Abstract
Background and Purpose
This study evaluated the clinical value of detachable-balloon embolization for traumatic carotid-cavernous fistula (TCCF), focusing on the frequency, risk factors, and retreatment of recurrence.
Methods
Fifty-eight patients with TCCF underwent transarterial detachable-balloon embolization between October 2004 and March 2011. The clinical follow-up was performed every 3 months until up to 3 years postprocedure. Each patient was placed in either the recurrence group or the nonrecurrence group according to whether a recurrence developed after the first procedure. The relevant factors including gender, fistula location, interval between trauma and the interventional procedure, blood flow in the carotid-cavernous fistula, number of balloons, and whether the internal carotid artery (ICA) was sacrificed were evaluated.
Results
All 58 TCCFs were successfully treated with transarterial balloon embolization, including 7 patients with ICA sacrifice. Recurrent fistulas occurred in seven patients during the follow-up period. Univariate analysis indicated that the interval between trauma and the interventional procedure (p=0.006) might be the main factor related to the recurrence of TCCF. The second treatments involved ICA sacrifice in two patients, fistula embolization with balloons in four patients, and placement of a covered stent in one patient.
Conclusions
Detachable balloons can still serve as the first-line treatment for TCCFs and recurrent TCCFs despite having a nonnegligible recurrence rate. Shortening the interval between trauma and the interventional procedure may reduce the risk of recurrence.
Keywords: traumatic carotid-cavernous fistula, detachable balloons, interventional procedure, recurrence
Introduction
Most traumatic carotid-cavernous fistulas (TCCFs) are abnormal shunts with a direct communication between the internal carotid artery (ICA) and the cavernous sinus, and are categorized as Barrow Type A based on the arterial supply.1,2 The classic manifestations of TCCFs include orbital bruit, conjunctival hyperemia, chemosis, proptosis, decreased visual acuity, and ocular motor palsy, occasionally combined with cerebral complications or recurrent epistaxis. The condition may be life-threatening if timely treatment is not employed.
Endovascular treatments with various embolization materials such as detachable balloons, microcoils, liquid adhesive, or covered stents are used to occlude TCCFs.3-8 Detachable balloons still represent an important therapeutic option due to their efficacy, safety, procedure simplicity, and cost-effectiveness (the last factor is especially important in some developing countries). However, there have been few systematic evaluations of risk factors for a recurrent TCCF (RTCCF) previously treated using detachable-balloon embolization.
In this study we retrospectively evaluated the clinical effectiveness of detachable balloons in the treatment of TCCFs at our institute. The frequency, risk factors, and retreatment rates of RTCCFs were also analyzed.
Methods
Patient group
The study protocol was approved by the ethics committee of the Institutional Review Board of the First Affiliated Hospital of Nanjing Medical University. All of the patients provided written informed consent for endovascular procedures and related clinical data collection before study entry. We retrospectively collected data from 58 TCCF patients who were treated by transarterial-balloon embolization at our institute between October 2004 and March 2011. The 58 cases consisted of 43 men and 15 women with a mean age of 43 years old (range, 15-68 years), all of whom had a history of trauma. The most common manifestations were orbital bruit (n=52), conjunctival hyperemia and chemosis (n=49), proptosis (n=48), decreased or lost visual acuity (n=21), and ptosis, ocular motor palsy, or recurrent epistaxis. The interval between trauma and the interventional procedure ranged from 11 to 170 days in 57 patients (mean, 48 days). The 58th patient represented an outlier, having received the procedure 12 years after the onset of the symptoms.
Procedure
All procedures were performed under local anesthesia via a femoral approach. All patients routinely received six-vessel cerebral angiography and a temporary-balloon-occlusion test in order to obtain precise information about the fistula, such as the flow pattern, venous drainage, and hemodynamic effects of the TCCFs, and to assess the extent of collateral blood flow and the compensatory ability of the circle of Willis before the initial treatment. Systemic heparinization was achieved by administering an intravenous bolus of heparin according to the patient's weight (approximately 0.6 mg/kg), with maintenance achieved by the continuous infusion of heparinized normal saline under pressure. The activated clotting time was maintained at a value that was approximately twice the baseline value. After the guiding catheters (8F) were placed in the ICA, the balloon-mounted microcatheter (Balt Medical Corporation, Somerset, NJ, France) was negotiated slowly into the fistula under flow guidance. In most cases the balloon (Balt Medical Corporation, Somerset, NJ, France) had to be partially inflated in order to achieve access to the fistula orifice. The balloon was inflated using standard hypertonic, water-soluble contrast material (Omnipaque 300, GE Healthcare, Shanghai, China). The location of the balloon was confirmed and the targeted fistula was characterized by injecting contrast agent through the guiding catheter. Once the balloon had been placed in the desired position, it was gradually inflated. Post-embolization cerebral angiography was performed to demonstrate whether the fistula had been completely obliterated. Occasionally it was necessary to reposition the balloon so as to fully close the fistula. Finally, the balloon was detached. In some cases more than one balloon was used to occlude large compartments of the cavernous sinus.
