by a novel mutation in the 5′ untranslated region: A study of its interaction with other genotypes in two families. Blood 87: 1170–1178 [PubMed] [Google Scholar]
- Ho PJ, Wickramasinghe SN, Rees DC, Lee MJ, Eden A, Thein SL 1997. Erythroblastic inclusions in dominantly inherited β thalassaemias. Blood 89: 322–328 [PubMed] [Google Scholar]
- Huang S-C, Benz EJJ 2001. Posttranscriptional factors influencing the hemoglobin content of the red cells. In Disorders of hemoglobin: Genetics, pathophysiology, and clinical management (ed. Steinberg MH, Forget BG, Higgs DR, Nagel RL), pp. 252–276 Cambridge University Press, Cambridge, UK [Google Scholar]
- Huang S-Z, Wong C, Antonarakis SE, Ro-Lein T, Lo WHY, Kazazian HHJ 1986. The same TATA box β-thalassemia mutation in Chinese and US Blacks: Another example of independent origins of mutation. Hum Genet 74: 162–164 [DOI] [PubMed] [Google Scholar]
- Huisman THJ 1997. Levels of Hb A2 in heterozygotes and homozygotes for β-thalassemia mutations: Influence of mutations in the CACCC and ATAAA motifs of the β-globin gene promoter. Acta Haematol 98: 187–194 [DOI] [PubMed] [Google Scholar]
- Humphries RK, Ley TJ, Anagnou NP, Baur AW, Nienhuis AW 1984. β0-39 thalassemia gene: A premature termination codon causes β-mRNA deficiency without affecting cytoplasmic β-mRNA stability. Blood 64: 23–32 [PubMed] [Google Scholar]
- Isken O, Maquat LE 2007. Quality control of eukaryotic mRNA: Safeguarding cells from abnormal mRNA function. Genes Dev 21: 1833–1856 [DOI] [PubMed] [Google Scholar]
- Jankovic L, Efremov GD, Josifovska O, Juricic D, Stoming TA, Kutlar A, Huisman THJ 1990. An initiation codon mutation as a cause of a β0-thalassemia. Hemoglobin 14: 169. [DOI] [PubMed] [Google Scholar]
- Kazazian HHJ, Orkin SH, Boehm CD, Goff SC, Wong C, Dowling CE, Newburger PE, Knowlton RG, Brown V, Donis-Keller H 1986. Characterisation of a spontaneous mutation to a β-thalassaemia allele. Am J Human Genet 38: 860–867 [PMC free article] [PubMed] [Google Scholar]
- Kazazian HHJ, Dowling CE, Hurwitz RL, Coleman M, Stopeck A, Adams JGI 1992. Dominant thalassemia-like phenotypes associated with mutations in exon 3 of the β-globin gene. Blood 79: 3014–3018 [PubMed] [Google Scholar]
- Kimberland ML, Divoky V, Prchal J, Schwahn U, Berger W, Kazazian HH Jr 1999. Full-length human L1 insertions retain the capacity for high frequency retrotransposition in cultured cells. Hum Mol Genet 8: 1557–1560 [DOI] [PubMed] [Google Scholar]
- Kioussis D, Vanin E, de Lange T, Flavell RA, Grosveld FG 1983. β-globin gene inactivation by DNA translocation in γβ-thalassaemia. Nature 306: 662–666 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Fukumaki Y, Komatsu N, Ohba Y, Miyaji T, Miura Y 1987. A novel globin structural mutant, Showa-Yakushiji (β110 Leu-Pro) causing a β-thalassemia phenotype. Blood 70: 1688–1691 [PubMed] [Google Scholar]
- Kulozik A, Yarwood N, Jones RW 1988. The Corfu δβ0 thalassemia: A small deletion acts at a distance to selectively abolish β globin gene expression. Blood 71: 457–462 [PubMed] [Google Scholar]
- Lam VMS, Xie SS, Tam JWO, Woo YK, Gu YL, Li AMC 1990. A new single nucleotide change at the initiation codon (ATG → AGG) identified in amplified genomic DNA of a Chinese β-thalassemic patient. Blood 75: 1207–1208 [PubMed] [Google Scholar]