If the above-mentioned procedure could not be implemented, the technique of ICA sacrifice was employed. After the first balloon was used to occlude the arterial segment bearing the fistula, a second balloon was placed proximal to the fistula to reduce the hemodynamic scouring effect. All patients were kept on absolute bed rest for up to 24 hours after the procedure, with head movement in particularly being restricted.
Follow-up
Patients were generally followed by clinical evaluations in the outpatient clinic or by phone calls every 3 months until up to 3 years after the procedure. The follow-up lasted 3 years in 39 of the 58 patients and the follow-up has lasted 6 month in the rest patient. During the follow-up period, angiography was not performed unless the symptoms or signs recurred.
Group division and statistical analysis
Each of the 58 patients was placed in either the recurrence group or the nonrecurrence group according to whether a recurrence developed after the first procedure. The relevant factors that were evaluated included gender, fistula location, whether the ICA was sacrificed, blood flow in the TCCF, the number of detachable balloons used, and the interval between trauma and the interventional procedure. The fistula location was classified into the ascending cavernous segment and the horizontal cavernous segment according to the ICA segmentation described by Lasjaunias and Santoyo-Vazquez.9
The blood flow in the TCCF was classified into three categories as described by Van Rooij et al.10 high, intermediate, and low. In high-flow carotid-cavernous fistulas (CCFs) all of the blood from the ICA entered the fistula without filling intracranial vessels, in intermediate-flow CCFs both the fistula and intracranial vessels received blood from the ICA, while in the low-flow CCFs only slow and sluggish filling of the cavernous sinus was apparent.
The seven cases in which the ICA was sacrificed were excluded when evaluating the blood flow in the CCF, number of balloons, and the interval between trauma and intervention; for the last factor the single recurrence case with an extremely long interval (12 years) was also excluded. The quantitative data were analyzed with a t test, and the enumeration data were analyzed with a Fisher's exact test. The significance level was defined as p<0.05. All calculations were performed with SPSS (version 17.0, Chicago, IL, USA).
Results
Angiography results of TCCFs
The general angiography data are summarized in Table 1.
Table 1.
Angiography data of all 58 patients
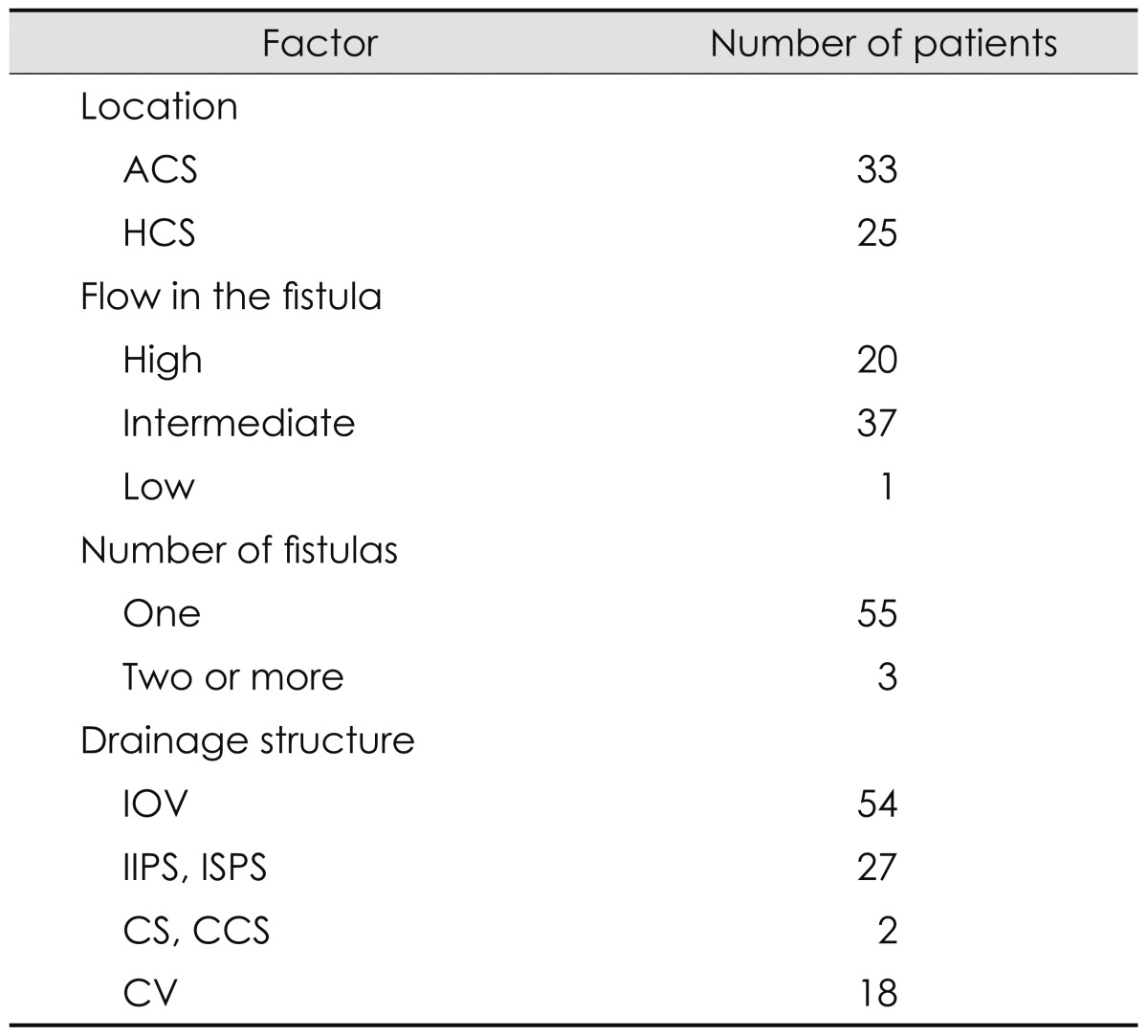
ACS: ascending cavernous segment, CCS: contralateral cavernous sinus, CS: circular sinus, CV: cortical vein, HCS: horizontal cavernous segment, IIPS: ipsilateral inferior petrosal sinus, IOV: ipsilateral ophthalmic vein, ISPS: ipsilateral superior petrosal sinus.
Detachable-balloon embolization results
All 58 patients were successfully treated using between 1 and 7 balloons (Fig. 1). The ipsilateral ICA was sacrificed in seven patients for the following reasons: the balloon could not pass the small fistula in two patients, five balloons ruptured during inflation in one patient, an enlarged cavernous sinus could not be completely occluded with multiple balloons in three patients (Fig. 2), and the ipsilateral ICA was obliterated distal to the fistula point in the seventh patient (Fig. 3). Symptoms related to the fistulas improved gradually after the procedure in all patients. Most of the patients suffered from endurable headache. No patients experienced severe complications related to the procedure such as hyperperfusion or premature detachment of the balloon. The patients were discharged at a mean of 4.8 days (range, 2-7 days) after the detachable-balloon embolization.
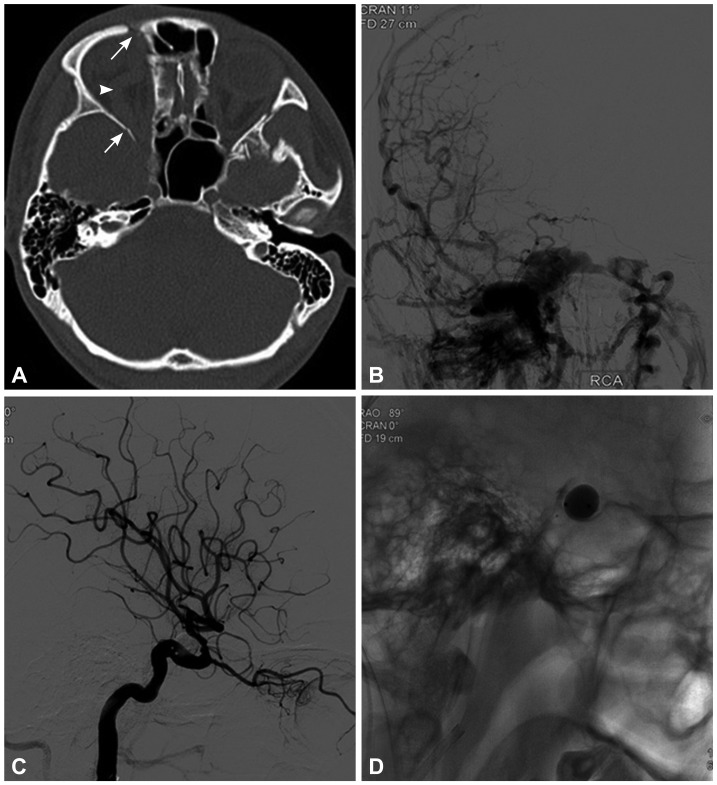
Fig. 1.