- Li DZ, Liao C, Li J 2009. A novel β-thalassemic allele due to a thirteen nucleotide deletion: Codons 54–58 (-T ATG GGC AAC CCT). Ann Hematol 88: 799–801 [DOI] [PubMed] [Google Scholar]
- Maragoudaki E, Kanavakis E, Trager-Synodinos J, Vrettou C, Tzetis M, Metxotou-Mavrommati A, Kattamis C 1999. Molecular, haematological and clinical studies of the −101 C → T substitution in the β-globin gene promoter in 25 β-thalassaemia intermedia patients and 45 heterozygotes. Br J Haematol 107: 699–706 [DOI] [PubMed] [Google Scholar]
- Moi P, Faa V, Marini MG, Asunis I, Ibba G, Cao A, Rosatelli MC 2004. A novel silent β-thalassemia mutation in the distal CACCC box affects the binding and responsiveness to EKLF. Br J Haematol 126: 881–884 [DOI] [PubMed] [Google Scholar]
- Metherall JE, Collins FS, Pan J, Weissman SM, Forget BG 1986. β0 thalassemia caused by a base substitution that creates an alternative splice acceptor site in an intron. EMBO J 5: 2551–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murru S, Loudianos G, Deiana M, Camaschella C, Sciarratta GV, Agosti S, Parodi MI, Cerruti P, Cao A, Pirastu M 1991. Molecular characterization of β-thalassemia intermedia in patients of Italian descent and identification of three novel β-thalassemia mutations. Blood 77: 1342–1347 [PubMed] [Google Scholar]
- Murru S, Loudianos G, Porcu S, Sciarratta GV, Agosti S, Parodi MI, Cao A, Pirastu M 1992. A β-thalassaemia phenotype not linked to the β-globin cluster in an Italian family. Br J Haematol 81: 283–287 [DOI] [PubMed] [Google Scholar]
- Nagy E, Maquat LE 1998. A rule for termination-codon position within intron-containing genes: When nonsense affects RNA abundance. Trends Biochem Sci 23: 198–199 [DOI] [PubMed] [Google Scholar]
- Ohba Y, Hattori Y, Harano T, Harano K, Fukumaki Y, Ideguchi H 1997. β-Thalassemia mutations in Japanese and Koreans. Hemoglobin 21: 191–200 [DOI] [PubMed] [Google Scholar]
- Olds RJ, Sura T, Jackson B, Wonke B, Hoffbrand AV, Thein SL 1991. A novel δ0 mutation in cis with Hb Knossos: A study of different genetic interactions in three Egyptian families. Br J Haematol 78: 430–436 [DOI] [PubMed] [Google Scholar]
- Öner R, Öner C, Wilson JB, Tamagnini GP, Ribeiro LML, Huisman THJ 1991. Dominant β-thalassaemia trait in a Portuguese family is caused by a deletion of (G) TGGCTGGTGT(G) and an insertion of (G)GCAG(G) in codons 134, 135, 136 and 137 of the β-globin gene. Br J Haematol 79: 306. [DOI] [PubMed] [Google Scholar]
- Orkin SH, Kazazian HHJ, Antonarakis SE, Goff SC, Boehm CD, Sexton JP, Waber PG, Giardina PJV 1982a. Linkage of β-thalassaemia mutations and β-globin gene polymorphisms with DNA polymorphisms in human β-globin gene cluster. Nature 296: 627–631 [DOI] [PubMed] [Google Scholar]
- Orkin SH, Kazazian HHJ, Antonarakis SE, Ostrer H, Goff SC, Sexton JP 1982b. Abnormal RNA processing due to the exon mutation of βE-globin gene. Nature 300: 768–769 [DOI] [PubMed] [Google Scholar]
- Orkin S, Antonarakis S, Loukopoulos D 1984. Abnormal processing of βKnossos RNA. Blood 64: 311–313 [PubMed] [Google Scholar]