A 23-year-old man who presented with right-sided proptosis, chemosis, orbital bruit, and decreased visual acuity that occurred 7 days after head blunt trauma. A: Computed tomography indicated right-sided proptosis (arrowhead), superior ophthalmic vein enlargement, and multiple orbital fractures (arrows). B: Cerebral angiography confirmed a high-flow direct carotid-cavernous fistulas that drained into the superior ophthalmic vein, inferior petrosal sinus, intercavernous sinus, and contralateral cavernous sinuses with cortical reflux. C and D: Immediate angiography after single-balloon embolization showed occlusion of the fistula and preservation of the internal carotid artery.
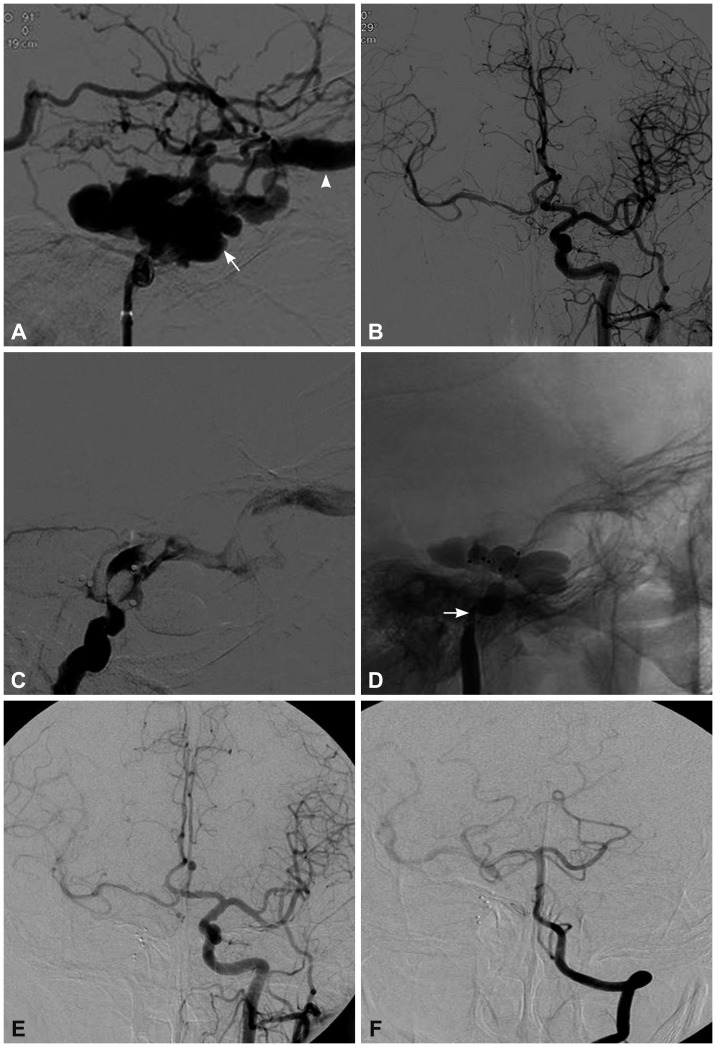
Fig. 2.
A 50-year-old man who presented with right-sided chemosis, proptosis, orbital bruit, and lost visual acuity that occurred 61 days after head blunt trauma. A: Lateral cerebral angiography showed a high-flow TCCF that mainly drained into the ophthalmic vein (arrow-head), and (B) contralateral cerebral angiography confirmed good hemodynamic compensation via the communicating artery. C: Angiography showed a residual fistula after embolizations using five balloons, of which two ballons were punctured during the procedure, and hence the ICA was sacrificed (arrow) (D). E and F: Cerebral angiography at the 2-month follow-up showed good compensation via the circle of Willis; the fistula had completely disappeared.
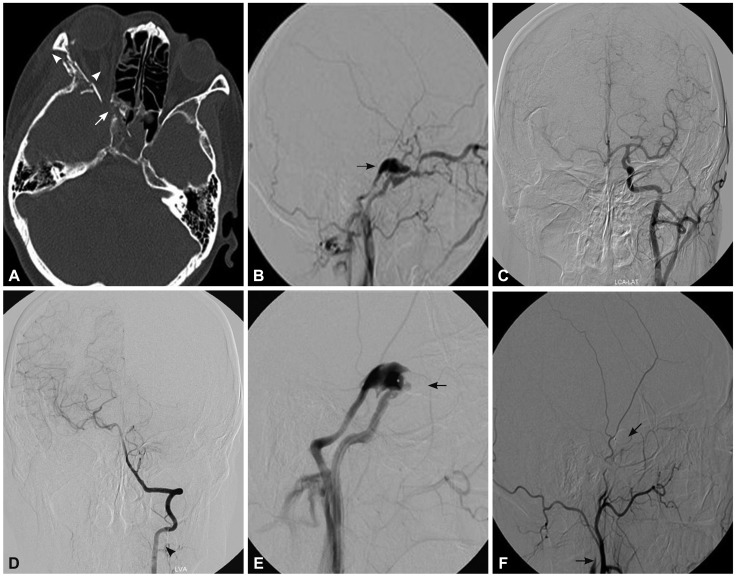
Fig. 3.
A 42-year-old woman who presented with right-sided proptosis, chemosis, orbital bruit, and headache 35 days after a vehicle accident. A: Computed tomography indicated right-sided proptosis, superior ophthalmic vein enlargement (arrowhead), and multiple orbital fractures (arrow). B: Cerebral angiography confirmed a high-flow direct CCF that drained into the superior ophthalmic vein and inferior petrosal sinus with cortical reflux (arrow). C and D: Cerebral angiography of the contralateral ICA confirmed good compensation of the circle of Willis. E: Cerebral angiography of the ipsilateral ICA after two-balloon embolization confirmed partial occlusion of the fistula and absence of the distal ICA (arrow), which prompted a diagnosis of obliteration of the ipsilateral ICA distal to the fistula point. F: The CCF was successfully treated after subsequent occlusion of the proximal ICA with a detachable balloon (arrow). CCF: carotid-cavernous fistula, ICA: internal carotid artery.
Symptoms such as orbital bruit were relieved immediately after the first procedure in all 58 patients, and symptoms such as chemosis or proptosis were relieved at 4-40 days after the procedure. However, some symptoms, such as lost visual acuity, were not relieved.
Recurrence of CCF
Seven of the 58 patients (12.1%) presented with recurrence of CCF during the follow-up. One balloon was used during the first procedure in all seven patients with RTCCF. The interval between the first procedure and recurrence ranged from 3 to 40 days (mean, 9.4 days). The seven patients received the second interventional procedure at between 2 and 7 days after symptomatic recurrence. Fluoroscopy during the second procedure showed that balloon deflation occurred in six patients (three of whom also had balloon migration), while only the metal marker was visible in the original position without contrast medium in the seventh patient. Four patients were treated successfully using a second detachable-balloon embolization with preservation of the ICA (Fig. 4). A covered stent was implanted in another hospital in one patient. The ICA was sacrificed in one patient because the shrunken balloon partially blocked the orifice of the fistula, which prevented a new balloon from being successfully navigated to the cavernous sinus. In the last patient, due to the venous route not being available, a procedure involving transarterial coils and Onyx embolization was planned. However, after two coils were placed in the cavernous sinus, the protected-balloon-mounted microcatheter could not be navigated distal to the ipsilateral ICA, so the ICA was sacrificed with coils. Symptoms related to the RTCCF improved gradually after the procedure in all seven patients, and there was no recurrence during the follow-up.