- Orkin SH, Cheng T-C, Antonarakis SE, Kazazian HHJ 1985. Thalassemia due to a mutation in the cleavage-polyadenylation signal of the human β-globin gene. EMBO J 4: 453–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco P, Peres MJ, Faustino P, Pischedda C, Gonçalves J, Carvajales-Ramos M, Seixas T, Martins MC, Moi P, Lavinha J 1995. β-thalassaemia unlinked to the β-globin gene interacts with sickle-cell trait in a Portuguese family. Br J Haematol 91: 85–89 [DOI] [PubMed] [Google Scholar]
- Park SS, Barnetson R, Kim SW, Weatherall DJ, Thein SL 1991. A spontaneous deletion of b33/34 Val in exon 2 of the b globin gene (Hb Korea) produces the phenotype of dominant β thalassaemia. Br J Haematol 78: 581. [DOI] [PubMed] [Google Scholar]
- Perseu L, Satta S, Moi P, Demartis FR, Manunza L, Sollaino MC, Barella S, Cao A, Galanello R 2011. KLF1 gene mutations cause borderline HbA2. Blood 118: 4454–4458 [DOI] [PubMed] [Google Scholar]
- Podda A, Galanello R, Maccioni L, Melis MA, Rosatelli C, Perseu L, Cao A 1991. Hemoglobin Cagliari (β60[E4] VAL → Glu): A novel unstable thalassemic hemoglobinopathy. Blood 77: 371–375 [PubMed] [Google Scholar]
- Prehu C, Pissard S, Al-Sheikh M, Le Niger C, Bachir D, Galacteros F, Wajcman H 2005. Two French Caucasian families with dominant thalassemia-like phenotypes due to hyper unstable hemoglobin variants: Hb Sainte Seve [codon 118 (-T)] and codon 127 [CAG → TAG (Gln → stop]). Hemoglobin 29: 229–233 [DOI] [PubMed] [Google Scholar]
- Ristaldi MS, Pirastu M, Murru S, Casula L, Loudianos G, Cao A, Sciarratta GV, Agosti S, Parodi MI, Leone D, et al. 1990. A spontaneous mutation produced a novel elongated β-globin chain structural variant (Hb Agnana) with a thalassemia-like phenotype. Blood 75: 1378–1380 [PubMed] [Google Scholar]
- Ristaldi MS, Casula S, Porcu S, Cao A 2001. Normal δ globin gene sequence in carrier of the silent-101 (C-T) β-thalassemia mutation with normal HbA2 level. Am J Hematol 67: 58. [DOI] [PubMed] [Google Scholar]
- Romao L, Inacio A, Santos S, Avila M, Faustino P, Pacheco P, Lavinha J 2000. Nonsense mutations in the human β-globin gene lead to unexpected levels of cytoplasmic mRNA accumulation. Blood 96: 2895–2901 [PubMed] [Google Scholar]
- Rooks H, Clark B, Best S, Rushton P, Oakley M, Thein OS, Cuthbert AC, Britland A, Ruf A, Thein SL 2012. A novel 506 kb deletion causing εγδβ thalassemia. Blood Cells Mol Dis 49: 121–127 [DOI] [PubMed] [Google Scholar]
- Rund D, Dowling C, Najjar K, Rachmilewitz EA, Kazazian HH Jr, Oppenheim A 1992. Two mutations in the β-globin polyadenylylation signal reveal extended transcripts and new RNA polyadenylylation sites. Proc Natl Acad Sci 89: 4324–4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rund D, Oron-Karni V, Filon D, Goldfarb A, Rachmilewitz E, Oppenheim A 1997. Genetic analysis of β-thalassaemia intermedia in Israel: Diversity of mechanisms and unpredictability of phenotype. Am J Hematol 54: 16–22 [DOI] [PubMed] [Google Scholar]
- Saba L, Meloni A, Sardu R, Travi M, Primignani P, Rosatelli MC, Cao A 1992. A novel β-thalassemia mutation (G → A) at the initiation codon of the β-globin gene. Human Mutation 1: 420–422 [DOI] [PubMed] [Google Scholar]
- Safaya S, Rieder RF, Dowling CE, Kazazian HHJ, Adams JGI 1989. Homozygous β-thalassemia without anemia. Blood 73: 324–328 [PubMed] [Google Scholar]