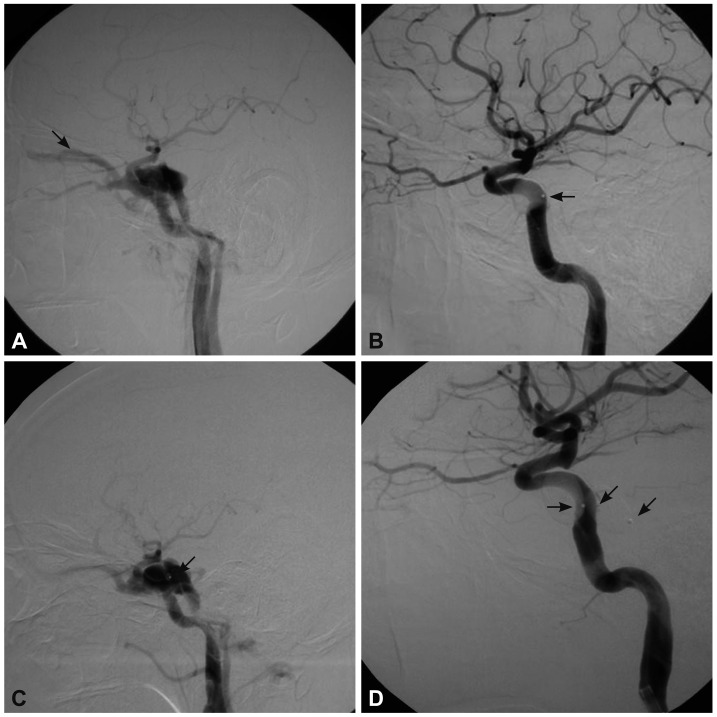
Fig. 4.
A 43-year-old man who presented with left-sided proptosis, chemosis, diplopia, and orbital bruit 70 days after a vehicle accident. A: Cerebral angiography confirmed an intermediate-flow direct CCF that drained into the superior ophthalmic vein (arrow). B: Immediate angiography after a single-balloon embolization showed occlusion of the fistula and preservation of the ICA (arrow). C: The patient developed a recurrent fistula on day 7 after the initial embolization, which was attributed to premature deflation and migration of the balloon (arrows) (D). Two detachable balloons were subsequently deployed, which resulted in successful treatment of the RTCCF (arrow). CCF: carotid-cavernous fistula, ICA: internal carotid artery, RTCCF: recurrent traumatic CCF.
Risk factors analysis
The relevant factors of all 58 patients are summarized in Table 2. Univariate analysis indicated that relevant factors including sex, fistula location, blood flow in the CCF, and the number of balloons may not be the main factors related to the recurrence of TCCFs, while the interval between trauma and the interventional procedure was a statistically significant factor (t=8.343, p=0.006).
Table 2.
Factors relevant to recurrence of a traumatic CCF managed with detachable balloons
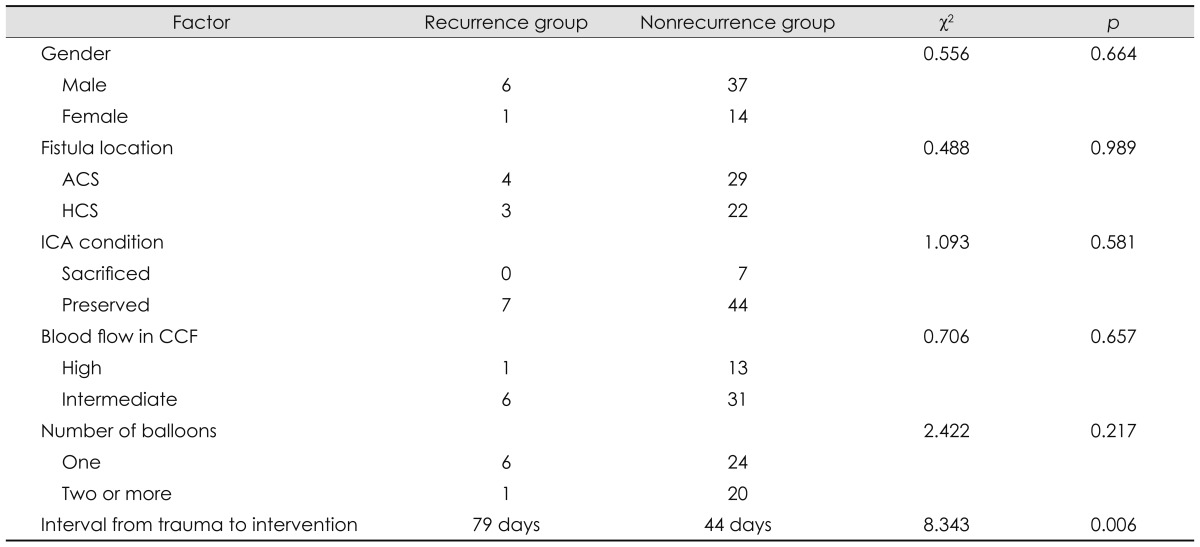
The seven cases in which the ICA was sacrificed were excluded when evaluating the blood flow in the CCF, number of balloons, and the interval between trauma and intervention; for the last factor the single case with an extremely long interval was also excluded. ACS: ascending cavernous segment, CCF: carotid-cavernous fistula, HCS: horizontal cavernous segment, ICA: internal carotid artery.
Discussion
TCCFs are rare complications that occur in 0.2-0.3% of craniomaxillofacial injuries.11 The goal in treating TCCFs is to occlude the site of communication between the ICA and the cavernous sinus while preserving the patency of the ICA.12 Nowadays such fistulas are occluded using various endovascular materials, including detachable balloons, microcoils, liquid adhesive, and covered stents. Detachable balloons still represent an important therapeutic option due to their efficacy, safety, procedural simplicity, and cost-effectiveness. In our series, the mean expenditure during the first procedure was US$ 2211 (range, US$ 1428-3264) and the mean length of stay was 4.8 days (range, 2-9 days), which indicates that detachable-balloon embolization is both relatively inexpensive and provides a speedy recovery.
Some disadvantages of detachable-balloon embolization remain to be resolved. The main disadvantage is the rate of ICA sacrifice; this was 12.1% in our 58 cases. The size of the fistula crucially affects the likelihood of successful occlusion of the fistula when using detachable balloons. Ideally the fistula should be larger than the deflated balloon,5 since a smaller fistula may affect the passage of the balloon and result in an unsuccessful procedure. The volume of the cavernous sinus is also very important. The presence of a markedly enlarged cavernous sinus makes it difficult to completely occlude the fistula, which may result in residual blood flow. In these situations the ICA may have to be sacrificed. However, continuing developments in embolization materials and techniques may lead to ICA sacrifice being unnecessary in the future.
Another disadvantage of detachable-balloon embolization for treatment of TCCFs is recurrence; the rate of this was also 12.1% in our 58 cases. Clarifying the risk factors is very important for the effective application of detachable balloons. Similar to the report of Lue et al.,13 the interval between trauma and the interventional procedure was markedly longer in our recurrence group than in the nonrecurrence group. An increasing interval decreases the stability of detachable balloons in an enlarged cavernous sinus, thus increasing the likelihood of recurrence. Therefore, based on our findings we suggest that the TCCF should be treated as quickly as possible in order to reduce the risk of recurrence.
Transarterial detachable-balloon embolization is still the first-line curative therapy for the retreatment of RTCCFs. However, the navigation of additional balloons may be difficult due to the presence of a deflated or migrated balloon partially blocking the orifice of the fistula or changing the structure of cavernous sinus, which might require the ICA to be sacrificed using detachable balloons during the retreatment procedure.
Due to their good manageability and the greater familiarity to neurointerventionalists, coils are nowadays more commonly applied in the treatment of TCCFs, especially for small fistulas with diameters of 2-3 mm.11,12,14 However, many coils would be needed when they are used alone for TCCFs with an enlarged cavernous sinus, thus making the procedure extremely expensive and increasing the probability of cranial nerve compression symptoms. Balloon-assisted or stent-assisted liquid adhesive embolization with or without coiling could be an effective treatment option.5,15 Balloons or coils slow the flow patterns in the cavernous sinus, serve as a scaffold for polymerization of the liquid adhesive, and form a physical barrier preventing further movement of the liquid adhesive. Covered stents have recently been considered a promising treatment choice of TCCFs, particularly for recurrent, residual, and multiple fistulas, and fistulas combined with pseudoaneurysms or dissections.11,16,17 However, the treatment limitations when using a covered stent in TCCF patients include the stiffness of the stent, the difficulty of navigation, and the absence of unified perioperative medication. Therefore, long-term follow-up and large-scale studies are warranted to clarify the long-term patency rate of covered stents.18
Conclusion
In developing countries, endovascular treatment with a detachable balloon is still the first-line choice for TCCFs in selected patients because of its efficacy, safety, procedural simplicity, and especially its cost-effectiveness, despite having a nonnegligible recurrence rate. The interval between trauma and the interventional procedure might be the main risk factor related to recurrence.