- Schoenberg DR, Maquat LE 2012. Regulation of cytoplasmic mRNA decay. Nat Rev Genet 13: 246–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schokker RC, Went LN, Bok J 1966. A new genetic variant of β-thalassaemia. Nature 209: 44–46 [DOI] [PubMed] [Google Scholar]
- Spritz RA, Jagadeeswaran P, Choudary PV, Biro PA, Elder JT, deRiel JK, Manley JL, Forget BG, Weissman SM 1981. Base substitution in an intervening sequence of a β+-thalassemic human globin gene. Proc Natl Acad Sci 78: 2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatoyannopoulos G, Woodson R, Papayannopoulou T, Heywood D, Kurachi MS 1974. Inclusion-body β-thalassemia trait. A form of β thalassemia producing clinical manifestation in simple heterozygotes. N Engl J Med 290: 939–943 [DOI] [PubMed] [Google Scholar]
- Swensen JJ, Agarwal AM, Esquilin JM, Swierczek S, Perumbeti A, Hussey D, Joiner CH, Pont-Kingdon G, Lyon E, Prchal JT 2010. Sickle cell disease due to uniparental disomy in a child who inherited sickle cell trait. Blood 116: 2822–2825 [DOI] [PubMed] [Google Scholar]
- Takeshita K, Forget BG, Scarpa A, Benz EJ Jr 1984. Intranuclear defect in β-globin mRNA accumulation due to a premature translation termination codon. Blood 64: 13–22 [PubMed] [Google Scholar]
- Thein SL 1998. β thalassaemia. In Baillière's clinical haematology, sickle cell disease and thalassaemia (ed. Rodgers GP), pp. 91–126 Baillière Tindall, London [Google Scholar]
- Thein SL 1999. Is it dominantly inherited β thalassaemia or just a β-chain variant that is highly unstable? Br J Haematol 107: 12–21 [DOI] [PubMed] [Google Scholar]
- Thein SL 2001. Structural Variants with a β-Thalassemia Phenotype. In Disorders of hemoglobin: Genetics, pathophysiology, and clinical management (ed. Steinberg MH, Forget BG, Higgs DR, Nagel RL), pp. 342–355 Cambridge University Press, Cambridge, UK [Google Scholar]
- Thein SL 2005. Pathophysiology of β thalassemia—A guide to molecular therapies. Hematology Am Soc Hematol Educ Program 31–37 [DOI] [PubMed] [Google Scholar]
- Thein SL, Wood WG 2009. The molecular basis of β thalassemia, δβ thalassemia, and hereditary persistence of fetal hemoglobin. In Disorders of hemoglobin: Genetics, pathophysiology, and clinical management (ed. Steinberg MH, Forget BG, Higgs DR, Nagel RL), pp. 323–356 Cambridge University Press, Cambridge, UK [Google Scholar]
- Thein SL, Old JM, Wainscoat JS, Petrou M, Modell B, Weatherall DJ 1984. Population and genetic studies suggest a single origin for the Indian deletion β0 thalassaemia. Br J Haematol 57: 271–278 [DOI] [PubMed] [Google Scholar]
- Thein SL, Hesketh C, Taylor P, Temperley IJ, Hutchinson RM, Old JM, Wood WG, Clegg JB, Weatherall DJ 1990. Molecular basis for dominantly inherited inclusion body β-thalassemia. Proc Natl Acad Sci 87: 3924–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein SL, Best S, Sharpe J, Paul B, Clark DJ, Brown MJ 1991. Hemoglobin Chesterfield (β28 Leu → Arg) produces the phenotype of inclusion body β thalassemia. Blood 77: 2791–2793 [PubMed] [Google Scholar]
- Thein SL, Wood WG, Wickramasinghe SN, Galvin MC 1993. β-Thalassemia unlinked to the β-globin gene in an English family. Blood 82: 961–967 [PubMed] [Google Scholar]