Acknowledgements
We thank Zhan Zhang and Jifu Wei of Nanjing Medical University for help with the statistical analyses and writing this paper, respectively.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Barnwell SL, O'Neill OR. Endovascular therapy of carotid cavernous fistulas. Neurosurg Clin N Am. 1994;5:485–495. [PubMed] [Google Scholar]
- 2.Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985;62:248–256. doi: 10.3171/jns.1985.62.2.0248. [DOI] [PubMed] [Google Scholar]
- 3.Serbinenko FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. 1974;41:125–145. doi: 10.3171/jns.1974.41.2.0125. [DOI] [PubMed] [Google Scholar]
- 4.Lewis AI, Tomsick TA, Tew JM., Jr Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with detachable balloons. Neurosurgery. 1995;36:239–245. doi: 10.1227/00006123-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Luo CB, Teng MM, Chang FC, Chang CY. Transarterial balloon-assisted n-butyl-2-cyanoacrylate embolization of direct carotid cavernous fistulas. AJNR Am J Neuroradiol. 2006;27:1535–1540. [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida K, Melake M, Oishi H, Yamamoto M, Arai H. Transvenous embolization of dural carotid cavernous fistulas: a series of 44 consecutive patients. AJNR Am J Neuroradiol. 2010;31:651–655. doi: 10.3174/ajnr.A1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Archondakis E, Pero G, Valvassori L, Boccardi E, Scialfa G. Angiographic follow-up of traumatic carotid cavernous fistulas treated with endovascular stent graft placement. AJNR Am J Neuroradiol. 2007;28:342–347. [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Xie X, You C, Zhang C, Cheng M, He M, et al. Placement of covered stents for the treatment of direct carotid cavernous fistulas. AJNR Am J Neuroradiol. 2009;30:1342–1346. doi: 10.3174/ajnr.A1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lasjaunias P, Santoyo-Vazquez A. Segmental agenesis of the internal carotid artery: angiographic aspects with embryological discussion. Anat Clin. 1984;6:133–141. doi: 10.1007/BF01773165. [DOI] [PubMed] [Google Scholar]
- 10.van Rooij WJ, Sluzewski M, Beute GN. Ruptured cavernous sinus aneurysms causing carotid cavernous fistula: incidence, clinical presentation, treatment, and outcome. AJNR Am J Neuroradiol. 2006;27:185–189. [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Li YD, Li MH, Tan HQ, Gu BX, Wang J, et al. Endovascular treatment of post-traumatic direct carotid-cavernous fistulas: A single-center experience. J Clin Neurosci. 2011;18:24–28. doi: 10.1016/j.jocn.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Gemmete JJ, Chaudhary N, Pandey A, Ansari S. Treatment of carotid cavernous fistulas. Curr Treat Options Neurol. 2010;12:43–53. doi: 10.1007/s11940-009-0051-3. [DOI] [PubMed] [Google Scholar]
- 13.Luo CB, Teng MM, Yen DH, Chang FC, Lirng JF, Chang CY. Endovascular embolization of recurrent traumatic carotid-cavernous fistulas managed previously with detachable balloons. J Trauma. 2004;56:1214–1220. doi: 10.1097/01.ta.0000131213.93205.57. [DOI] [PubMed] [Google Scholar]
- 14.Bavinzski G, Killer M, Gruber A, Richling B. Treatment of post-traumatic carotico-cavernous fistulae using electrolytically detachable coils: technical aspects and preliminary experience. Neuroradiology. 1997;39:81–85. doi: 10.1007/s002340050371. [DOI] [PubMed] [Google Scholar]
- 15.Luo CB, Teng MM, Chang FC, Chang CY. Traumatic indirect carotid cavernous fistulas: angioarchitectures and results of transarterial embolization by liquid adhesives in 11 patients. Surg Neurol. 2009;71:216–222. doi: 10.1016/j.surneu.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 16.Li MH, Li YD, Tan HQ, Luo QY, Cheng YS. Treatment of distal internal carotid artery aneurysm with the willis covered stent: a prospective pilot study. Radiology. 2009;253:470–477. doi: 10.1148/radiol.2532090037. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Lan ZG, Xie XD, You C, He M. Traumatic carotid-cavernous fistulas treated with covered stents: experience of 12 cases. World Neurosurg. 2010;73:514–519. doi: 10.1016/j.wneu.2010.02.062. [DOI] [PubMed] [Google Scholar]
- 18.Hoit DA, Schirmer CM, Malek AM. Stent graft treatment of cerebrovascular wall defects: intermediate-term clinical and angiographic results. Neurosurgery. 2008;62:ONS380–ONS388. doi: 10.1227/01.neu.0000326022.08973.b2. [DOI] [PubMed] [Google Scholar]