- Traeger-Synodinos J, Tzetis M, Kanavakis E, Metaxotou-Mavromati A, Kattamis C 1991. The Corfu δβ thalassaemia mutation in Greece: Haematological phenotype and prevalence. Br J Haematol 79: 302–305 [DOI] [PubMed] [Google Scholar]
- Treisman R, Orkin SH, Maniatis T 1983. Specific transcription and RNA splicing defects in five cloned β-thalassaemia genes. Nature 302: 591–596 [DOI] [PubMed] [Google Scholar]
- Varawalla NY, Old JM, Weatherall DJ 1991. Rare β-thalassaemia mutations in Asian Indians. Br J Haematol 79: 640–644 [DOI] [PubMed] [Google Scholar]
- Verhovsek M, Shah NR, Wilcox I, Koenig SC, Barros T, Thornburg CD, Steinberg MH, Luo HY, Chui DH 2012. Severe fetal and neonatal hemolytic anemia due to a 198 kb deletion removing the complete β-globin gene cluster. Pediatr Blood Cancer 59: 941–944 [DOI] [PubMed] [Google Scholar]
- Viprakasit V, Gibbons RJ, Broughton BC, Tolmie JL, Brown D, Lunt P, Winter RM, Marinoni S, Stefanini M, Brueton L, et al. 2001. Mutations in the general transcription factor TFIIH result in β-thalassaemia in individuals with trichothiodystrophy. Hum Mol Genet 10: 2797–2802 [DOI] [PubMed] [Google Scholar]
- Wainscoat JS, Thein SL, Wood WG, Weatherall DJ, Tzotos S, Kanavakis E, Metaxatou-Mavromati A, Kattamis C 1985. A novel deletion in the b globin gene complex. Ann NY Acad Sci 445: 20–27 [DOI] [PubMed] [Google Scholar]
- Wajcman H, Lahary A, Prome D, Kister J, Riou J, Godart C, Prehu C, Traeger-Synodinos J, Papassotiriou I, Galacteros F 2001. Hb Mont Saint Aignan [β128(H6)Ala → Pro]: A new unstable variant leading to chronic microcytic anemia. Hemoglobin 25: 57–65 [DOI] [PubMed] [Google Scholar]
- Weatherall DJ, Clegg JB 2001. The thalassaemia syndromes. Blackwell Science, Oxford [Google Scholar]
- Weatherall DJ, Clegg JB, Knox-Macaulay HHM, Bunch C, Hopkins CR, Temperley IJ 1973. A genetically determined disorder with features both of thalassaemia and congenital dyserythropoietic anaemia. Br J Haematol 24: 681–702 [DOI] [PubMed] [Google Scholar]
- Westaway D, Williamson R 1981. An intron nucleotide sequence variant in a cloned β+-thalassemia globin gene. Nucl Acid Res 9: 1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Dowling CE, Saiki RK, Higuchi RG, Erlich HA, Kazazian HHJ 1987. Characterization of β-thalassaemia mutations using direct genomic sequencing of amplified single copy DNA. Nature 330: 384–386 [DOI] [PubMed] [Google Scholar]
- Wong SC, Stoning TA, Efremov GD, Huisman THJ 1989. High frequencies of a rearrangement (+ATA; −T) at −530 to the β-globin gene in different populations indicate the absence of a correlation with a silent β-thalassemia determinant. Hemoglobin 13: 1–5 [DOI] [PubMed] [Google Scholar]
- Yang KG, Kutlar F, George E, Wilson JB, Kutlar A, Stoming TA, Gonzalez-Redondo JM, Huisman THJ 1989. Molecular characterization of β-globin gene mutations in Malay patients with Hb E-β-thalassaemia and thalassaemia major. Br J Haematol 72: 73–80 [DOI] [PubMed] [Google Scholar]
- Yu C, Niakan KK, Matsushita M, Stamatoyannopoulos G, Orkin SH, Raskind WH 2002. X-linked thrombocytopenia with thalassemia from a mutation in the amino finger of GATA-1 affecting DNA binding rather than FOG-1 interaction. Blood 100: 2040–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]